Tamm Plasmon Polariton Biosensors Based on Porous Silicon: Design, Validation and Analysis
Abstract
:1. Introduction
2. Materials and Methods
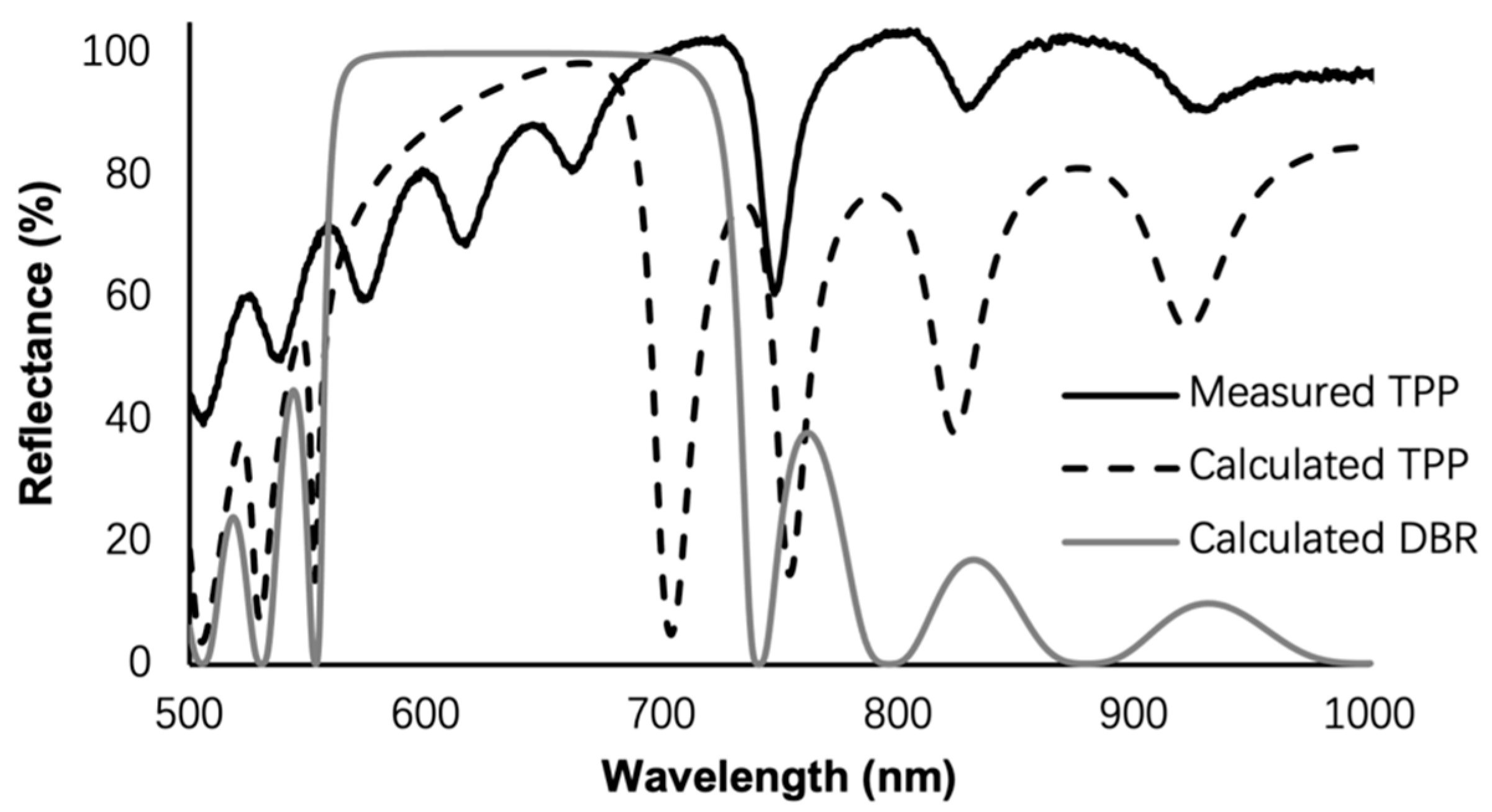
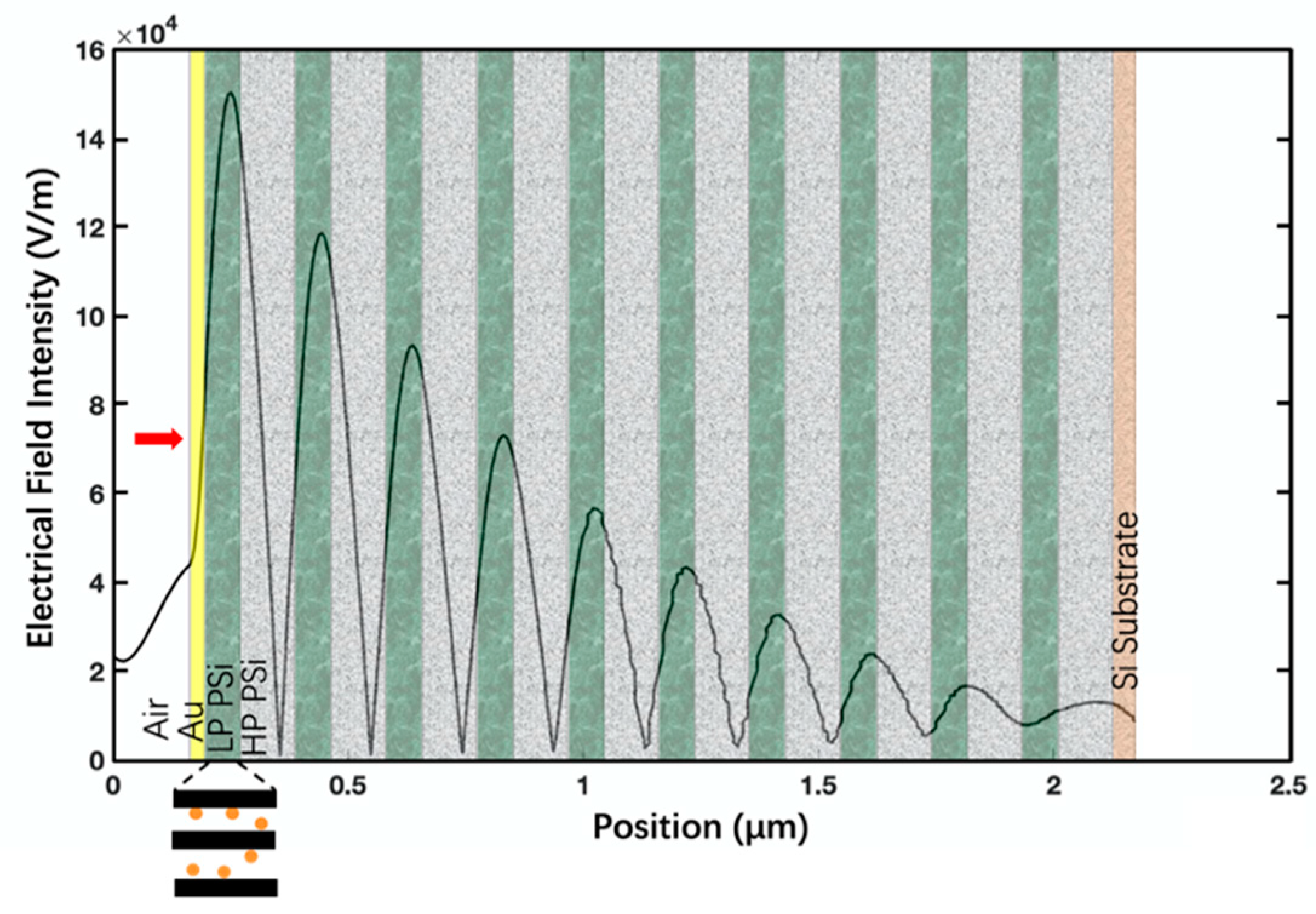
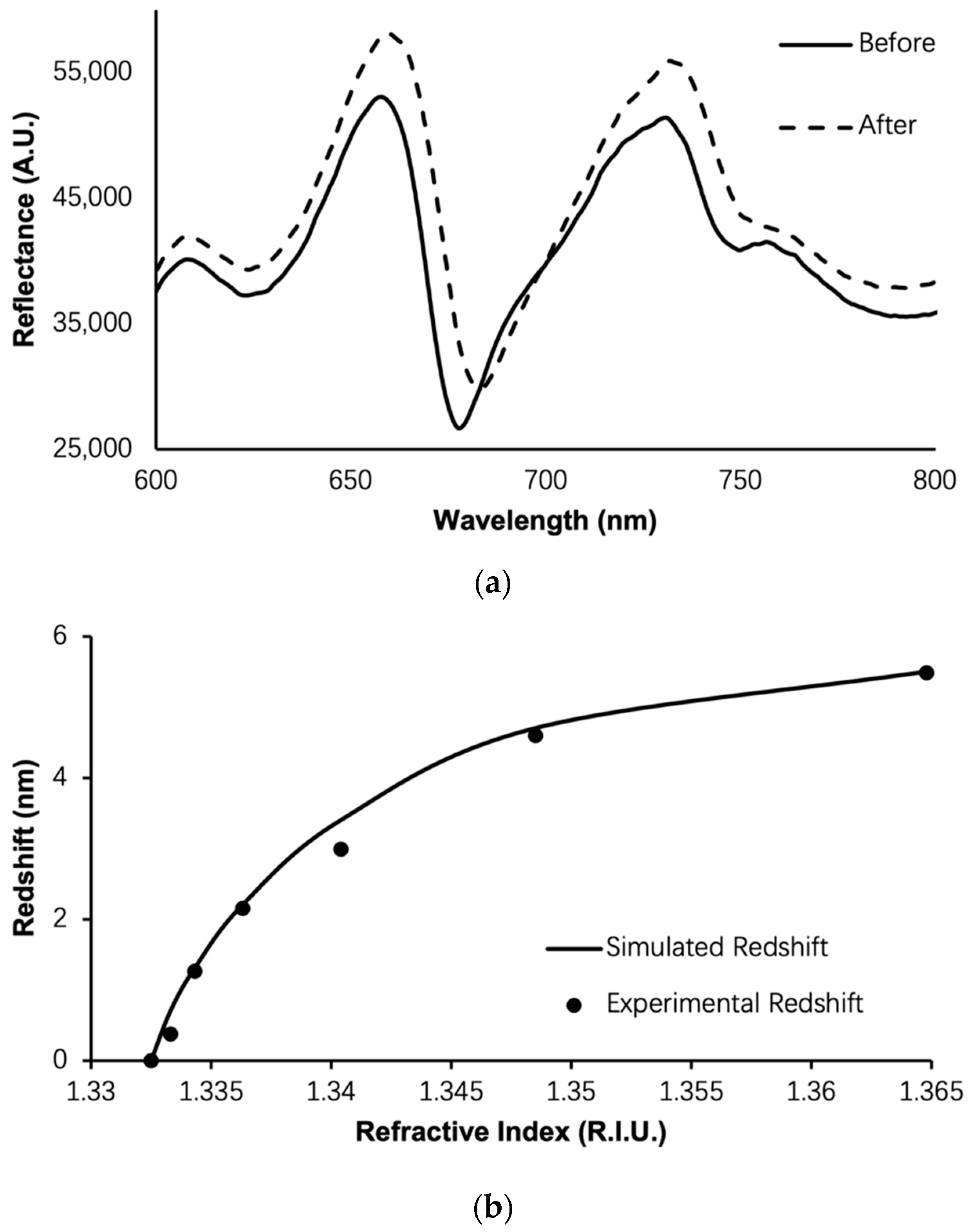
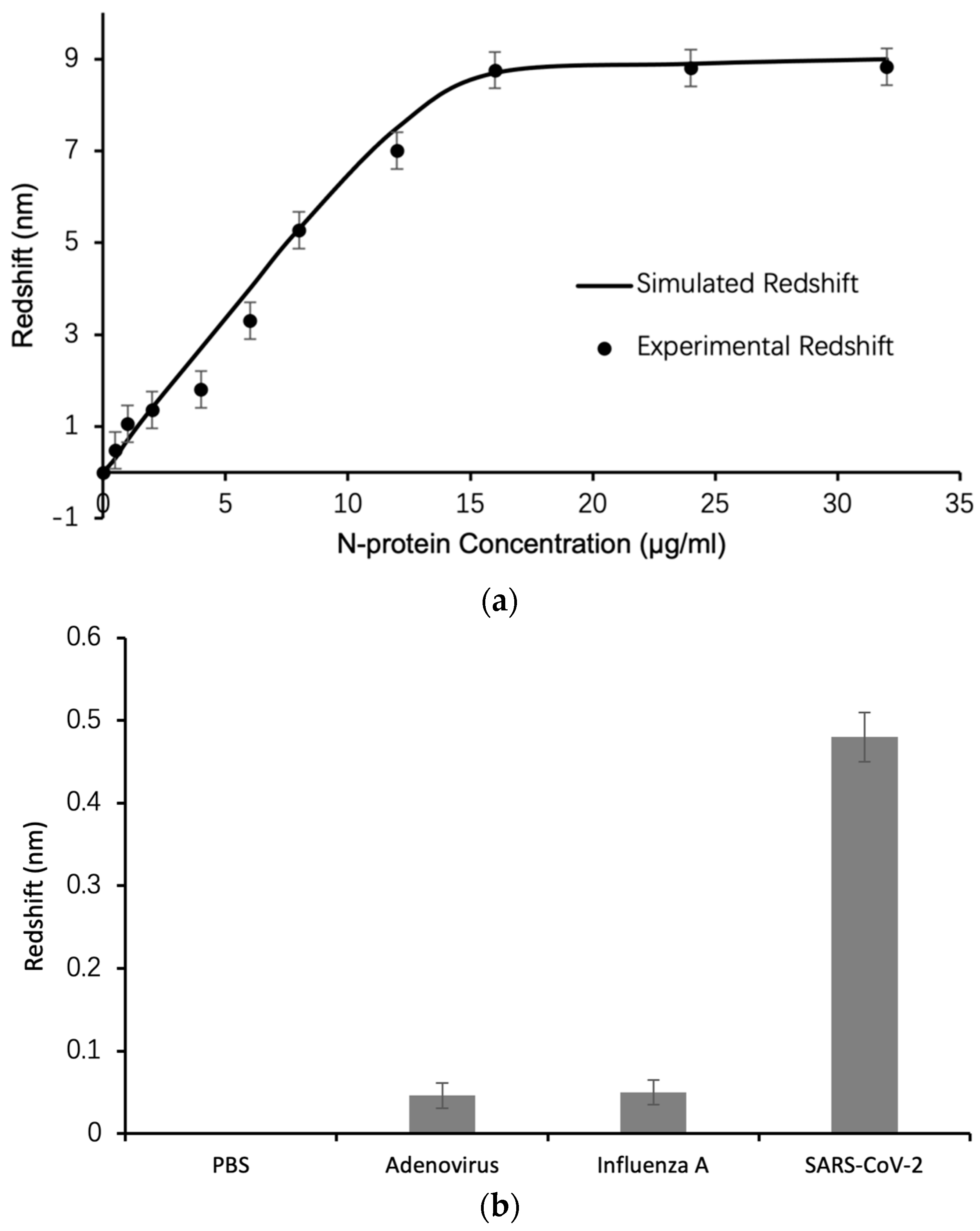
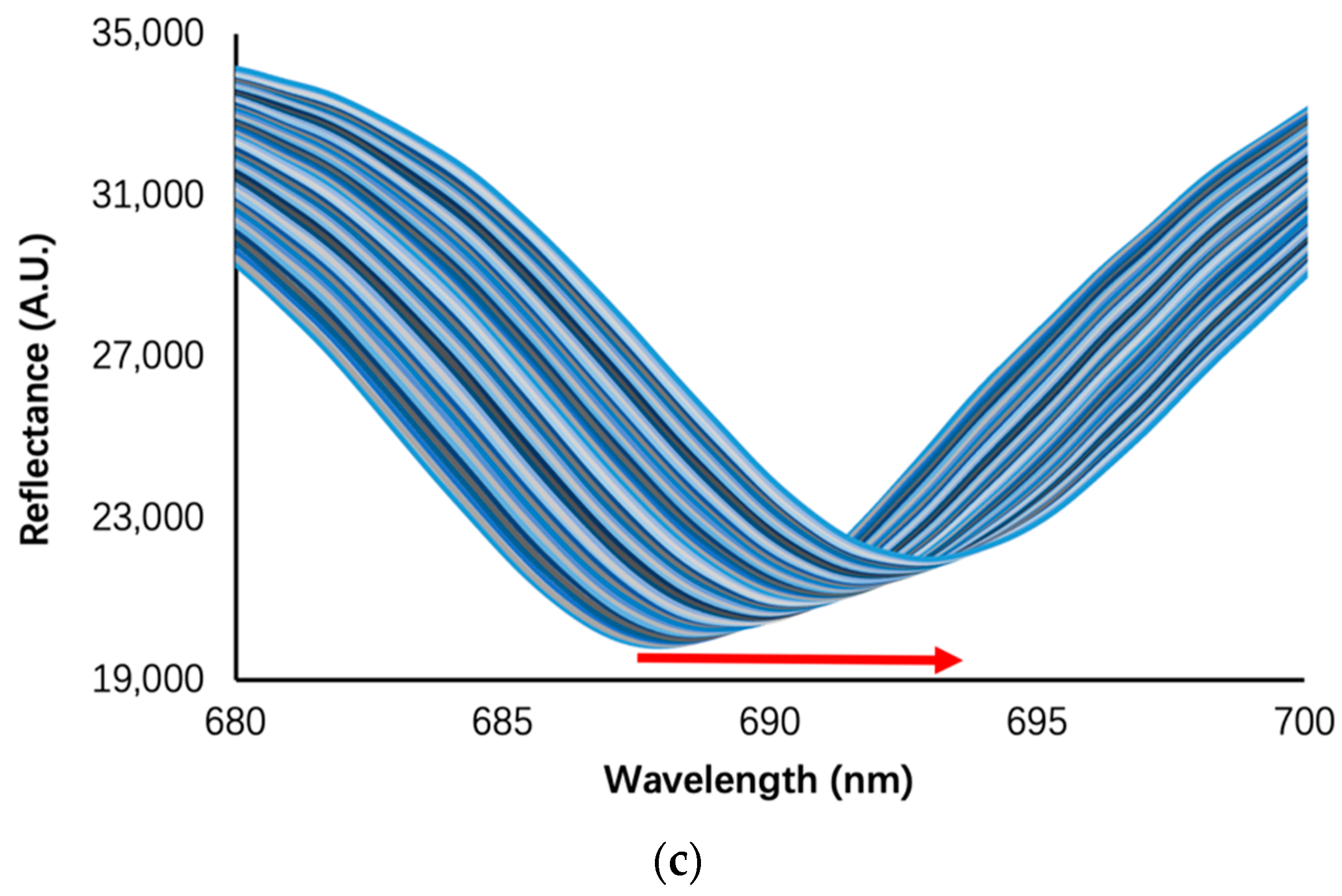
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bahavarnia, F.; Hasanzadeh, M.; Sadighbayan, D.; Seidi, F. Recent Progress and Challenges on the Microfluidic Assay of Pathogenic Bacteria Using Biosensor Technology. Biomimetics 2022, 7, 175. [Google Scholar] [CrossRef]
- Singh, P.; Pandey, V.K.; Srivastava, S.; Singh, R. A systematic review on recent trends and perspectives of biosensors in food industries. J. Food Saf. 2023, 43, e13071. [Google Scholar] [CrossRef]
- Herrera-Domínguez, M.; Morales-Luna, G.; Mahlknecht, J.; Cheng, Q.; Aguilar-Hernández, I.; Ornelas-Soto, N. Optical Biosensors and Their Applications for the Detection of Water Pollutants. Biosensors 2023, 13, 370. [Google Scholar] [CrossRef]
- Uhuo, O.V.; Waryo, T.T.; Januarie, K.C.; Nwambaekwe, K.C.; Ndipingwi, M.M.; Ekwere, P.; Iwuoha, E.I. Bioanalytical methods encompassing label-free and labeled tuberculosis aptasensors: A review. Anal. Chim. Acta 2022, 1234, 340326. [Google Scholar] [CrossRef] [PubMed]
- Uniyal, A.; Srivastava, G.; Pal, A.; Taya, S.; Muduli, A. Recent Advances in Optical Biosensors for Sensing Applications: A Review. Plasmonics 2023, 18, 735–750. [Google Scholar] [CrossRef]
- Li, B.C.; Zhang, R.C.; Bi, R.Z.; Olivo, M. Applications of Optical Fiber in Label-Free Biosensors and Bioimaging: A Review. Biosensors 2023, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Khonina, S.N.; Voronkov, G.S.; Grakhova, E.P.; Kazanskiy, N.L.; Kutluyarov, R.V.; Butt, M.A. Polymer Waveguide-Based Optical Sensors-Interest in Bio, Gas, Temperature, and Mechanical Sensing Applications. Coatings 2023, 13, 549. [Google Scholar] [CrossRef]
- Ning, S.P.; Chang, H.C.; Fan, K.C.; Hsiao, P.Y.; Feng, C.H.; Shoemaker, D.; Chen, R.T. A point-of-care biosensor for rapid detection and differentiation of COVID-19 virus (SARS-CoV-2) and influenza virus using subwavelength grating micro-ring resonator. Appl. Phys. Rev. 2023, 10, 021410. [Google Scholar] [CrossRef]
