A Floating Capsule Electrochemical System for In Situ and Multichannel Ion-Selective Sensing
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Materials
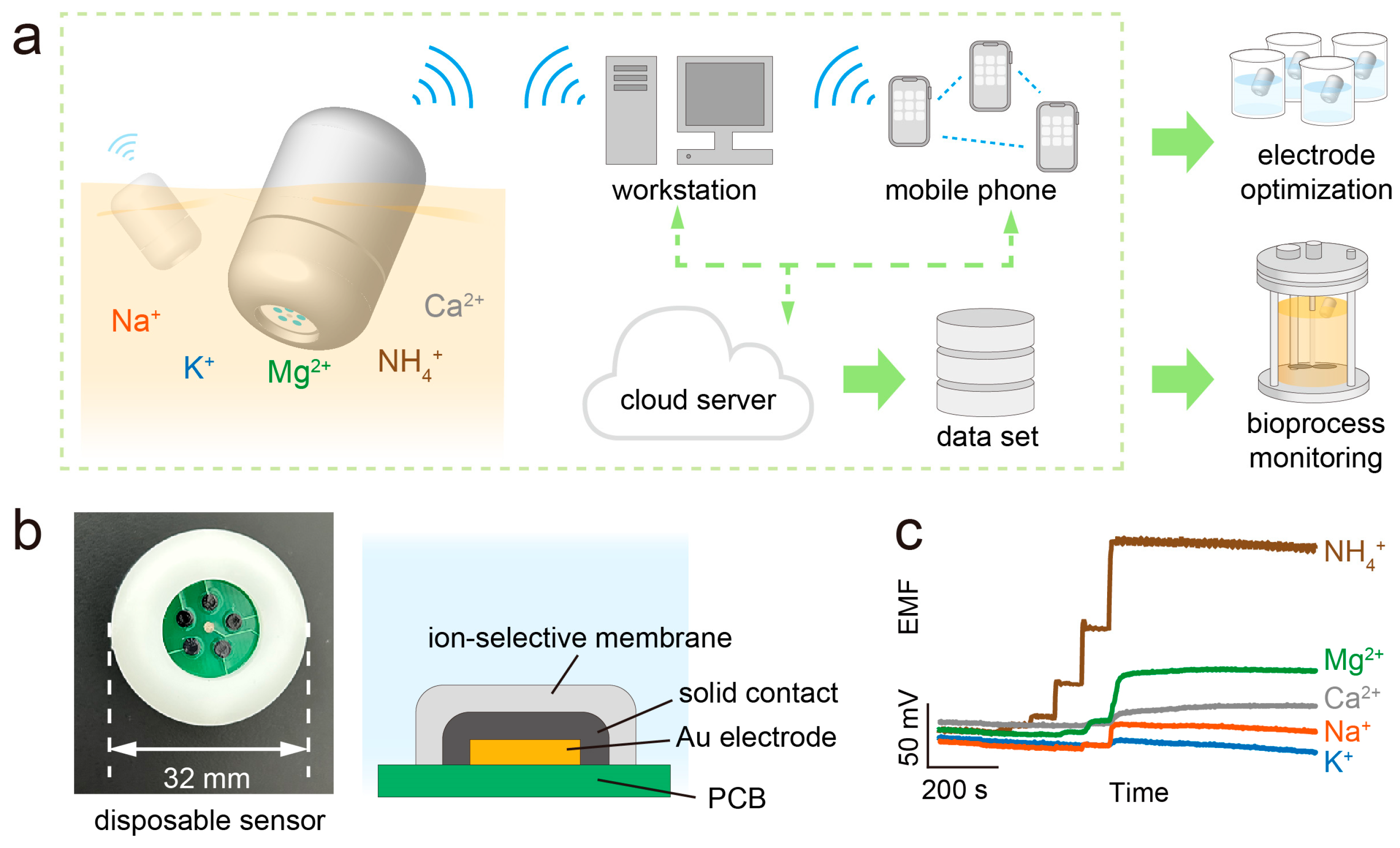
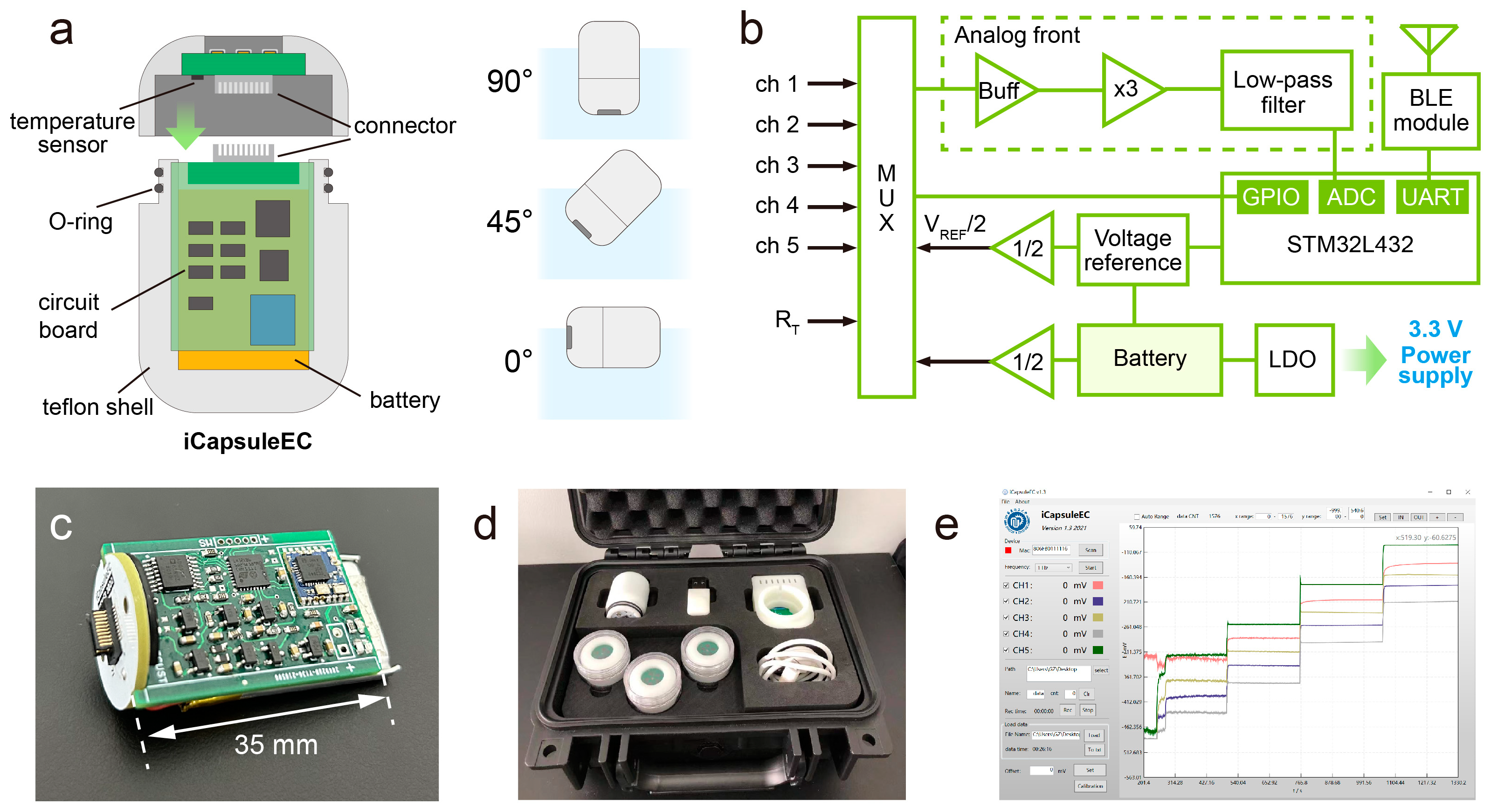
2.2. Design of the iCapsuleEC System
2.3. Hardware and Software
2.4. Prepreparation of the PCB-Au/SC/ISE-Based Sensor
2.5. Testing for Potential Measurement
2.6. Measurement of Inorganic Ions in Sample Solution
3. Results and Discussion
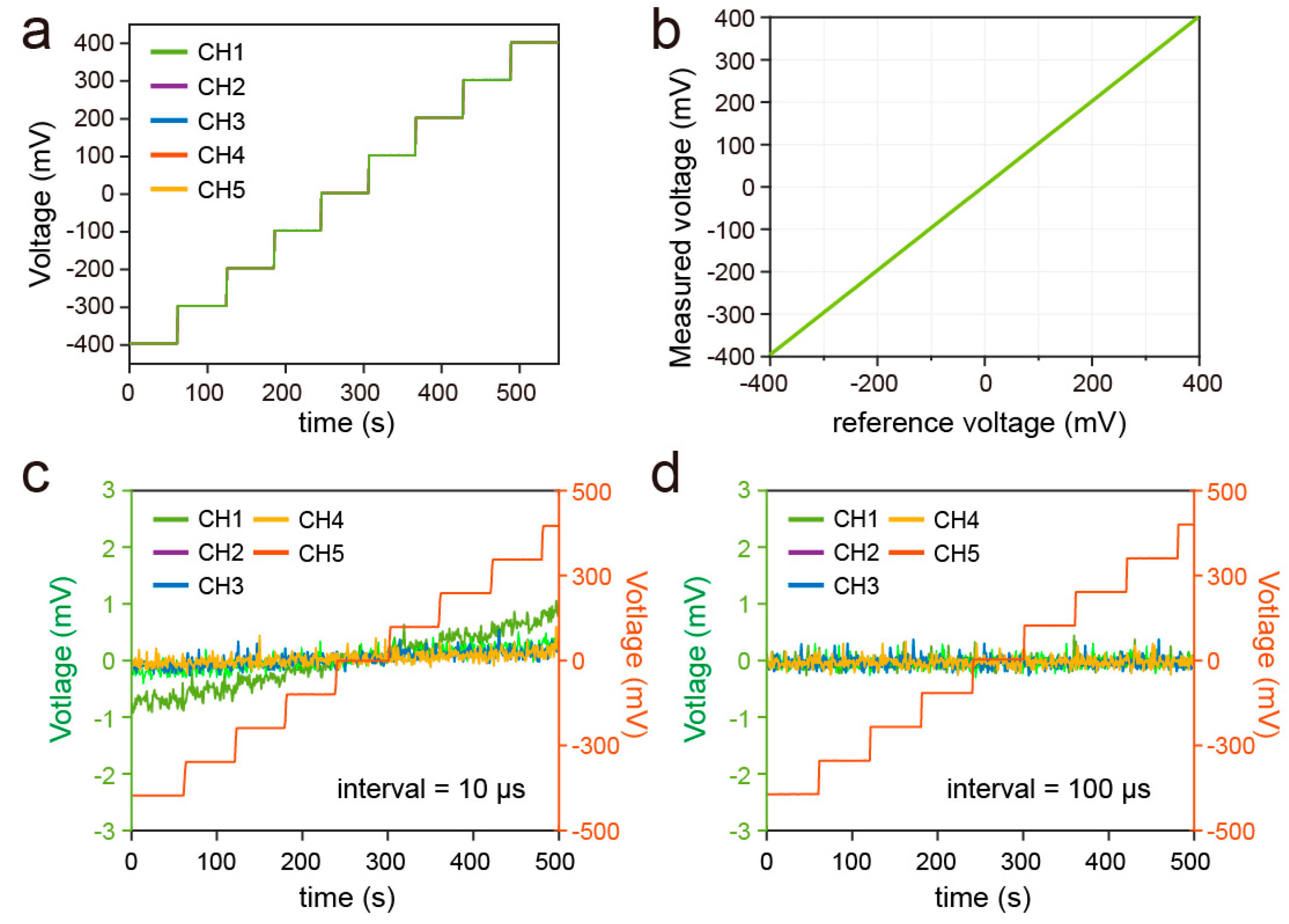
3.1. Accuracy of the iCapsuleEC for Multichannel Measurement
3.2. Optimization of the Solid-Contact Layer for the SC-ISEs
3.3. Performance for Detection of K+, NH4+, Na+, Ca2+, and Mg2+
3.4. Multichannel Analysis in Simulated Cell Culture Media
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Weber, E.W.; Maus, M.V.; Mackall, C.L. The Emerging Landscape of Immune Cell Therapies. Cell 2020, 181, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.T.L.; Nguyen, Q.T.; Phan, T.T.K.; Nguyen, G.H.; Le, P.T.T.; Hoang, V.T.; Forsyth, N.R.; et al. Stem cell-based therapy for human diseases. Signal Transduct. Target. Ther. 2022, 7, 272. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Chen, X.; Dowbaj, A.M.; Sljukic, A.; Bratlie, K.; Lin, L.; Fong, E.L.S.; Balachander, G.M.; Chen, Z.; Soragni, A.; et al. Organoids. Nat. Rev. Methods Primers 2022, 2, 94. [Google Scholar] [CrossRef] [PubMed]
- Reyes, S.J.; Durocher, Y.; Pham, P.L.; Henry, O. Modern Sensor Tools and Techniques for Monitoring, Controlling, and Improving Cell Culture Processes. Processes 2022, 10, 189. [Google Scholar] [CrossRef]
