Surface Functionalised Optical Fibre for Detection of Hydrogen Sulphide
Abstract
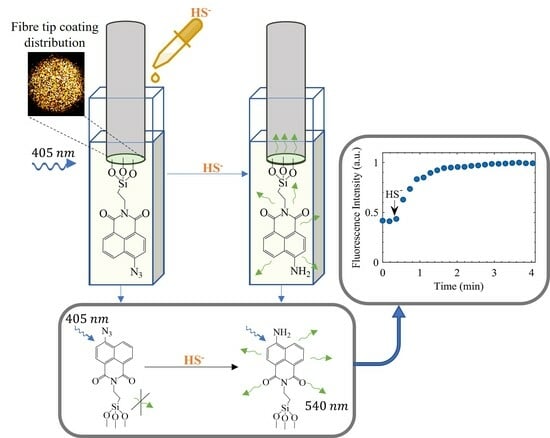
:1. Introduction
2. Materials and Methods
2.1. General Experimental
2.2. Synthesis of the Fluorescent Probes
2.2.1. 6-Bromo-2-(2-methoxyethyl)-1H-benz[de]isoquinoline-1,3(2H)-dione (4)
2.2.2. 6-Azido-2-(2-methoxyethyl)-1H-benzo[de]isoquinoline-1,3(2H)-dione (1)
2.2.3. 6-Bromo-2-(3-(triethoxysilyl)propyl)-1H-benzo[de]isoquinoline-1,3(2H)-dione (5)
2.2.4. 6-Azido-2-(3-(triethoxysilyl)propyl)-1H-benzo[de]isoquinoline-1,3(2H)-dione (2)
2.3. Surface Functionalisation and Coating of the Optical Fibres
2.4. X-ray Photoelectron Spectroscopy
2.5. Spectral Measurements of Free Fluorophore in Solution
2.6. Optical Fibre-Based Sensing Setup
3. Results and Discussion
3.1. Synthesis
3.2. Coating of Optical Fibres and Characterisation
3.3. Spectral Response of Free Fluorophore in Solution
3.4. Detection of Hydrosulphide Ion with the Optical Fibre-Based Sensing Setup
3.5. pH Effects on the Probe Response
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Usha, S.P.; Mishra, S.K.; Gupta, B.D. Fibre optic hydrogen sulfide gas sensors utilizing ZnO thin film/ZnO nanoparticles: A comparison of surface plasmon resonance and lossy mode resonance. Sens. Act. B Chem. 2015, 218, 196–204. [Google Scholar] [CrossRef]
- Yoo, S.Y.; Gopala, L.; Kang, C.; Lee, M.H. Hydrogen sulfide-activatable fluorescence turn-on azide-containing naphthalimide derivative. Bull. Korean Chem. Soc. 2022, 43, 1231–1235. [Google Scholar] [CrossRef]
- Lippert, A.R.; New, E.J.; Chang, C.J. Reaction-based fluorescent probes for selective imaging of hydrogen sulfide in living cells. J. Am. Chem. Soc. 2011, 133, 10078–10080. [Google Scholar] [CrossRef]
- Cha, Y.; Gopala, L.; Lee, M.H. A bio-friendly biotin-coupled and azide-functionalized naphthalimide for real-time endogenous hydrogen sulfide analysis in living cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 291, 122385. [Google Scholar] [CrossRef]
- Zhang, L.; Li, S.; Hong, M.; Xu, Y.; Wang, S.; Liu, Y.; Qian, Y.; Zhao, J. A colorimetric and ratiometric fluorescent probe for the imaging of endogenous hydrogen sulphide in living cells and sulphide determination in mouse hippocampus. Org. Biomol. Chem. 2014, 12, 5115–5125. [Google Scholar] [CrossRef]
- Qiao, Q.; Zhao, M.; Lang, H.; Mao, D.; Cui, J.; Xu, Z. A turn-on fluorescent probe for imaging lysosomal hydrogen sulfide in living cells. RSC Adv. 2014, 4, 25790–25794. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, T.; Liu, J.T.; Miao, J.Y.; Zhao, B.X. A new ratiometric fluorescent probe for rapid, sensitive and selective detection of endogenous hydrogen sulfide in mitochondria. Chem. Commun. 2016, 52, 3131–31349. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Hessler, W.; Henary, M. H2S Sensors: Synthesis, Optical Properties, and Selected Biomedical Applications under Visible and NIR Light. Molecules 2023, 28, 1295. [Google Scholar] [CrossRef] [PubMed]
- Khattak, S.; Rauf, M.A.; Khan, N.H.; Zhang, Q.Q.; Chen, H.J.; Muhammad, P.; Ansari, M.A.; Alomary, M.N.; Jahangir, M.; Zhang, C.Y.; et al. Hydrogen sulfide biology and its role in cancer. Molecules 2022, 27, 3389. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.H.; Chu, Y.H.; Lin, K.T. The hidden role of hydrogen sulfide metabolism in cancer. Int. J. Mol. Sci. 2021, 22, 6562. [Google Scholar] [CrossRef]
- Shackelford, R.E.; Mohammad, I.Z.; Meram, A.T.; Kim, D.; Alotaibi, F.; Patel, S.; Ghali, G.E.; Kevil, C.G. Molecular functions of hydrogen sulfide in cancer. Pathophysiology 2021, 28, 437–456. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Sharma, S.; Singh, R.; Rajput, S.; Chattopadhyay, N.; Tewari, D.; Joshi, K.B.; Verma, S. A naphthalimide-based peptide conjugate for concurrent imaging and apoptosis induction in cancer cells by utilizing endogenous hydrogen sulfide. Chem. Sci. 2021, 12, 16085–16091. [Google Scholar] [CrossRef] [PubMed]
- Akbari, M.; Sogutdelen, E.; Juriasingani, S.; Sener, A. Hydrogen sulfide: Emerging role in bladder, kidney, and prostate malignancies. Oxidative Med. Cell. Longev. 2019, 2019, 2360945. [Google Scholar] [CrossRef] [PubMed]
- Furne, J.; Saeed, A.; Levitt, M.D. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1479–R1485. [Google Scholar] [CrossRef]
- Duc, C.; Boukhenane, M.L.; Wojkiewicz, J.L.; Redon, N. Hydrogen sulfide detection by sensors based on conductive polymers: A review. Front. Mater. 2020, 7, 215. [Google Scholar] [CrossRef]
- Lawrence, N.S.; Davis, J.; Jiang, L.; Jones, T.G.; Davies, S.N.; Compton, R.G. The electrochemical analog of the methylene blue reaction: A novel amperometric approach to the detection of hydrogen sulfide. Electroanalysis 2000, 12, 1453–1460. [Google Scholar] [CrossRef]
- Ali, F.I.; Awwad, F.; Greish, Y.E.; Mahmoud, S.T. Hydrogen sulfide (H2S) gas sensor: A review. IEEE Sens. J. 2018, 19, 2394–2407. [Google Scholar] [CrossRef]
- Pandey, S.K.; Kim, K.H.; Tang, K.T. A review of sensor-based methods for monitoring hydrogen sulfide. TrAc Trends Anal. Chem. 2012, 32, 87–99. [Google Scholar] [CrossRef]
- Allsop, T.; Neal, R. A review: Application and Implementation of optic fibre sensors for gas detection. Sensors 2021, 21, 6755. [Google Scholar] [CrossRef]
- Choi, S.A.; Park, C.S.; Kwon, O.S.; Giong, H.K.; Lee, J.S.; Ha, T.H.; Lee, C.S. Structural effects of naphthalimide-based fluorescent sensor for hydrogen sulfide and imaging in live zebrafish. Sci. Rep. 2016, 6, 26203. [Google Scholar] [CrossRef]
- Yu, F.; Han, X.; Chen, L. Fluorescent probes for hydrogen sulfide detection and bioimaging. Chem. Commun. 2014, 50, 12234–122493. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Cheng, Y.; Dai, C.; King, A.L.; Predmore, B.L.; Lefer, D.J.; Wang, B. A fluorescent probe for fast and quantitative detection of hydrogen sulfide in blood. Angew. Chem. 2011, 123, 9846–9849. [Google Scholar] [CrossRef]
- Elsayed, S.; de la Torre, C.; Santos-Figueroa, L.E.; Marin-Hernandez, C.; Martinez-Manez, R.; Sancenon, F.; Costero, A.M.; Gil, S.; Parra, M. Azide and sulfonylazide functionalized fluorophores for the selective and sensitive detection of hydrogen sulfide. Sens. Act. B Chem. 2015, 207, 987–994. [Google Scholar] [CrossRef]
- Wang, X.; An, L.; Tian, Q.; Cui, K. Recent progress in H 2 S activated diagnosis and treatment agents. RSC Adv. 2019, 9, 33578–33588. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, X.; Lu, S.; Zhang, L.; Feng, L.; Zhong, S.; Zhang, N.; Bing, T.; Shangguan, D. Ratiometric detection and imaging of hydrogen sulfide in mitochondria based on a cyanine/naphthalimide hybrid fluorescent probe. Analyst 2020, 145, 6549–6555. [Google Scholar] [CrossRef]
- Henthorn, H.A.; Pluth, M.D. Mechanistic insights into the H2S-mediated reduction of aryl azides commonly used in H2S detection. J. Am. Chem. Soc. 2015, 137, 15330–15336. [Google Scholar] [CrossRef]
- Xu, Q.; He, L.; Wei, H.; Lin, W. An ICT-based hydrogen sulfide sensor with good water solubility for fluorescence imaging in living cells. J. Fluoresc. 2018, 28, 5–11. [Google Scholar] [CrossRef]
- Mowbray, S.E.; Amiri, A.M. A brief overview of medical fiber optic biosensors and techniques in the modification for enhanced sensing ability. Diagnostics 2019, 9, 23. [Google Scholar] [CrossRef]
- Singh, L.; Agarwal, N.; Barthwal, H.; Arya, B.; Singh, T. Application of Fiber Optics in Bio-Sensing. In Fiber Optics-Technology and Applications; IntechOpen: London, UK, 2021; Chapter 6; ISBN 978183969626. [Google Scholar] [CrossRef]
- Li, C.; Xiao, L.; Yang, X.; Feng, W. Ag/APTES/CuxO (x = 1, 2)-MGS-coated no-core fibre surface plasmon resonance gas sensor and its application in hydrogen sulfide detection. IEEE Sens. J. 2021, 22, 2182–2189. [Google Scholar] [CrossRef]
- Chen, R.; Lan, G.; Wang, N.; Yan, W.; Yi, J.; Wei, W. Highly sensitive fibre-optic SPR sensor with surface coated TiO2/MWCNT composite film for hydrogen sulfide gas detection. J. Phys. D Appl. Phys. 2021, 55, 105108. [Google Scholar] [CrossRef]
- Lopez, J.D.; Keley, M.; Dante, A.; Werneck, M.M. Optical fibre sensor coated with copper and iron oxide nanoparticles for hydrogen sulfide sensing. Opt. Fibre Technol. 2021, 67, 102731. [Google Scholar] [CrossRef]
- Chen, R.; Liu, W.; Huang, G.; Wang, D.; Qin, X.; Feng, W. Hydrogen sulfide sensor based on tapered fibre sandwiched between two molybdenum disulfide/citric acid composite membrane coated long-period fibre gratings. Appl. Opt. 2018, 57, 9755–9759. [Google Scholar] [CrossRef]
- Qin, X.; Feng, W.; Yang, X.; Wei, J.; Huang, G. Molybdenum sulfide/citric acid composite membrane-coated long period fibre grating sensor for measuring trace hydrogen sulfide gas. Sens. Act. B Chem. 2018, 272, 60–68. [Google Scholar] [CrossRef]
- Feng, X.; Feng, W.; Tao, C.; Deng, D.; Qin, X.; Chen, R. Hydrogen sulfide gas sensor based on graphene-coated tapered photonic crystal fibre interferometer. Sens. Act. B Chem. 2017, 247, 540–545. [Google Scholar] [CrossRef]
- Huang, G.; Li, Y.; Chen, C.; Yue, Z.; Zhai, W.; Li, M.; Yang, B. Hydrogen sulfide gas sensor based on titanium dioxide/amino-functionalized graphene quantum dots coated photonic crystal fibre. J. Phys. D Appl. Phys. 2020, 53, 325102. [Google Scholar] [CrossRef]
- De Acha, N.; Socorro-Leránoz, A.B.; Elosúa, C.; Matías, I.R. Trends in the design of intensity-based optical fiber biosensors (2010–2020). Biosensors 2021, 11, 197. [Google Scholar] [CrossRef]
- Li, Y.; Luo, S.; Gui, Y.; Wang, X.; Tian, Z.; Yu, H. Difunctional hydrogel optical fiber fluorescence sensor for continuous and simultaneous monitoring of glucose and pH. Biosensors 2023, 13, 287. [Google Scholar] [CrossRef]
- Gong, J.; Tanner, M.G.; Venkateswaran, S.; Stone, J.M.; Zhang, Y.; Bradley, M. A hydrogel-based optical fibre fluorescent pH sensor for observing lung tumor tissue acidity. Anal. Chim. Acta 2020, 1134, 136–143. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Sun, T.; Grattan, K.T. A turn-on fluorescence-based fibre optic sensor for the detection of mercury. Sensors 2019, 19, 2142. [Google Scholar] [CrossRef]
- Sarkar, P.K.; Halder, A.; Adhikari, A.; Polley, N.; Darbar, S.; Lemmens, P.; Pal, S.K. DNA-based fiber optic sensor for direct in-vivo measurement of oxidative stress. Sens. Act. B Chem. 2018, 255, 2194–2202. [Google Scholar] [CrossRef]
- Epstein, J.R.; Walt, D.R. Fluorescence-based fibre optic arrays: A universal platform for sensing. Chem. Soc. Rev. 2003, 32, 203–214. [Google Scholar] [CrossRef]
- Smith, S.; Goodge, K.; Delaney, M.; Struzyk, A.; Tansey, N.; Frey, M. A comprehensive review of the covalent immobilization of biomolecules onto electrospun nanofibers. Nanomaterials 2020, 10, 2142. [Google Scholar] [CrossRef]
- Kandimalla, V.B.; Tripathi, V.S.; Ju, H. Biosensors based on immobilization of biomolecules in sol-gel matrices. In Electrochemical Sensors, Biosensors and their Biomedical Applications; Chapter 16; Academic Press: Cambridge, MA, USA, 2008; pp. 503–529. ISBN 9780123737380. [Google Scholar] [CrossRef]
- Hao, G.; Xu, Z.P.; Li, L. Manipulating extracellular tumour pH: An effective target for cancer therapy. RSC Adv. 2018, 8, 22182–22192. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Shanti, A. Effect of exogenous ph on cell growth of breast cancer cells. Int. J. Mol. Sci. 2021, 22, 9910. [Google Scholar] [CrossRef]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR chemical shifts of common laboratory solvents as trace impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef]
- Betancourt, F.; Valente, A.; Yan, H. 1, 8-Naphthalimide derivatives as probes for protein surface hydrophobicity. J. Photochem. Photobiol. A Chem. 2021, 418, 113386. [Google Scholar] [CrossRef]
- Montoya, L.A.; Pluth, M.D. Selective turn-on fluorescent probes for imaging hydrogen sulfide in living cells. Chem. Commun. 2012, 48, 4767–4769. [Google Scholar] [CrossRef]
- Rouhani, S.; Haghgoo, S. A novel fluorescence nanosensor based on 1, 8-naphthalimide-thiophene doped silica nanoparticles, and its application to the determination of methamphetamine. Sens. Act. B Chem. 2015, 209, 957–965. [Google Scholar] [CrossRef]
- Mousli, Y.; Rouvière, L.; Traboulsi, I.; Hunel, J.; Buffeteau, T.; Heuzé, K.; Vellutini, L.; Genin, E. Hydrosilylation of azide-containing olefins as a convenient access to azidoorganotrialkoxysilanes for self-assembled monolayer elaboration onto silica by spin coating. Chemistryselect 2018, 3, 7333–7339. [Google Scholar] [CrossRef]
- Multar, E.; Daud, S.; Rohizad, S.N.A.; Halid, N.T. Functionalized fibre optics for glucose detection. J. Phys. Conf. Ser. 2020, 1484, 012010. [Google Scholar] [CrossRef]
- Tosi, D.; Sypabekova, M.; Bekmurzayeva, A.; Molardi, C.; Dukenbayev, K. Optical Fibre Biosensors: Device Platforms, Biorecognition, Applications, 1st ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 253–282. [Google Scholar] [CrossRef]
- Sypabekova, M.; Hagemann, A.; Rho, D.; Kim, S. 3-Aminopropyltriethoxysilane (APTES) deposition methods on oxide surfaces in solution and vapor phases for biosensing applications. Biosensors 2022, 13, 36. [Google Scholar] [CrossRef]
- Zhu, M.; Lerum, M.Z.; Chen, W. How to prepare reproducible, homogeneous, and hydrolytically stable aminosilane-derived layers on silica. Langmuir 2012, 28, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Pasternack, R.M.; Rivillon Amy, S.; Chabal, Y.J. Attachment of 3-(aminopropyl) triethoxysilane on silicon oxide surfaces: Dependence on solution temperature. Langmuir 2008, 24, 12963–12971. [Google Scholar] [CrossRef] [PubMed]
- Chastain, J.; King, R.C., Jr. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer: Waltham, MA, USA, 1992; Volume 40, p. 221. [Google Scholar]
- Major, G.H.; Fernandez, V.; Fairley, N.; Smith, E.F.; Linford, M.R. Guide to XPS data analysis: Applying appropriate constraints to synthetic peaks in XPS peak fitting. J. Vac. Sci. Technol. A Vacuum, Surfaces, Film. 2022, 40, 063201. [Google Scholar] [CrossRef]
- Dalby, K.N.; Nesbitt, H.W.; Zakaznova-Herzog, V.P.; King, P.L. Resolution of bridging oxygen signals from O 1s spectra of silicate glasses using XPS: Implications for O and Si speciation. Geochim. Cosmochim. Acta 2007, 71, 4297–4313. [Google Scholar] [CrossRef]
- Miller, D.J.; Biesinger, M.C.; McIntyre, N.S. Interactions of CO2 and CO at fractional atmosphere pressures with iron and iron oxide surfaces: One possible mechanism for surface contamination? Surf. Interface Anal. 2002, 3, 299–305. [Google Scholar] [CrossRef]
- Min, H.; Girard-Lauriault, P.L.; Gross, T.; Lippitz, A.; Dietrich, P.; Unger, W.E. Ambient-ageing processes in amine self-assembled monolayers on microarray slides as studied by ToF-SIMS with principal component analysis, XPS, and NEXAFS spectroscopy. Anal. Bioanal. Chem. 2012, 403, 613–623. [Google Scholar] [CrossRef]
- Brandner, S.; Kratky, T.; Holtz, K.; Becker, T.; Jekle, M. Controlling glass bead surface functionality-Impact on network formation in natural edible polymer systems. Compos. Sci. Technol. 2021, 211, 108864. [Google Scholar] [CrossRef]
- Barral, K.; Moorhouse, A.D.; Moses, J.E. Efficient conversion of aromatic amines into azides: A one-pot synthesis of triazole linkages. Org. Lett. 2007, 9, 1809–1811. [Google Scholar] [CrossRef]
- Jothi, D.; Munusamy, S.; Iyer, S.K. A new sensitive “turn-on” fluorescent probe based on naphthalimide: Application in visual recognition of hydrogen sulfide in environmental samples and living cells. J. Photochem. Photobiol. Chem. 2021, 420, 113491. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L. A novel “turn-on” fluorescent probe based on hydroxy functionalized naphthalimide as a logic platform for visual recognition of H2S in environment and living cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 235, 118331. [Google Scholar] [CrossRef] [PubMed]









| C 1s (%) | O 1s (%) | N 1s (%) | Si 2p (%) | |
|---|---|---|---|---|
| Control optical fibre | 13.91 ± 1.53 | 55.75 ± 1.09 | 0 | 30.33 ± 0.43 |
| Coated optical fibre | 45.05 ± 5.85 | 31.93 ± 3.90 | 3.15 ± 0.18 | 19.86 ± 1.84 |
| Element | Chemical Bond Contribution | Coated (%) | Control (%) |
|---|---|---|---|
| C 1s | C-C/C-H | 77.36 ± 4.37 | 89.18 ± 1.58 |
| C-O/C-N | 14.64 ± 2.70 | 8.62 ± 1.09 | |
| C=O | 7.99 ± 1.81 | 2.19 ± 0.48 | |
| O 1s | Si-O-Si | 91.35 ± 2.51 | 97.26 ± 0.19 |
| O-C | 0.88 ± 0.65 | 2.74 ± 0.19 | |
| N-C=O | 7.74 ± 1.58 | 0 | |
| Si 2p | SiO2 | 79.18 ± 2.88 | 100 |
| Si-C | 20.81 ± 2.88 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baghapour, S.; Nehema, J.; Zhang, W.Q.; Warren-Smith, S.C.; Hickey, S.M.; Plush, S.E.; Afshar Vahid, S. Surface Functionalised Optical Fibre for Detection of Hydrogen Sulphide. Biosensors 2023, 13, 949. https://doi.org/10.3390/bios13110949
Baghapour S, Nehema J, Zhang WQ, Warren-Smith SC, Hickey SM, Plush SE, Afshar Vahid S. Surface Functionalised Optical Fibre for Detection of Hydrogen Sulphide. Biosensors. 2023; 13(11):949. https://doi.org/10.3390/bios13110949
Chicago/Turabian StyleBaghapour, Shaghayegh, Jasmine Nehema, Wen Qi Zhang, Stephen C. Warren-Smith, Shane M. Hickey, Sally E. Plush, and Shahraam Afshar Vahid. 2023. "Surface Functionalised Optical Fibre for Detection of Hydrogen Sulphide" Biosensors 13, no. 11: 949. https://doi.org/10.3390/bios13110949