- Wang, R.D.; Yan, M.L.; Jiang, M.; Li, Y.; Kang, X.; Hu, M.X.; Liu, B.B.; He, Z.Q.; Kong, D.P. Label-free and selective cholesterol detection based on multilayer functional structure coated fiber fabry-perot interferometer probe. Anal. Chim. Acta 2023, 1252, 341051. [Google Scholar] [CrossRef]
- Miyan, H.; Agrahari, R.; Gowre, S.K.; Jain, P.K.; Mahto, M. Computational study of 2D photonic crystal based biosensor for SARS-COV-2 detection. Meas. Sci. Technol. 2023, 34, 074004. [Google Scholar] [CrossRef]
- Taya, S.A.; Doghmosh, N.; Almawgani, A.H.M.; Hindi, A.T.; Colak, I.; Alqanoo, A.A.M.; Patel, S.K.; Pal, A. Surface Plasmon Resonance Biosensor Based on STO and Graphene Sheets for Detecting Two Commonly Used Buffers: TRIS-Borate-EDTA and Dulbecco Phosphate Buffered Saline. Plasmonics 2023, 18, 1695–1703. [Google Scholar] [CrossRef]
- Coco, A.S.; Campos, F.V.; Diaz, C.A.R.; Guimaraes, M.C.C.; Prado, A.R.; de Oliveira, J.P. Localized Surface Plasmon Resonance-Based Nanosensor for Rapid Detection of Glyphosate in Food Samples. Biosensors 2023, 13, 512. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Rong, G.G.; Zhao, J.W.; Zhang, S.L.; Zhu, Y.X.; He, B.Y. A Nanoscale Porous Silicon Microcavity Biosensor for Novel Label-Free Tuberculosis Antigen-Antibody Detection. Nano 2012, 7, 1250049. [Google Scholar] [CrossRef]
- Rong, G.G.; Zheng, Y.Q.; Li, X.Q.; Guo, M.Z.; Su, Y.; Bian, S.M.; Dang, B.B.; Chen, Y.; Zhang, Y.J.; Shen, L.H.; et al. A high-throughput fully automatic biosensing platform for efficient COVID-19 detection. Biosens. Bioelectron. 2023, 220, 114861. [Google Scholar] [CrossRef] [PubMed]
- Rong, G.; Najmaie, A.; Sipe, J.E.; Weiss, S.M. Nanoscale porous silicon waveguide for label-free DNA sensing. Biosens. Bioelectron. 2008, 23, 1572–1576. [Google Scholar] [CrossRef]
- Rong, G.G.; YZheng, Q.; Yang, X.; Bao, K.J.; Xia, F.; Ren, H.H.; Bian, S.M.; Li, L.; Zhu, B.W.; Sawan, M. A Closed-Loop Approach to Fight Coronavirus: Early Detection and Subsequent Treatment. Biosensors 2022, 12, 900. [Google Scholar] [CrossRef]
- Weiss, S.M.; Rong, G.G. Porous Silicon Waveguides for Small Molecule Detection. Nanosci. Nanotechnol. Chem. Biol. Def. 2009, 1016, 185–194. [Google Scholar]
- Kaliteevski, M.; Iorsh, I.; Brand, S.; Abram, R.A.; Chamberlain, J.M.; Kavokin, A.V.; Shelykh, I.A. Tamm plasmon-polaritons: Possible electromagnetic states at the interface of a metal and a dielectric Bragg mirror. Phys. Rev. B 2007, 76, 165415. [Google Scholar] [CrossRef]
- Sasin, M.E.; Seisyan, R.P.; Kalitteevski, M.A.; Brand, S.; Abram, R.A.; Chamberlain, J.M.; Egorov, A.Y.; Vasil’ev, A.P.; Mikhrin, V.S.; Kavokin, A.V. Tamm plasmon polaritons: Slow and spatially compact light. Appl. Phys. Lett. 2008, 92, 251112. [Google Scholar] [CrossRef]
- Lu, H.; Li, Y.W.; Jia, H.; Li, Z.W.; Ma, D.; Zhao, J.L. Induced reflection in Tamm plasmon systems. Opt. Express 2019, 27, 5383–5392. [Google Scholar] [CrossRef]
- Normani, S.; Carboni, F.F.; Lanzani, G.; Scotognella, F.; Paterno, G.M. The impact of Tamm plasmons on photonic crystals technology. Phys. B-Condens. Matter 2022, 645, 414253. [Google Scholar] [CrossRef]
- Toanen, V.; Symonds, C.; Benoit, J.M.; Gassenq, A.; Lemaitre, A.; Bellessa, J. Room-Temperature Lasing in a Low-Loss Tamm Plasmon Cavity. ACS Photonics 2020, 7, 2952–2957. [Google Scholar] [CrossRef]
- Pyatnov, M.V.; Bikbaev, R.G.; Timofeev, I.V.; Vetrov, S.Y. Model of a tunable hybrid Tamm mode-liquid crystal device. Appl. Opt. 2020, 59, 6347–6351. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.J.; Huang, Y.; Zhong, S.C.; Lin, T.L.; Zhang, Z.H.; Zeng, Q.M.; Yao, L.G.; Yu, Y.J.; Peng, Z.K. Tamm plasmon polaritons induced active terahertz ultra-narrowband absorbing with MoS2. Opt. Laser Technol. 2022, 156, 108581. [Google Scholar] [CrossRef]
- Zhang, X.L.; Song, J.F.; Li, X.B.; Feng, J.; Sun, H.B. Optical Tamm states enhanced broad-band absorption of organic solar cells. Appl. Phys. Lett. 2012, 101, 243901. [Google Scholar] [CrossRef]
- Elsayed, H.A.; Taha, T.A.; Algarni, S.A.; Ahmed, A.M.; Mehaney, A. Evolution of optical Tamm states in a 1D photonic crystal comprising a nanocomposite layer for optical filtering and reflecting purposes. Opt. Quantum Electron. 2022, 54, 312. [Google Scholar] [CrossRef]
- Ruan, B.X.; Li, M.; Liu, C.; Gao, E.D.; Zhang, Z.B.; Chang, X.; Zhang, B.H.; Li, H.J. Slow-light effects based on the tunable Fano resonance in a Tamm state coupled graphene surface plasmon system. Phys. Chem. Chem. Phys. 2023, 25, 1685–1689. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.M.; Elsayed, H.A.; Mehaney, A. High-Performance Temperature Sensor Based on One-dimensional Pyroelectric Photonic Crystals Comprising Tamm/Fano Resonances. Plasmonics 2021, 16, 547–557. [Google Scholar] [CrossRef]
- Sabra, W.; Elsayed, H.A.; Mehaney, A.; Aly, A.H. Numerical optimization of 1D superconductor photonic crystals pressure sensor for low temperatures applications. Solid State Commun. 2022, 343, 114671. [Google Scholar] [CrossRef]
- Hao, H.Y.; Xing, F.; Li, L. Plasmon-based optical sensors for high-sensitivity surface deformation detection of silver and gold. Appl. Nanosci. 2020, 10, 3939–3944. [Google Scholar] [CrossRef]
- Zaky, Z.A.; Al-Dossari, M.; Zohny, E.I.; Aly, A.H. Refractive index sensor using Fibonacci sequence of gyroidal graphene and porous silicon based on Tamm plasmon polariton. Opt. Quantum Electron. 2023, 55, 6. [Google Scholar] [CrossRef]
- Sansierra, M.C.; Morrone, J.; Cornacchiulo, F.; Fuertes, M.C.; Angelome, P.C. Detection of Organic Vapors Using Tamm Mode Based Devices Built from Mesoporous Oxide Thin Films. Chemnanomat 2019, 5, 1289–1295. [Google Scholar] [CrossRef]
- Zaky, Z.A.; Hanafy, H.; Panda, A.; Pukhrambam, P.D.; Aly, A.H. Design and Analysis of Gas Sensor Using Tailorable Fano Resonance by Coupling Between Tamm and Defected Mode Resonance. Plasmonics 2022, 17, 2103–2111. [Google Scholar] [CrossRef]
- Liu, Y.M.; Zheng, Q.W.; Yuan, H.X.; Wang, S.P.; Yin, K.Q.; Dai, X.Y.; Zou, X.; Jiang, L.Y. High Sensitivity Terahertz Biosensor Based on Mode Coupling of a Graphene/Bragg Reflector Hybrid Structure. Biosensors 2021, 11, 377. [Google Scholar] [CrossRef]
- Panda, A.; Pukhrambam, P.D. Study of Metal-Porous GaN-Based 1D Photonic Crystal Tamm Plasmon Sensor for Detection of Fat Concentrations in Milk. Micro Nanoelectron. Devices Circuits Syst. 2023, 904, 415–425. [Google Scholar]
- Maji, P.S.; Shukla, M.K.; Das, R. Blood component detection based on miniaturized self-referenced hybrid Tamm-plasmon-polariton sensor. Sens. Actuators B-Chem. 2018, 255, 729–734. [Google Scholar] [CrossRef]
- Su, M.Y.; Li, K.S.; Wang, C.F.; Wu, L.M.; Yang, S.; Lin, Q.W.; Li, Y.; Tang, L.P.; Zhou, R.L. Tamm-plasmon-polariton biosensor based on one-dimensional topological photonic crystal. Results Phys. 2023, 48, 106454. [Google Scholar] [CrossRef]
- Hou, Q.R.; Chi, N. Calculation of surface areas for porous silicon. Mod. Phys. Lett. B 1999, 13, 1005–1009. [Google Scholar] [CrossRef]
- Northen, T.R.; Woo, H.K.; Northen, M.T.; Nordström, A.; Uritboonthail, W.; Turner, K.L.; Siuzdak, G. High surface area of porous silicon drives desorption of intact molecules. J. Am. Soc. Mass Spectrom. 2007, 18, 1945–1949. [Google Scholar] [CrossRef]
- Li, C.; Liao, H.L.; Huang, R.; Wang, Y.Y. A CMOS-compatible silicon substrate optimization technique and its application in radio frequency crosstalk isolation. Chin. Phys. B 2008, 17, 2730–2738. [Google Scholar]
- Juneau-Fecteau, A.; Frechette, L.G. Tamm plasmon-polaritons in a metal coated porous silicon photonic crystal. Opt. Mater. Express 2018, 8, 2774–2781. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Mehaney, A. Ultra-high sensitive 1D porous silicon photonic crystal sensor based on the coupling of Tamm/Fano resonances in the mid-infrared region. Sci. Rep. 2019, 9, 6973. [Google Scholar] [CrossRef] [PubMed]
- Juneau-Fecteau, A.; Savin, R.; Boucherif, A.; Frechette, L.G. A practical Tamm plasmon sensor based on porous Si. AIP Adv. 2021, 11, 065305. [Google Scholar] [CrossRef]
- Vyunishev, A.M.; Bikbaev, R.G.; Svyakhovskiy, S.E.; Timofeev, I.V.; Pankin, P.S.; Evlashin, S.A.; Vetrov, S.Y.; Myslivets, S.A.; Arkhipkin, V.G. Broadband Tamm plasmon polariton. J. Opt. Soc. Am. B-Opt. Phys. 2019, 36, 2299–2305. [Google Scholar] [CrossRef]
- Jeng, S.C. Applications of Tamm plasmon-liquid crystal devices. Liq. Cryst. 2020, 47, 1223–1231. [Google Scholar] [CrossRef]
- Kumari, A.; Kumar, S.; Shukla, M.K.; Kumar, G.; Maji, P.S.; Vijaya, R.; Das, R. Coupling to Tamm-plasmon-polaritons: Dependence on structural parameters. J. Phys. D-Appl. Phys. 2018, 51, 255103. [Google Scholar] [CrossRef]
- Khardani, M.; Bouaicha, M.; Bessais, B. Bruggeman effective medium approach for modelling optical properties of porous silicon: Comparison with experiment. Phys. Status Solidi C-Curr. Top. Solid State Phys. 2007, 4, 1986–1990. [Google Scholar] [CrossRef]
- Guerra, G.; Mousavi, S.M.A.; Taranta, A.; Fokoua, E.N.; Santagiustina, M.; Galtarossa, A.; Poletti, F.; Palmieri, L. Unified Coupled-Mode Theory for Geometric and Material Perturbations in Optical Waveguides. J. Light. Technol. 2022, 40, 4714–4727. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhou, X.H. A colorimetric sandwich-type bioassay for SARS-CoV-2 using a hACE2-based affinity peptide pair. J. Hazard. Mater. 2022, 425, 127923. [Google Scholar] [CrossRef]
- Passler, N.C.; Paarmann, A. Generalized 4 × 4 matrix formalism for light propagation in anisotropic stratified media: Study of surface phonon polaritons in polar dielectric heterostructures. J. Opt. Soc. Am. B-Opt. Phys. 2017, 34, 2128–2139. [Google Scholar] [CrossRef]
- Li, X.W.; Xiong, M.Y.; Deng, Q.L.; Guo, X.B.; Li, Y.R. The utility of SARS-CoV-2 nucleocapsid protein in laboratory diagnosis. J. Clin. Lab. Anal. 2022, 36, e24534. [Google Scholar] [CrossRef]
- Litchfield, M.; Brookes, P.; Ojrzynska, A.; Kavi, J.; Dawood, R. Comparison of the clinical sensitivity and specificity of two commercial RNA SARS-CoV-2 assays. Int. J. Infect. Dis. 2022, 118, 194–196. [Google Scholar] [CrossRef]
- Mistry, D.A.; Wang, J.Y.; Moeser, M.E.; Starkey, T.; Lee, L.Y.W. A systematic review of the sensitivity and specificity of lateral flow devices in the detection of SARS-CoV-2. BMC Infect. Dis. 2021, 21, 828. [Google Scholar] [CrossRef]
- Fox, T.; Geppert, J.; Dinnes, J.; Scandrett, K.; Bigio, J.; Sulis, G.; Hettiarachchi, D.; Mathangasinghe, Y.; Weeratunga, P.; Wickramasinghe, D.; et al. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst. Rev. 2022. [Google Scholar] [CrossRef]
- Gong, P.; Antrim, A.K.; Bickman, S.R.; Cooley, E.G.; Chung, S.H. Sandwich Hybridization Assay for In Situ Real-Time Cyanobacterial Detection and Monitoring: A Review. Biosensors 2022, 12, 640. [Google Scholar] [CrossRef] [PubMed]

| Structure | Porosity | Current Density | Etch Time | Bruggeman Effective R.I. | Thickness |
|---|---|---|---|---|---|
| LP PSi | 52% | 5 mA/cm2 | 20 s | 2.08 | 100 nm |
| HP PSi | 76% | 48 mA/cm2 | 6 s | 1.41 | 150 nm |
| No. | Step Name | Step Operation |
|---|---|---|
| 1 | Chip cleaning | Clean the biosensor chips using a plasma cleaning machine, ethanol, isopropanol, and ultrapure water, and then place them in a 96 well plate container for later use. |
| 2 | Sulfhydryl proteinA modified chip | Dilute mercapto proteinA (Xlement Cat. No. G60001) with ultrapure water to a working concentration of 50 μg/mL, take an appropriate amount of mercapto proteinA solution and add it to the biosensor chip surface. Leave at 4 °C overnight or 37 °C for 2 h. |
| 3 | Preparation of coating antibody solution | Take COVID-19 N-protein antibody (Xlement Cat. No. C10002), use coupling buffer solution (Xlement Cat. No. S20029) to prepare 50 μg/mL of coating antibody solution. |
| 4 | Chip directed immobilization of antibodies | Take an appropriate amount of 50 μg/mL of coating antibody solution and apply on the surface of the biosensor chip and react at 37 °C (with shaking) for 20–30 min. After reaction completes, clean the biosensor chip twice with Phosphate-buffered saline (PBS) buffer solution (pH~7.4). |
| 5 | Closure | Take an appropriate amount of sealing solution (Xlement Cat. No. G30004) and add it to the surface of the biosensor chip. Leave it at 37 °C for 30 min. After removing and drying the sealing solution, the chips can be used directly for bioanalytical detection assay. Alternatively, perform the following steps before storing chips for future use. |
| 6 | Protection | Take an appropriate amount of protective solution (Xlement Cat. No. G30006) and add it to the surface of the biosensor chip. Place it at 37 °C for 30 min, and then remove and dry the protective solution. |
| 7 | Chip drying | Place the modified chip in a 37 °C oven and dry for 5 min. |
| 8 | Plastic sealing | Use a sealing machine to vacuum seal the wrapped chips and refrigerate them for storage. |
| 9 | Storage | The sealed chip is generally stable with shelf life of 3 days at 37 °C and 6 months at 4 °C. Store the vacuum sealed chips in dry and dark conditions can also extend the shelf life. |
| 10 | Biosensing | Open the chip sealing. Drop 20 µL of N-protein solution (Xlement Cat. No. C10002) in varying concentrations in PBS buffer on biosensor surface. Put on cover glass and take spectral measurement with fiber spectrometer. |
| Technologies | Target | Sensitivity | Specificity | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Reverse transcription polymerase chain reaction (RT-PCR) [52] | Specific gene sequence, such as ORF1ab | >90% | Nearly 100% | Accurate result, current gold standard, high throughput. | Need clean environment, complex equipment, and staff training, slow turnaround |
| Antigen detection by lateral flow [53] | Viral proteins such as N-protein Or S-protein | 37.7–99.2% | 92.4–100% | Rapid and onsite detection, no need for equipment. | Results not accurate due to dynamic antigen secretion and sensitivity limitations. |
| Antibody detection by lateral flow [54] | Immune globulin such as IgG or IgM | 41.1–95% | 98.6–99.8% | Rapid and onsite detection, no need for equipment. | Positive results require further verification by RT-PCR or CT scan. |
| TPP biosensor (this work) | N-protein | >90% | >95% | Rapid and onsite detection, high throughput. | Need handheld or desktop equipment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rong, G.; Sawan, M. Tamm Plasmon Polariton Biosensors Based on Porous Silicon: Design, Validation and Analysis. Biosensors 2023, 13, 1026. https://doi.org/10.3390/bios13121026
Rong G, Sawan M. Tamm Plasmon Polariton Biosensors Based on Porous Silicon: Design, Validation and Analysis. Biosensors. 2023; 13(12):1026. https://doi.org/10.3390/bios13121026
Chicago/Turabian StyleRong, Guoguang, and Mohamad Sawan. 2023. "Tamm Plasmon Polariton Biosensors Based on Porous Silicon: Design, Validation and Analysis" Biosensors 13, no. 12: 1026. https://doi.org/10.3390/bios13121026






