Biological Impact of γ-Fe2O3 Magnetic Nanoparticles Obtained by Laser Target Evaporation: Focus on Magnetic Biosensor Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Magnetic Nanoparticles and Ferrofluid
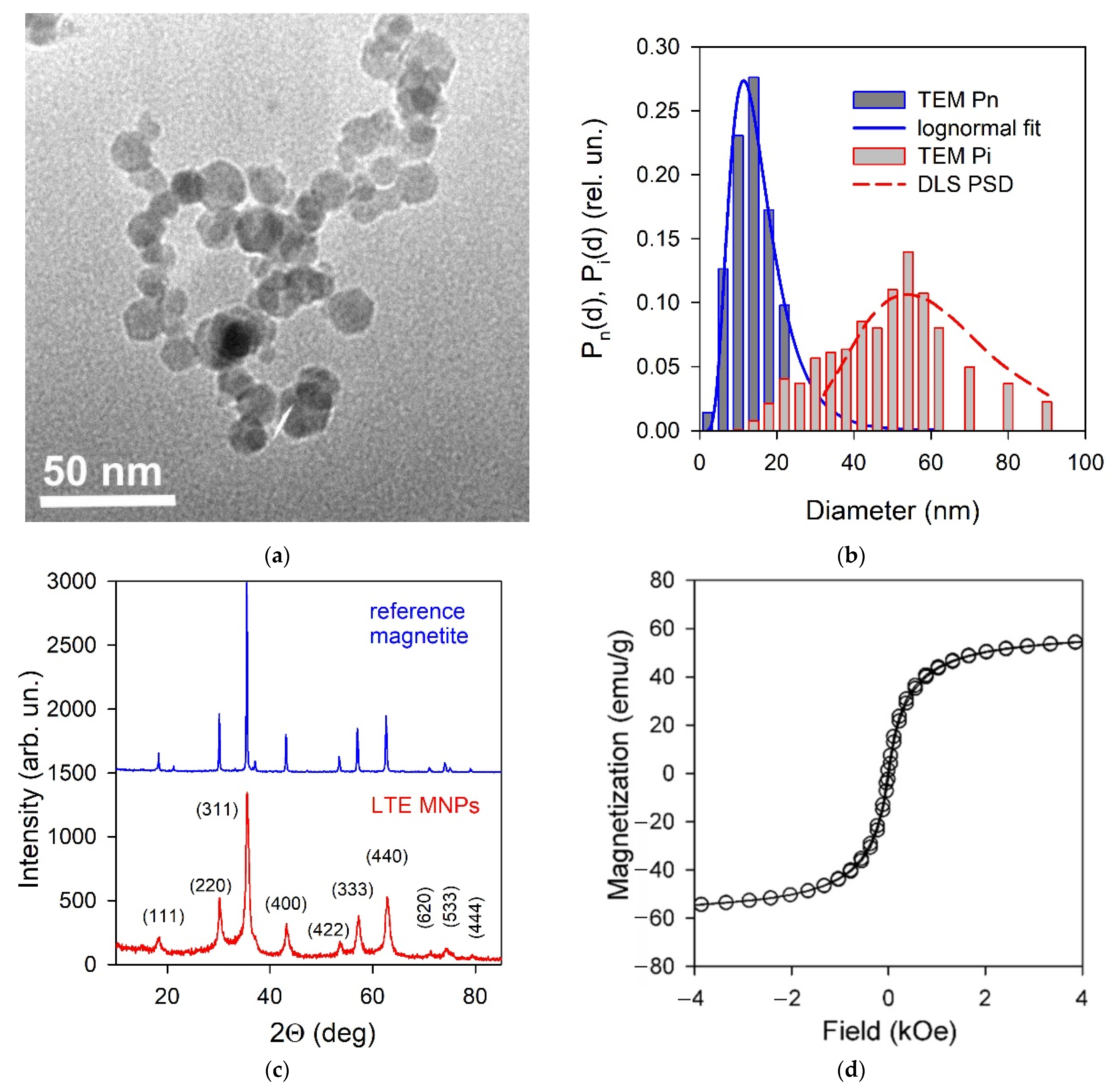
2.2. Characterization of Magnetic Nanoparticltes
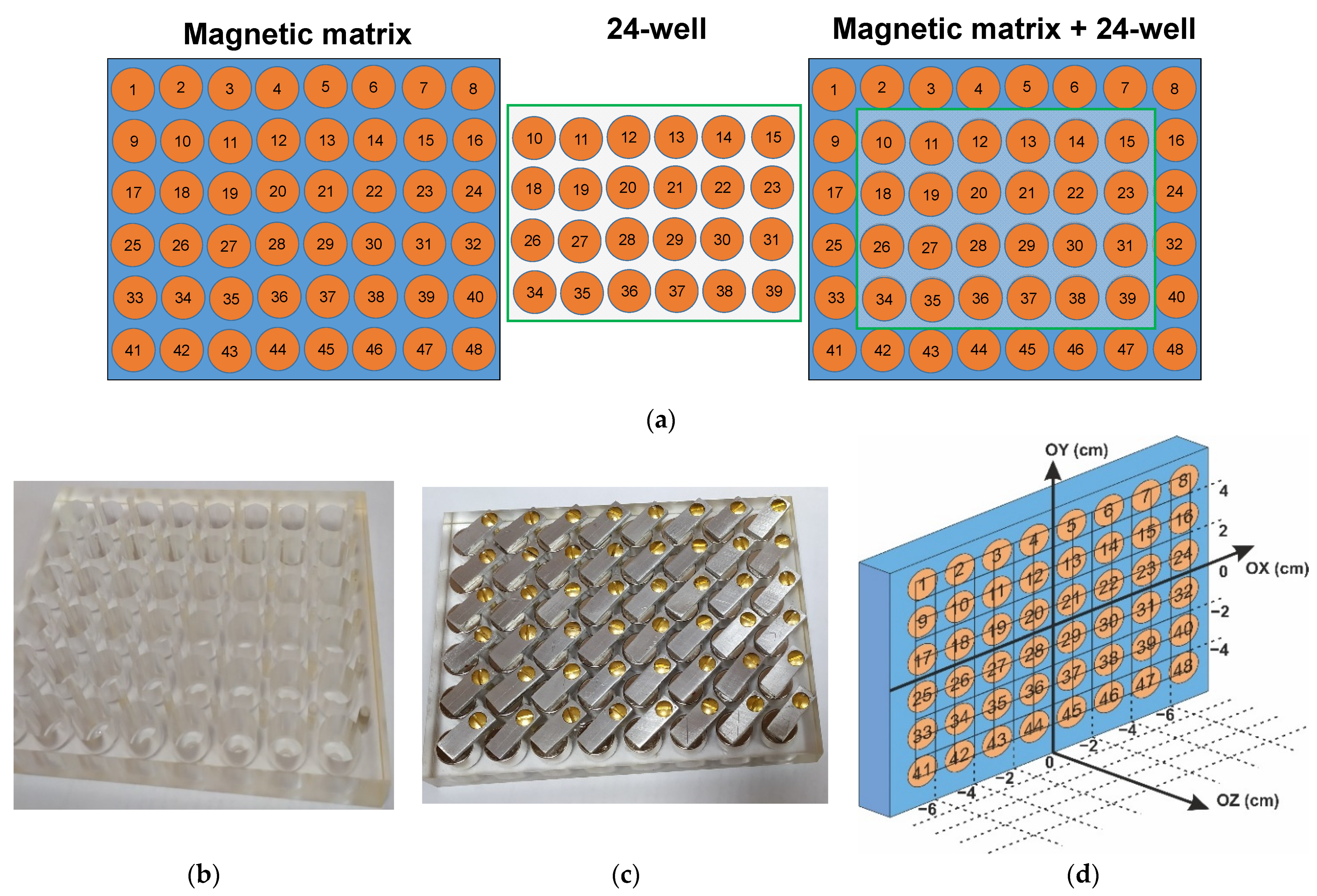
2.3. Design and Characterization of Magnetic Matrix
2.4. Design of Experiments with Cell Cultures
2.4.1. Cell Culture
2.4.2. Testing of MNPs Cytotoxicity
2.4.3. Proliferation Assay
2.4.4. Cell Counting
2.4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4.6. Prussian Blue Staining
2.4.7. Colorimetric Test for Collagen Deposition Measuring
2.4.8. Statistics
3. Results
3.1. Properties of γ-Fe2O3 LTE Magnetic Nanoparticles and Ferrofluid
3.2. Biological Activity of MNPs
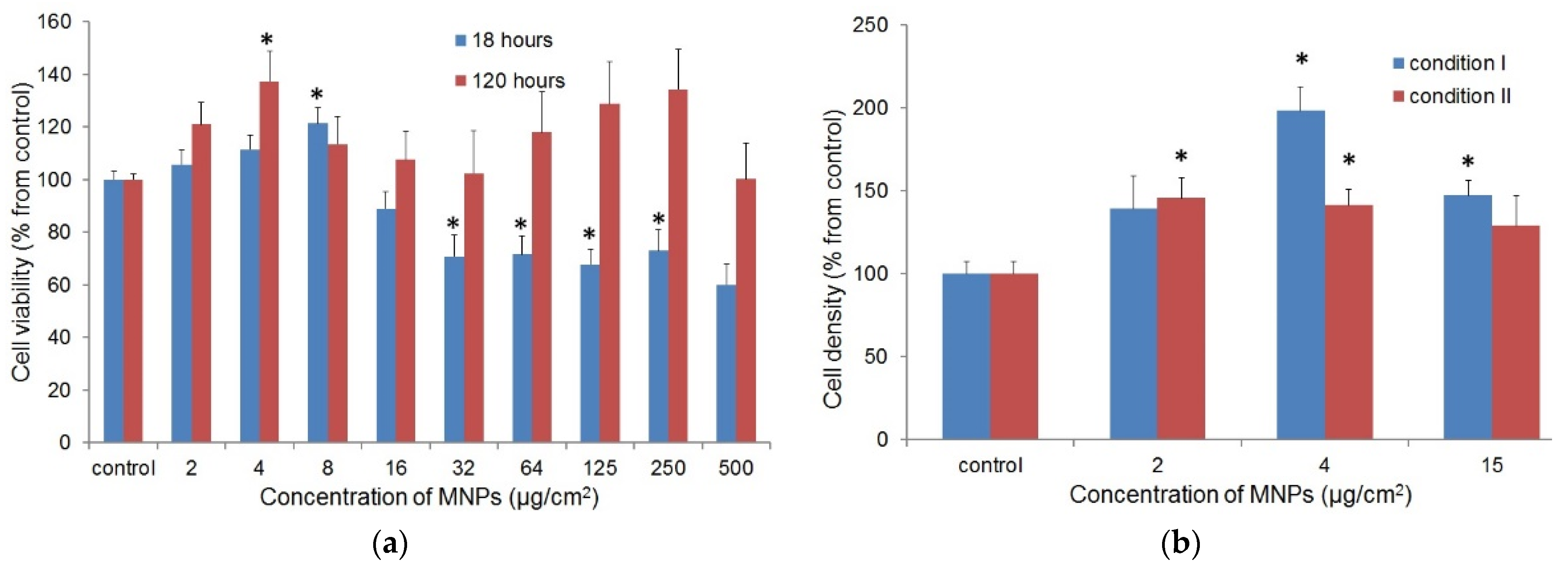
3.2.1. Cellular Uptake and the Cytotoxic Effect of γ-Fe2O3 LTE MNPs on Fibroblasts
3.2.2. The Effect of MNPs on the Proliferation of Fibroblasts
3.2.3. Cytokine Release
3.2.4. Collagen Secretion and Deposition
3.2.5. Effect of Magnetic Field on the Proliferation and Secretory Activity of Fibroblasts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baselt, D.R.; Lee, G.U.; Natesan, M.; Metzger, S.W.; Sheehan, P.E.; Colton, R.A. A biosensor based on magnetoresistance technology. Biosens. Bioelectron. 1998, 13, 731–739. [Google Scholar] [CrossRef]
- Llandro, J.; Palfreyman, J.J.; Ionescu, A.; Barnes, C.H.W. Magnetic biosensor technologies for medical applications: A review. Med. Biol. Eng. Comput. 2010, 48, 977–998. [Google Scholar] [CrossRef] [PubMed]
- Kurlyandskaya, G.V.; Levit, V.I. Advanced materials for drug delivery and biosensorsbased on magnetic label detection. Mater. Sci. Eng. C 2007, 27, 495–503. [Google Scholar] [CrossRef]
- Kurlyandskaya, G.V.; Novoselova, I.P.; Schupletsova, V.V.; Andrade, R.; Dunec, N.A.; Litvinova, L.S.; Safronov, A.P.; Yurova, K.A.; Kulesh, N.A.; Dzyuman, A.N.; et al. Nanoparticles for magnetic biosensing systems. J. Magn. Magn. Mater. 2017, 431, 249–254. [Google Scholar] [CrossRef]
- Blanc-Béguin, F.; Nabily, S.; Gieraltowski, J.; Turzo, A.; Querellou, S.; Salaun, P.Y. Cytotoxicity and GMI bio-sensor detection of maghemite nanoparticles internalized into cells. Magn. Magn. Mater. 2009, 321, 192–197. [Google Scholar] [CrossRef]
- Kurlyandskaya, G.V.; Portnov, D.S.; Beketov, I.V.; Larrañaga, A.; Safronov, A.P.; Orue, I.; Medvedev, A.I.; Chlenova, A.A.; Sanchez-Ilarduya, M.B.; Martinez-Amesti, A.; et al. Nanostructured materials for magnetic biosensing. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2017, 1861, 1494–1506. [Google Scholar] [CrossRef]
- Uchiyama, T.; Mohri, K.; Honkura, Y.; Panina, L.V. Recent advances of pico-Tesla resolution magneto-impedance sensor based on amorphous wire CMOS IC MI Sensor. IEEE Trans. Magn. 2012, 48, 3833–3839. [Google Scholar] [CrossRef]
- Nakai, T.A. Uniform magnetic field generator combined with a thin-film magneto-impedance sensor capable of human body scans. Sensors 2022, 22, 3120. [Google Scholar] [CrossRef]
- Melnikov, G.Y.; Lepalovskij, V.N.; Svalov, A.V.; Safronov, A.P.; Kurlyandskaya, G.V. Thin film sensor for detecting of stray fields of magnetic particles in blood vessel. Sensors 2021, 21, 3621. [Google Scholar] [CrossRef]
- Skinner, W.S.; Zhang, S.; Guldberg, R.E.; Ong, K.G. Magnetoelastic sensor optimization for improving mass monitoring. Sensors 2022, 22, 827. [Google Scholar] [CrossRef]
- Shekhar, S.; Karipott, S.S.; Guldberg, R.E.; Ong, K.G. Magnetoelastic sensors for real-time tracking of cell growth. Biotechnol. Bioeng. 2021, 118, 2380–2385. [Google Scholar] [CrossRef] [PubMed]
- Beato, J.; Pérez-Landazábal, J.; Gómez-Polo, C. Enhanced magnetic nanoparticle detection sensitivity in non-linear magnetoimpedance-based sensor. IEEE Sens. J. 2018, 18, 8701–8708. [Google Scholar] [CrossRef]
- Gloag, L.; Mehdipour, M.; Chen, D.; Tilley, R.D.; Gooding, J.J. Advances in the application of magnetic nanoparticles for sensing. Adv. Mater. 2019, 31, 1904385. [Google Scholar] [CrossRef] [PubMed]
- Grossman, J.H.; McNeil, S.E. Nanotechnology in cancer medicine. Phys. Today 2012, 65, 38–42. [Google Scholar] [CrossRef] [Green Version]
- Zamani Kouhpanji, M.R.; Stadler, B.J.H. A guideline for effectively synthesizing and characterizing magnetic nanoparticles for advancing nanobiotechnology: A review. Sensors 2020, 20, 2554. [Google Scholar] [CrossRef] [PubMed]
- Tishin, A.M.; Shtil, A.A.; Pyatakov, A.P.; Zverev, V.I. Developing antitumor magnetic hyperthermia: Principles, materials and devices. Recent Pat. Anti-Cancer Drug Discov. 2016, 11, 360–375. [Google Scholar] [CrossRef]
- Wang, L.; Yan, Y.; Wang, M.; Yang, H.; Zhou, Z.; Peng, C.; Yang, S. An integrated nanoplatform for theranostics via multifunctional core-shell ferrite nanocubes. J. Mater. Chem. B 2016, 4, 1908–1914. [Google Scholar] [CrossRef]
- Ali, A.; Shah, T.; Ullah, R.; Zhou, P.; Guo, M.; Ovais, M.; Tan, Z.; Rui, Y. Review on recent progress in magnetic nanoparticles: Synthesis, characterization, and diverse applications. Front. Chem. 2021, 9, 629054. [Google Scholar] [CrossRef]
- Buznikov, N.A.; Safronov, A.P.; Orue, I.; Golubeva, E.V.; Lepalovskij, V.N.; Svalov, A.V.; Chlenova, A.A.; Kurlyandskaya, G.V. Modelling of magnetoimpedance response of thin film sensitive element in the presence of ferrogel: Next step toward development of biosensor for in tissue embedded magnetic nanoparticles detection. Biosens. Bioelectron. 2018, 117, 366–372. [Google Scholar] [CrossRef]
- Cao, Q.L.; Fan, Q.; Chen, Q.; Liu, C.T.; Han, X.T.; Li, L. Recent advances in manipulation of micro- and nano-objects with magnetic fields at small scales. Mater. Horiz. 2020, 7, 638–666. [Google Scholar] [CrossRef]
- Rocha-Santos, T.A.P. Sensors and biosensors based on magnetic nanoparticles. Trends Anal. Chem. 2014, 62, 28–36. [Google Scholar] [CrossRef]
- Ramaswamy, B.; Kulkarni, S.D.; Villar, P.S.; Smith, R.S.; Eberly, C.; Araneda, R.C.; Depireux, D.A.; Shapiro, B. Movement of magnetic nanoparticles in brain tissue: Mechanisms and impact on normal neuronal function. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1821–1829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, S.D.; Gwenin, V.V.; Gwenin, C.D. Magnetic functionalized nanoparticles for biomedical, drug delivery and imaging applications. Nanoscale Res. Lett. 2019, 14, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prilepskii, A.Y.; Fakhardo, A.F.; