A Fiber-Optical Dosimetry Sensor for Gamma-Ray Irradiation Measurement in Biological Applications
Abstract
:1. Introduction
2. Materials and Methods
3. The Result and Discussion
3.1. The FOD Holder Design Consideration
3.2. The FOD Sample Consideration
3.3. The TL Dose Response
4. The Absorbed Dose Distribution Calculations
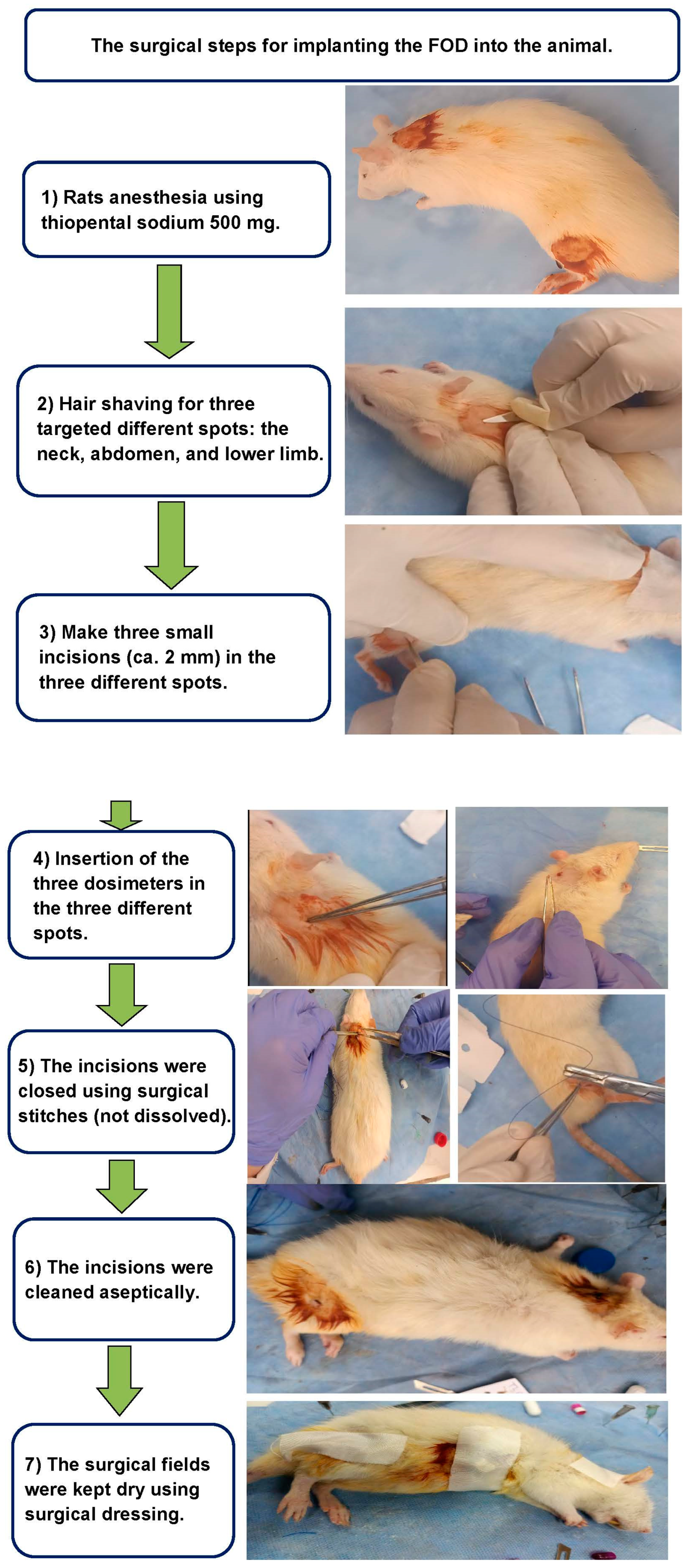
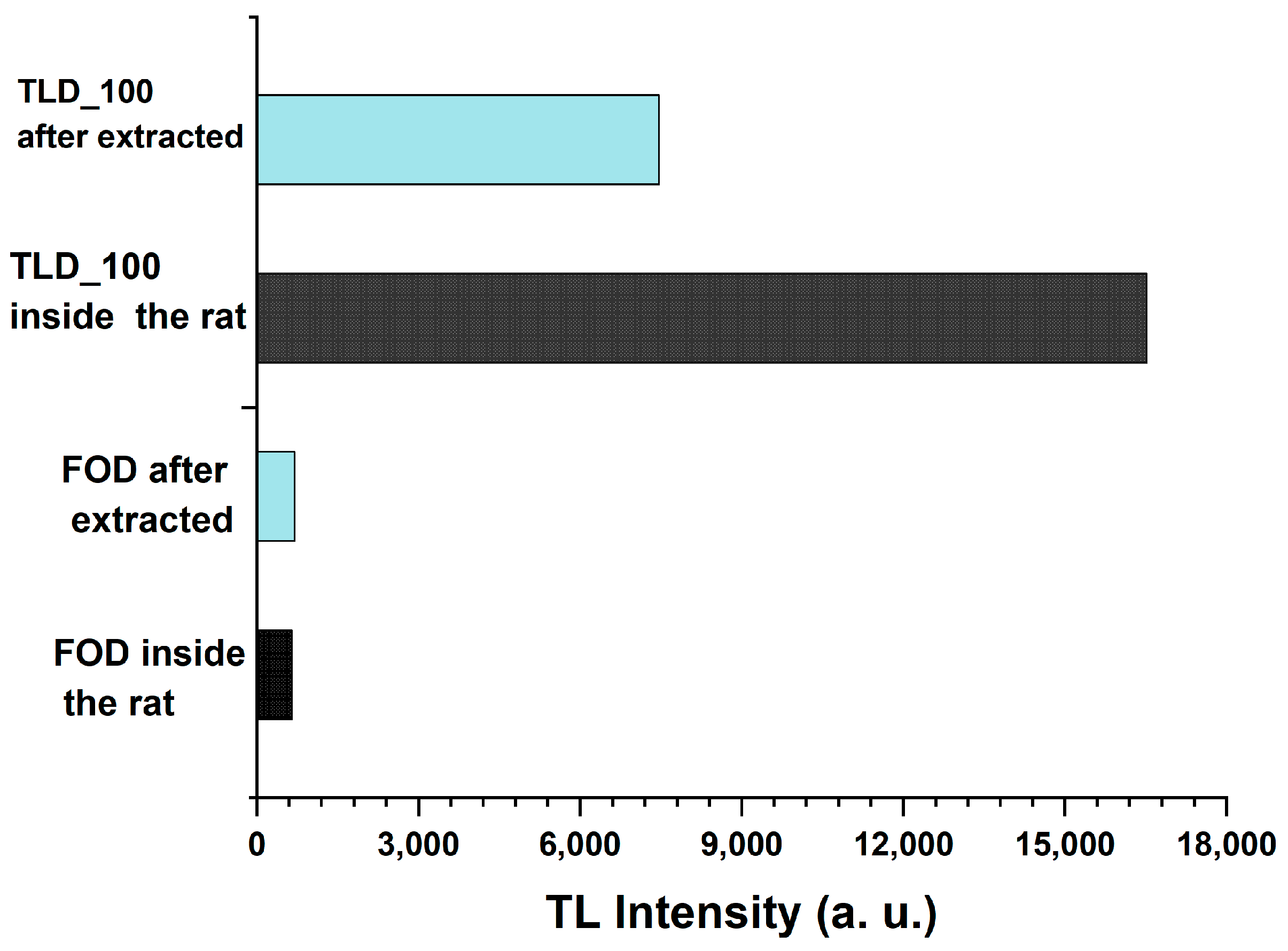
5. The FOD Transplanted Inside Animals
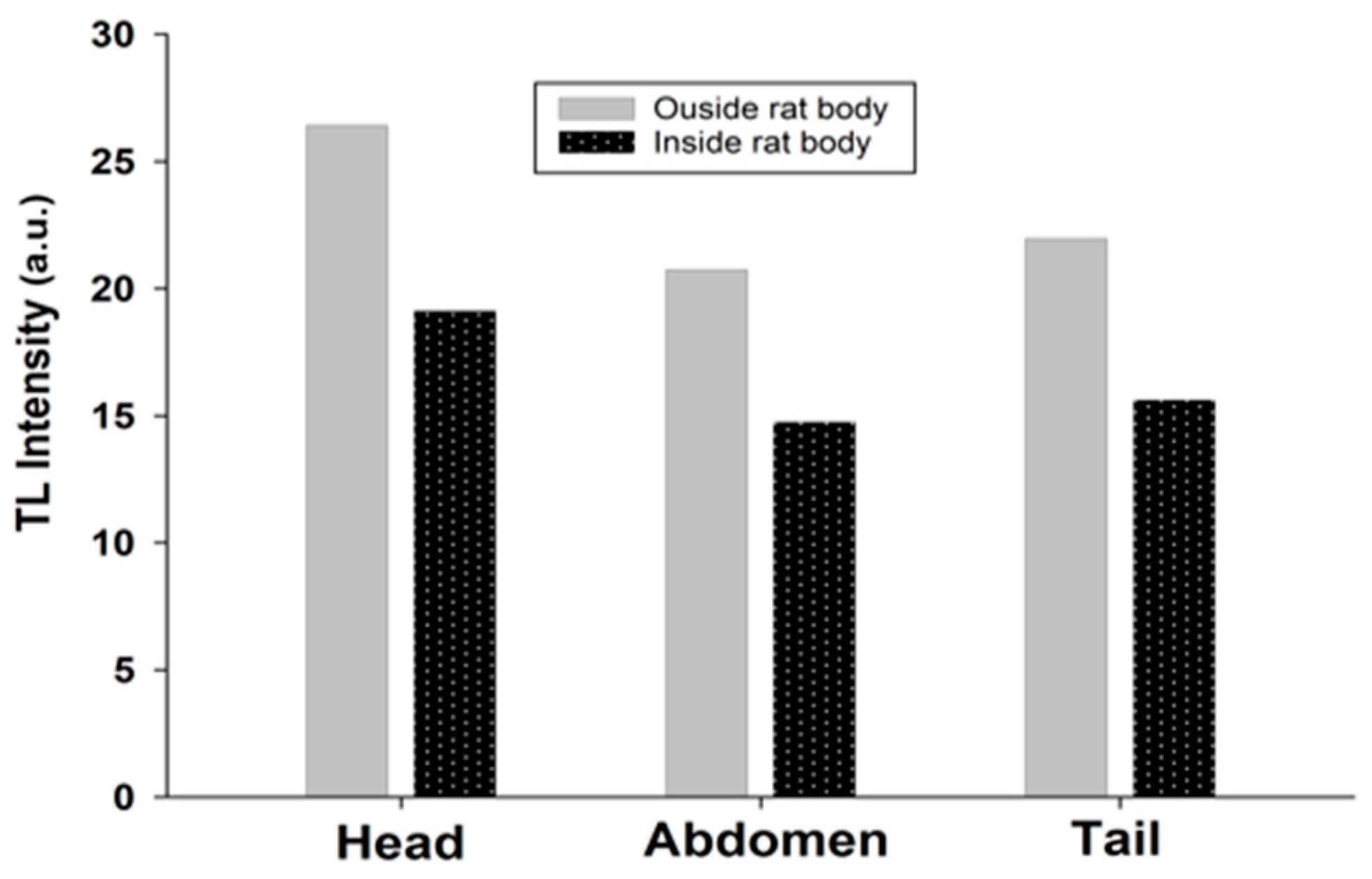
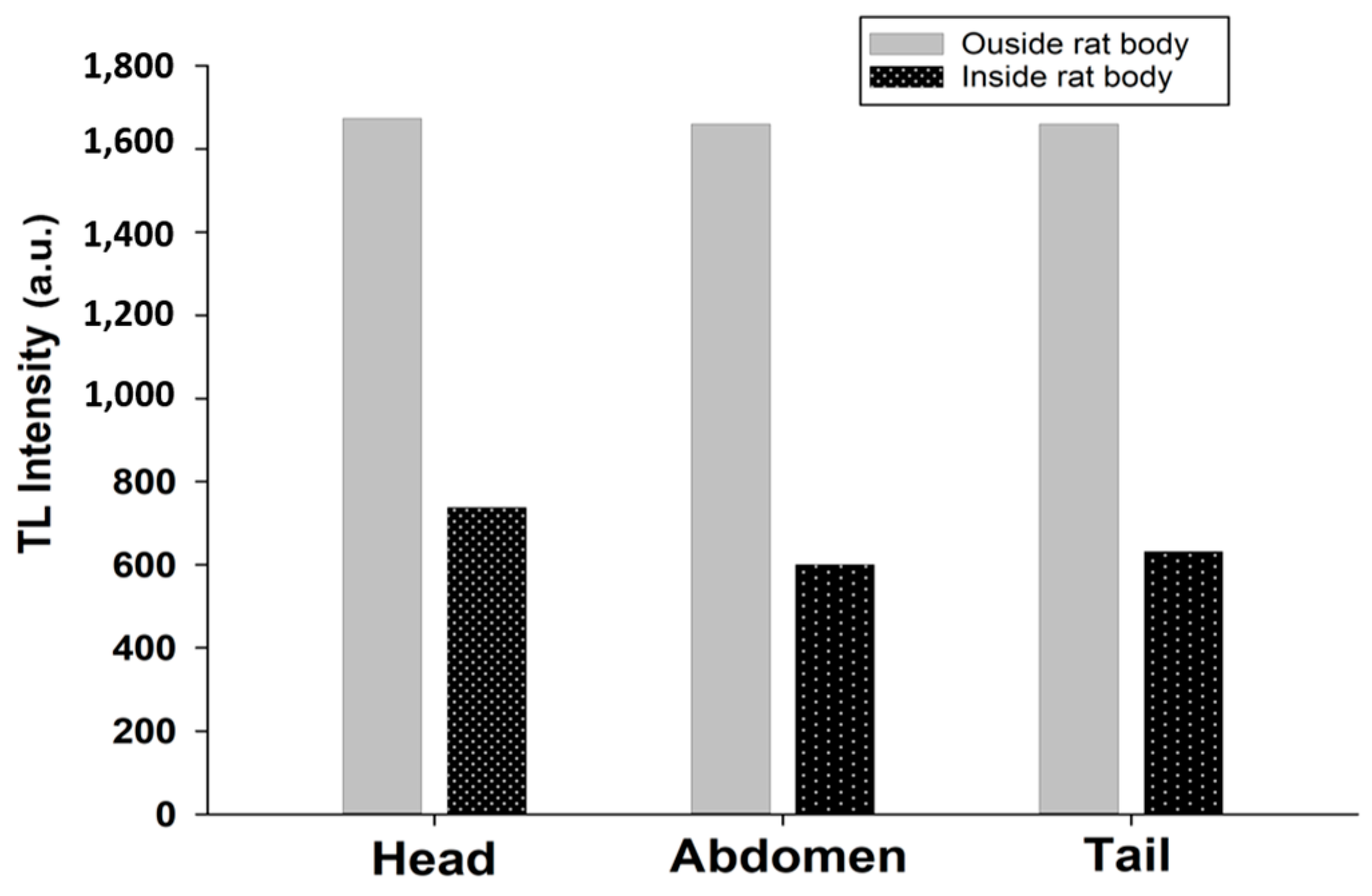
6. Dose Assessment
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yukihara, E.G.; McKeever, S.W.S.; Andersen, C.E.; Bos, A.J.J.; Bailiff, I.K.; Yoshimura, E.M.; Sawakuchi, G.O.; Bossin, L.; Christensen, J.B. Luminescence Dosimetry. Nat. Rev. Methods Prim. 2022, 2, 26. [Google Scholar] [CrossRef]
- Murthy, K.V.R. Thermoluminescence and Its Applications: A Review. Defect Diffus. Forum 2014, 347, 35–73. [Google Scholar] [CrossRef]
- Knoll, G.F. Radiation Detection and Measurement; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Kase, K. The Dosimetry of Ionizing Radiation; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Yasin, M.; Harun, S.W.; Arof, H. Selected Topics on Optical Fiber Technology; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar]
- Shaaban, A.; Du, Y.-C. An Optical Universal Plasmon-Based Biosensor for Virus Detection. J. Med. Biol. Eng. 2023, 43, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Hashim, S.; Al-Ahbabi, S.; Bradley, D.A.; Webb, M.; Jeynes, C.; Ramli, A.T.; Wagiran, H. The Thermoluminescence Response of Doped SiO2 Optical Fibres Subjected to Photon and Electron Irradiations. Appl. Radiat. Isot. 2009, 67, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Mattem, P.L.; Watkins, L.M.; Skoog, C.D.; Brandon, J.R.; Barsis, E.H. The Effects of Radiation on the Absorption and Luminescence of Fiber Optic Waveguides and Materials. IEEE Trans. Nucl. Sci. 1974, 21, 81–95. [Google Scholar] [CrossRef]
- Okamoto, K.; Toh, K.; Nagata, S.; Tsuchiya, B.; Suzuki, T.; Shamoto, N.; Shikama, T. Temperature Dependence of Radiation Induced Optical Transmission Loss in Fused Silica Core Optical Fibers. J. Nucl. Mater. 2004, 329, 1503–1506. [Google Scholar] [CrossRef]
- Regnier, E.; Flammer, I.; Girard, S.; Gooijer, F.; Achten, F.; Kuyt, G. Low-Dose Radiation-Induced Attenuation at Infrared Wavelengths for P-Doped, Ge-Doped and Pure Silica-Core Optical Fibres. IEEE Trans. Nucl. Sci. 2007, 54, 1115–1119. [Google Scholar] [CrossRef]
- Rahman, A.K.M.M.; Zubair, H.T.; Begum, M.; Abdul-rashid, H.A.; Amouzad, G.; Amin, Y.M.; Maah, M.J.; Bradley, D.A. Germanium-Doped Optical Fi Ber for Real-Time Radiation Dosimetry. Radiat. Phys. Chem. 2015, 116, 170–175. [Google Scholar] [CrossRef]
- Girard, S.; Kuhnhenn, J.; Gusarov, A.; Brichard, B.; Van Uffelen, M.; Ouerdane, Y.; Boukenter, A.; Marcandella, C. Radiation Effects on Silica-Based Optical Fibers: Recent Advances and Future Challenges. IEEE Trans. Nucl. Sci. 2013, 60, 2015–2036. [Google Scholar] [CrossRef]
- Zubair, H.T.; Begum, M.; Moradi, F.; Rahman, A.K.M.M.; Mahdiraji, G.A.; Oresegun, A.; Louay, G.T.; Omar, N.Y.M.; Khandaker, M.U.; Adikan, F.R.M.; et al. Recent Advances in Silica Glass Optical Fiber for Dosimetry Applications. IEEE Photonics J. 2020, 12, 1–25. [Google Scholar] [CrossRef]
- Yasin, M.; Harun, S.W.; Arof, H. Fiber Optic Sensors; CRC press: Boca Raton, FL, USA, 2017; ISBN 9789533079226. [Google Scholar]
- Moradi, F.; Mahdiraji, G.A.; Khandaker, M.U.; Ung, N.M.; Mahamd Adikan, F.R.; Khellaf, I.; Bradley, D.A. Investigation on Various Types of Silica Fibre as Thermoluminescent Sensors for Ultra-High Dose Radiation Dosimetry. Sens. Actuators A Phys. 2018, 273, 197–205. [Google Scholar] [CrossRef]
- Ghomeishi, M.; Mahdiraji, G.A.; Adikan, F.R.M.; Ung, N.M.; Bradley, D.A. Sensitive Fibre-Based Thermoluminescence Detectors for High Resolution In-Vivo Dosimetry. Sci. Rep. 2015, 5, 13309. [Google Scholar] [CrossRef] [PubMed]
- Sporea, D.; Sporea, A.; OKeeffe, S.; McCarthy, D.; Lewis, E. Optical Fibers and Optical Fiber Sensors Used in Radiation Monitoring. In Selected Topics on Optical Fiber Technology; InTech: Vienna, Austria, 2012. [Google Scholar] [CrossRef]
- Kovačević, M.S.; Djordjevich, A.; Savović, S.; Bajić, J.S.; Stupar, D.Z.; Slankamenac, M.P.; Kovačević, M. Measurement of 60CO Gamma Radiation Induced Attenuation in Multimode Step-Index POF at 530 Nm. Nucl. Technol. Radiat. Prot. 2013, 28, 158–162. [Google Scholar] [CrossRef]
- Espinosa, G.; Golzarri, J.I.; Bogard, J.; García-Macedo, J. Commercial Optical Fibre as TLD Material. Radiat. Prot. Dosimetry 2006, 119, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Thermo Scientific. Thermoluminescence (TL) Reader. Available online: https://www.thermofisher.com/order/catalog/product/3500TLDDS3 (accessed on 2 December 2023).
- Bos, A.J.J. Thermoluminescence as a Research Tool to Investigate Luminescence Mechanisms. Materials 2017, 10, 1357. [Google Scholar] [CrossRef] [PubMed]
- Meditron TLD-100TM Thermoluminescent Dosimetry Material. Available online: https://www.meditron.ch/++INDUSTRIAL++/index.php/passive-dosimeters/tld-materials/product/250-tld-100-tm-thermoluminescent-dosimetry-material (accessed on 2 December 2023).
- Issa, F.; Latip, N.A.A.; Bradley, D.A.; Nisbet, A. Ge-Doped Optical Fibres as Thermoluminescence Dosimeters for Kilovoltage X-Ray Therapy Irradiations. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2011, 652, 834–837. [Google Scholar] [CrossRef]
- Search, H.; Journals, C.; Contact, A.; Iopscience, M.; Address, I.P. Assessment of Ge-Doped Optical Fibres Subjected to x-Ray Irradiation. J. Phys. Conf. Ser. 2014, 546, 012017. [Google Scholar] [CrossRef]
- Hashim, S.; Omar, S.S.C.; Ibrahim, S.A.; Hassan, W.M.S.W.; Ung, N.M.; Mahdiraji, G.A.; Bradley, D.A.; Alzimami, K. Thermoluminescence Response of Flat Optical Fiber Subjected to 9 MeV Electron Irradiations. Radiat. Phys. Chem. 2015, 106, 46–49. [Google Scholar] [CrossRef]
- Entezam, A.; Khandaker, M.U.; Amin, Y.M.; Ung, N.M.; Maah, J.; Bradley, D.A. Thermoluminescence Response of Ge-Doped SiO2 Fibres to Electrons, X-and γ-Radiation. Radiat. Phys. Chem. 2016, 121, 115–121. [Google Scholar] [CrossRef]
- Begum, M.; Rahman, A.K.M.M.; Abdul-Rashid, H.A.; Wan Abdullah, W.S.; Mohd Noor, N.; Zulkifli, M.I.; Muhamad-Yasin, S.Z.; Mat-Sharif, K.A.; Tamchek, N.; Mat Nawi, S.N.; et al. Thermoluminescence Response of Ge-Doped Optical Fiber Dosimeters with Different Core Sizes. In Proceedings of the 2013 IEEE 4th International Conference on Photonics (ICP), Melaka, Malaysia, 28–30 October 2013; pp. 291–293. [Google Scholar]
- Hassan, M.F.; Rahman, W.N.W.A.; Fadzil, M.S.A.; Tominaga, T.; Geso, M.; Akasaka, H.; Bradley, D.A.; Noor, N.M. The Thermoluminescence Response of Ge-Doped Flat Fibre for Proton Beam Measurements: A Preliminary Study. J. Phys. Conf. Ser. 2017, 851, 12034. [Google Scholar] [CrossRef]
- Hassan, M.F.; Rahman, W.; Tominaga, T.; Geso, M.; Akasaka, H.; Bradley, D.A.; Noor, N.M. Ge-Doped Silica Fibre for Proton Beam Dosimetry. Radiat. Phys. Chem. 2019, 165, 108390. [Google Scholar] [CrossRef]
- Abdul Rahman, A.T.; Nisbet, A.; Bradley, D.A. Dose-Rate and the Reciprocity Law: TL Response of Ge-Doped SiO2 Optical Fibers at Therapeutic Radiation Doses. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2011, 652, 891–895. [Google Scholar] [CrossRef]
- Ramli, A.T.; Bradley, D.A.; Hashim, S.; Wagiran, H. The Thermoluminescence Response of Doped SiO2 Optical Fibres Subjected to Alpha-Particle Irradiation. Appl. Radiat. Isot. 2009, 67, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Hashim, S.; Bradley, D.A.; Saripan, M.I.; Ramli, A.T.; Wagiran, H. The Thermoluminescence Response of Doped SiO2 Optical Fibres Subjected to Fast Neutrons. Appl. Radiat. Isot. 2010, 68, 700–703. [Google Scholar] [CrossRef]
- Rodrigues, L.C.V.; Stefani, R.; Brito, H.F.; Felinto, M.C.F.C.; Hls, J.; Lastusaari, M.; Laamanen, T.; Malkamki, M. Thermoluminescence and Synchrotron Radiation Studies on the Persistent Luminescence of BaAl2O4:Eu2+,Dy3+. J. Solid State Chem. 2010, 183, 2365–2371. [Google Scholar] [CrossRef]
- Wahib, N.; Zulkepely, N.N.; Nawi, S.N.M.; Amin, Y.M.; Ling, Y.S.; Sani, S.F.A.; Maah, M.J.; Bradley, D.A. Gamma Irradiated Thermoluminescence Response of Ge-Doped SiO2 Fibre. Appl. Radiat. Isot. 2015, 105, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Mahdirajia, G.A.; Ghomeishi, M.; Dermosesian, E.; Hashim, S.; Ung, N.M.; Adikan, F.R.M.; Bradley, D.A. Optical Fiber Based Dosimeter Sensor: Beyond TLD-100 Limits. Sens. Actuators A Phys. 2015, 222, 48–57. [Google Scholar] [CrossRef]
- Yusoff, A.L.; Hugtenburg, R.P.; Bradley, D.A. Review of Development of a Silica-Based Thermoluminescence Dosimeter. Radiat. Phys. Chem. 2005, 74, 459–481. [Google Scholar] [CrossRef]
- Kuo, P.-L.; Yen, L.B.; Du, Y.-C.; Chen, P.-F.; Tsai, P.-Y. Combination of xgboost analysis and rule-based method for intrapartum cardiotocograph classification. J. Med. Biol. Eng. 2021, 41, 534–542. [Google Scholar] [CrossRef]
- Flexilicate Sdn Bhd Optical Fiber. Available online: https://flexilicate.com (accessed on 2 December 2023).
- Al-Charm Ti-6A1-4V. Available online: https://www.al-charm.com.tw (accessed on 2 December 2023).
- Izewska, J.; Andreo, P. The IAEA/WHO TLD Postal Programme for Radiotherapy Hospitals. Radiother. Oncol. 2000, 54, 65–72. [Google Scholar] [CrossRef]
- Izewska, J.; Novotny, J.; Van Dam, J.; Dutreix, A.; der Schueren, E. The Influence of the IAEA Standard Holder on Dose Evaluated from TLD Samples. Phys. Med. Biol. 1996, 41, 465. [Google Scholar] [CrossRef] [PubMed]
- Iżewska, J.; Georg, D.; Bera, P.; Thwaites, D.; Arib, M.; Saravi, M.; Sergieva, K.; Li, K.; Yip, F.G.; Mahant, A.K.; et al. A Methodology for TLD Postal Dosimetry Audit of High-Energy Radiotherapy Photon Beams in Non-Reference Conditions. Radiother. Oncol. 2007, 84, 67–74. [Google Scholar] [CrossRef]
- Noor, N.M.; Hussein, M.; Kadni, T.; Bradley, D.A.; Nisbet, A. Characterization of Ge-Doped Optical Fibres for MV Radiotherapy Dosimetry. Radiat. Phys. Chem. 2014, 98, 33–41. [Google Scholar] [CrossRef]
- Lam, S.E.; Bradley, D.A.; Khandaker, M.U. Small-Field Radiotherapy Photon Beam Output Evaluation: Detectors Reviewed. Radiat. Phys. Chem. 2021, 178, 108950. [Google Scholar] [CrossRef]
- Chen, R.; Mckeever, S.W.S. Theory of Thermoluminescence and Related Phenomena Thermally and Optically Stimulated Luminescence: A Simulation Approach Reuven Chen Vasilis Pagonis; World Scientific Pub Co. Pte Ltd.: Singapore, 1997. [Google Scholar] [CrossRef]
- Ermis, E.E.; Pilicer, F.B.; Pilicer, E.; Celiktas, C. A Comprehensive Study for Mass Attenuation Coefficients of Different Parts of the Human Body through Monte Carlo Methods. Nucl. Sci. Tech. 2016, 27, 54. [Google Scholar] [CrossRef]
- Böhlen, T.T.; Cerutti, F.; Chin, M.P.W.; Fassò, A.; Ferrari, A.; Ortega, P.G.; Mairani, A.; Sala, P.R.; Smirnov, G.; Vlachoudis, V. The FLUKA Code: Developments and Challenges for High Energy and Medical Applications. Nucl. Data Sheets 2014, 120, 211–214. [Google Scholar] [CrossRef]
- Ferrari, A.; Ranft, J.; Sala, P.R.; Fassò, A. FLUKA: A Multi-Particle Transport Code (Program Version 2005); CERN: Geneva, Switzerland, 2005. [Google Scholar]
- Allison, J.; Amako, K.; Apostolakis, J.E.A.; Araujo, H.; Dubois, P.A.; Asai, M.; Barrand, G.; Capra, R.; Chauvie, S.; Chytracek, R.; et al. Geant4 Developments and Applications. IEEE Trans. Nucl. Sci. 2006, 53, 270–278. [Google Scholar] [CrossRef]
- Sai, P.; Pappu, S.; Jayasree, V.Y. Numerical Computation of SAR in Human Head with Transparent Shields Using Transmission Line Method. Prog. Electromagn. Res. M 2021, 105, 31–44. [Google Scholar]
- Askar, M.A.; El Shawi, O.E.; Mansour, N.A.; Hanafy, A.M. Breast Cancer Suppression by Curcumin-Naringenin-Magnetic-Nano-Particles: In Vitro and in Vivo Studies. Tumor Biol. 2021, 43, 225–247. [Google Scholar] [CrossRef]






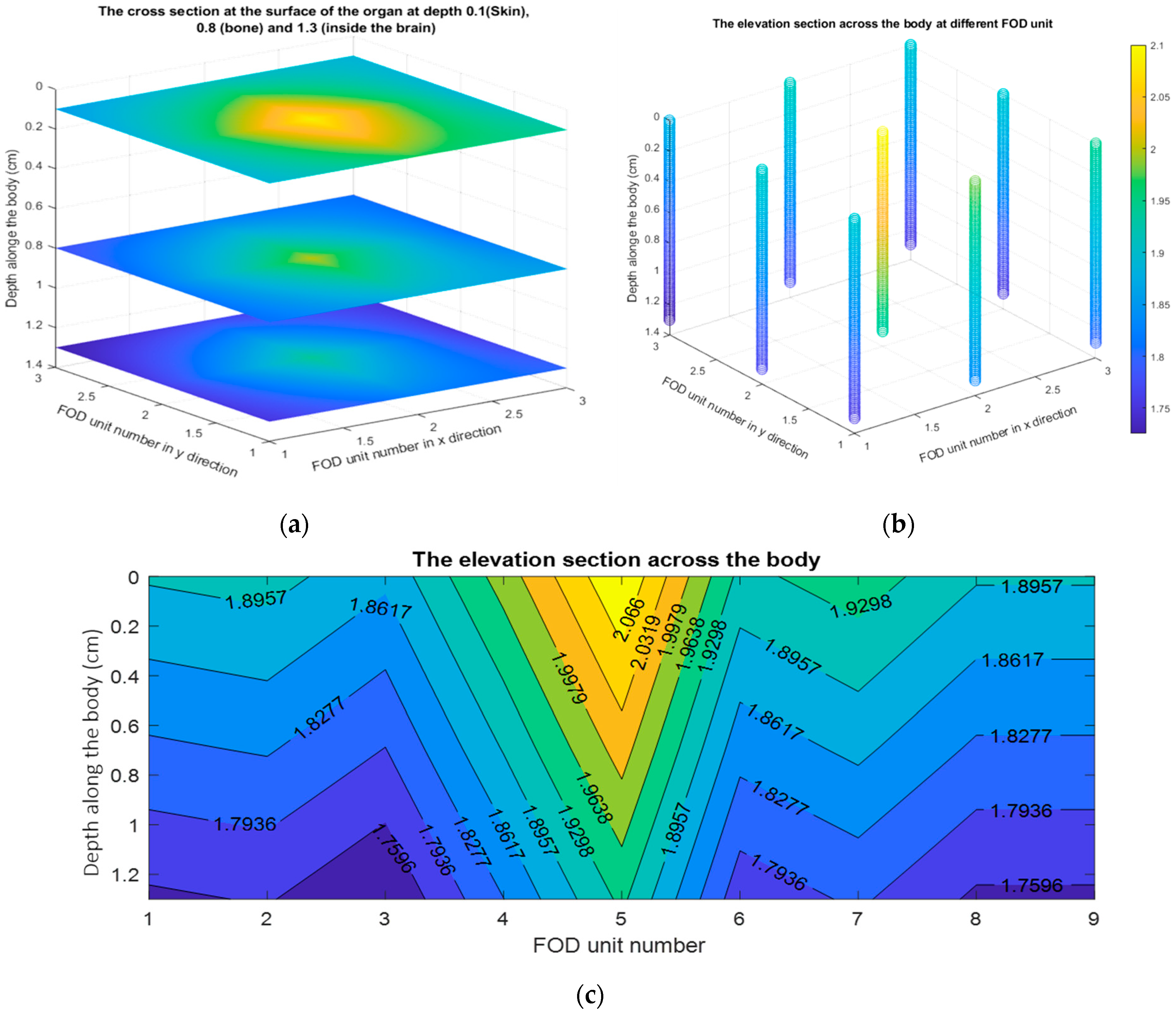
| Tissue Type | The Calculated Mass Attenuation Coefficients (ρ) through XCOM Program [44] |
|---|---|
| Skin | 0.06257 |
| Bone | 0.06043 |
| Brain | 0.06314 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsharkawi, A.S.A.; Alazab, H.A.; Sayed, M.; Askar, M.A.; Abdelrahman, I.Y.; Arafa, A.A.; Saleh, H.I.; Gomaa, L.R.; Du, Y.-C. A Fiber-Optical Dosimetry Sensor for Gamma-Ray Irradiation Measurement in Biological Applications. Biosensors 2023, 13, 1010. https://doi.org/10.3390/bios13121010
Elsharkawi ASA, Alazab HA, Sayed M, Askar MA, Abdelrahman IY, Arafa AA, Saleh HI, Gomaa LR, Du Y-C. A Fiber-Optical Dosimetry Sensor for Gamma-Ray Irradiation Measurement in Biological Applications. Biosensors. 2023; 13(12):1010. https://doi.org/10.3390/bios13121010
Chicago/Turabian StyleElsharkawi, Adel Shaaban Awad, Huda A. Alazab, Mahmoud Sayed, Mostafa A. Askar, Ibrahim Y. Abdelrahman, Amany A. Arafa, Hassan I. Saleh, Lotfy R. Gomaa, and Yi-Chun Du. 2023. "A Fiber-Optical Dosimetry Sensor for Gamma-Ray Irradiation Measurement in Biological Applications" Biosensors 13, no. 12: 1010. https://doi.org/10.3390/bios13121010