- Jiang, W.; Xu, Y.; Li, C.; Lv, X.; Wang, D. Effect of inorganic salts on the growth and Cd2+ bioaccumulation of Zygosaccharomyces rouxii cultured under Cd2+ stress. Bioresour. Technol. 2013, 128, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Marison, I.W.; von Stockar, U. The importance of ammonia in mammalian cell culture. J. Biotechnol. 1996, 46, 161–185. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Harcum, S.W. Effects of elevated ammonium on glycosylation gene expression in CHO cells. Metab. Eng. 2006, 8, 123–132. [Google Scholar] [CrossRef]
- Yang, M.; Butler, M. Effects of ammonia on CHO cell growth, erythropoietin production, and glycosylation. Biotechnol. Bioeng. 2000, 68, 370–380. [Google Scholar] [CrossRef]
- Todtenberg, N.; Schmitz-Hertzberg, S.-T.; Schmalz, K.; Klatt, J.; Jorde, F.; Juttner, B.; Kraemer, R. Autonomous Sensor Capsule for Usage in Bioreactors. IEEE Sens. J. 2015, 15, 4093–4102. [Google Scholar] [CrossRef]
- Zimmermann, R.; Fiabane, L.; Gasteuil, Y.; Volk, R.; Pinton, J.-F. Characterizing flows with an instrumented particle measuring Lagrangian accelerations. New J. Phys. 2013, 15, 015018. [Google Scholar] [CrossRef]
- Stine, J.M.; Beardslee, L.A.; Sathyam, R.M.; Bentley, W.E.; Ghodssi, R. Electrochemical Dissolved Oxygen Sensor-Integrated Platform for Wireless In Situ Bioprocess Monitoring. Sens. Actuators B Chem. 2020, 320, 128381. [Google Scholar] [CrossRef]
- Udomsom, S.; Budwong, A.; Wongsa, C.; Sangngam, P.; Baipaywad, P.; Manaspon, C.; Auephanwiriyakul, S.; Theera-Umpon, N.; Paengnakorn, P. Automatic Programmable Bioreactor with pH Monitoring System for Tissue Engineering Application. Bioengineering 2022, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Murthy, G.S. Bioreactor control systems in the biopharmaceutical industry: A critical perspective. Syst. Microbiol. Biomanuf. 2021, 2, 91–112. [Google Scholar] [CrossRef]
- Ding, J.; Qin, W. Recent advances in potentiometric biosensors. TrAC Trends Anal. Chem. 2020, 124, 115803. [Google Scholar] [CrossRef]
- Cuartero, M.; Crespo, G.A. All-solid-state potentiometric sensors: A new wave for in situ aquatic research. Curr. Opin. Electrochem. 2018, 10, 98–106. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Z.; Cai, R.; Zheng, H.; Yu, J.; Zhang, Y.; Gu, Z. Microneedle-based transdermal detection and sensing devices. Lab. Chip 2023, 23, 869–887. [Google Scholar] [CrossRef] [PubMed]
- Garland, N.T.; McLamore, E.S.; Cavallaro, N.D.; Mendivelso-Perez, D.; Smith, E.A.; Jing, D.; Claussen, J.C. Flexible Laser-Induced Graphene for Nitrogen Sensing in Soil. ACS Appl. Mater. Interfaces 2018, 10, 39124–39133. [Google Scholar] [CrossRef]
- Ali, M.A.; Wang, X.; Chen, Y.; Jiao, Y.; Mahal, N.K.; Moru, S.; Castellano, M.J.; Schnable, J.C.; Schnable, P.S.; Dong, L. Continuous Monitoring of Soil Nitrate Using a Miniature Sensor with Poly(3-octyl-thiophene) and Molybdenum Disulfide Nanocomposite. ACS Appl. Mater. Interfaces 2019, 11, 29195–29206. [Google Scholar] [CrossRef]
- Urbanowicz, M.; Pijanowska, D.G.; Jasiński, A.; Ekman, M.; Bocheńska, M.K. A miniaturized solid-contact potentiometric multisensor platform for determination of ionic profiles in human saliva. J. Solid. State Electrochem. 2019, 23, 3299–3308. [Google Scholar] [CrossRef]
- Liu, H.; Gu, Z.; Zhao, Q.; Li, S.; Ding, X.; Xiao, X.; Xiu, G. Printed circuit board integrated wearable ion-selective electrode with potential treatment for highly repeatable sweat monitoring. Sens. Actuators B Chem. 2022, 355, 131102. [Google Scholar] [CrossRef]
- Mou, L.; Xia, Y.; Jiang, X. Epidermal Sensor for Potentiometric Analysis of Metabolite and Electrolyte. Anal. Chem. 2021, 93, 11525–11531. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Y.; Zhang, M.; Zhang, F.; He, P. Home Detection Technique for Na+ and K+ in Urine Using a Self-Calibrated all-Solid-State Ion-Selective Electrode Array Based on Polystyrene-Au Ion-Sensing Nanocomposites. Anal. Chem. 2021, 93, 8318–8325. [Google Scholar] [CrossRef]
- Liu, H.; Gu, Z.; Liu, Y.; Xiao, X.; Xiu, G. Validation of the Application of Solid Contact Ion-Selective Electrode for Off-Body Sweat Ion Monitoring. Biosensors 2022, 12, 229. [Google Scholar] [CrossRef]
- Vázquez, M.; Bobacka, J.; Ivaska, A.; Lewenstam, A. Influence of oxygen and carbon dioxide on the electrochemical stability of poly(3,4-ethylenedioxythiophene) used as ion-to-electron transducer in all-solid-state ion-selective electrodes. Sens. Actuators B Chem. 2002, 82, 7–13. [Google Scholar] [CrossRef]
- Vázquez, M.; Danielsson, P.; Bobacka, J.; Lewenstam, A.; Ivaska, A. Solution-cast films of poly(3,4-ethylenedioxythiophene) as ion-to-electron transducers in all-solid-state ion-selective electrodes. Sens. Actuators B Chem. 2004, 97, 182–189. [Google Scholar] [CrossRef]
- Veder, J.P.; De Marco, R.; Clarke, G.; Jiang, S.P.; Prince, K.; Pretsch, E.; Bakker, E. Water uptake in the hydrophilic poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) solid-contact of all-solid-state polymeric ion-selective electrodes. Analyst 2011, 136, 3252–3258. [Google Scholar] [CrossRef]
- Shao, Y.; Yao, Y.; Jiang, C.; Zhao, F.; Liu, X.; Ying, Y.; Ping, J. Two-dimensional MXene nanosheets (types Ti3C2Tx and Ti2CTx) as new ion-to-electron transducers in solid-contact calcium ion-selective electrodes. Mikrochim. Acta 2019, 186, 750. [Google Scholar] [CrossRef]
- Joon, N.K.; He, N.; Ruzgas, T.; Bobacka, J.; Lisak, G. PVC-Based Ion-Selective Electrodes with a Silicone Rubber Outer Coating with Improved Analytical Performance. Anal. Chem. 2019, 91, 10524–10531. [Google Scholar] [CrossRef]
- Kucherenko, I.S.; Sanborn, D.; Chen, B.; Garland, N.; Serhan, M.; Forzani, E.; Gomes, C.; Claussen, J.C. Ion-Selective Sensors Based on Laser-Induced Graphene for Evaluating Human Hydration Levels Using Urine Samples. Adv. Mater. Technol. 2020, 5, 1901037. [Google Scholar] [CrossRef]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q. All-solid-state Ca2+ Ion-selective Electrode with Black Phosphorus and Reduced Graphene Oxide as the Mediator Layer. Int. J. Electrochem. Sci. 2019, 14, 4933–4945. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, W.-K.; Roh, M.-Y.; Kang, C.-I.; Park, J.-M.; Sudduth, K.A. Automated sensing of hydroponic macronutrients using a computer-controlled system with an array of ion-selective electrodes. Comput. Electron. Agric. 2013, 93, 46–54. [Google Scholar] [CrossRef]
- Gu, Z.; Liu, H.X.; Ying, Y.L.; Xiu, G.; Long, Y.T. A thumb-size electrochemical system for portable sensors. Analyst 2018, 143, 2760–2764. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Chen, L.; Yang, Y.; Li, D.; Qian, Z.; Liang, S.; Yan, L.; Li, L.H.; Wu, M.; Fang, H. Two-dimensional Na-Cl crystals of unconventional stoichiometries on graphene surface from dilute solution at ambient conditions. Nat. Chem. 2018, 10, 776–779. [Google Scholar] [CrossRef]
- Chen, L.; Shi, G.; Shen, J.; Peng, B.; Zhang, B.; Wang, Y.; Bian, F.; Wang, J.; Li, D.; Qian, Z.; et al. Ion sieving in graphene oxide membranes via cationic control of interlayer spacing. Nature 2017, 550, 380–383. [Google Scholar] [CrossRef]
- Li, L.; Wang, F.; Zhu, J.; Wu, W. The facile synthesis of layered Ti2C MXene/carbon nanotube composite paper with enhanced electrochemical properties. Dalton Trans. 2017, 46, 14880–14887. [Google Scholar] [CrossRef]
- Tang, J.; Mathis, T.S.; Kurra, N.; Sarycheva, A.; Xiao, X.; Hedhili, M.N.; Jiang, Q.; Alshareef, H.N.; Xu, B.; Pan, F.; et al. Tuning the Electrochemical Performance of Titanium Carbide MXene by Controllable In Situ Anodic Oxidation. Angew. Chem. Int. Ed. Engl. 2019, 58, 17849–17855. [Google Scholar] [CrossRef]
- Umezawa, Y.; Bühlmann, P.; Umezawa, K.; Tohda, K.; Amemiya, S. Potentiometric Selectivity Coefficients of Ion-Selective Electrodes. Part I. Inorganic Cations (Technical Report). 2000, 72, 1851–2082. [Google Scholar] [CrossRef]
- Xue, M.; Mackin, C.; Weng, W.H.; Zhu, J.; Luo, Y.; Luo, S.L.; Lu, A.Y.; Hempel, M.; McVay, E.; Kong, J.; et al. Integrated biosensor platform based on graphene transistor arrays for real-time high-accuracy ion sensing. Nat. Commun. 2022, 13, 5064. [Google Scholar] [CrossRef]



| ISE | Ionophore | Ionic Additive | Plasticizer | Main Solid Component | Reference |
|---|---|---|---|---|---|
| K+ | 1.00 wt% valinomycin | 0.40 wt% Na-TFPB | 65.70 wt% DOS | 32.90 wt% PVC | [28] |
| NH4+ | 1.00 wt% nonactin | 0.50 wt% KTpClPB | 33.00 wt% DOS | 65.50 wt% PVC | [29] |
| Na+ | 1.00 wt% Na ionophore X | 0.55 wt% Na-TFPB | 65.45 wt% DOS | 33.00 wt% PVC | [30] |
| Ca2+ | 1.00 wt% ETH 5324 | 0.50 wt% Na-TFPB | 65.50 wt% DOS | 33.00 wt% PVC | [31] |
| Mg2+ | 1.40 wt% Mg ionophore I | 1.00 wt% KTpClPB | 64.50 wt% 2-NPOE | 33.10 wt% PVC | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Ding, A.; Zhou, J.-L.; Yan, B.-Y.; Gu, Z.; Wang, H.-F. A Floating Capsule Electrochemical System for In Situ and Multichannel Ion-Selective Sensing. Biosensors 2023, 13, 914. https://doi.org/10.3390/bios13100914
Yang J, Ding A, Zhou J-L, Yan B-Y, Gu Z, Wang H-F. A Floating Capsule Electrochemical System for In Situ and Multichannel Ion-Selective Sensing. Biosensors. 2023; 13(10):914. https://doi.org/10.3390/bios13100914
Chicago/Turabian StyleYang, Jie, Ao Ding, Jia-Le Zhou, Bing-Yong Yan, Zhen Gu, and Hui-Feng Wang. 2023. "A Floating Capsule Electrochemical System for In Situ and Multichannel Ion-Selective Sensing" Biosensors 13, no. 10: 914. https://doi.org/10.3390/bios13100914





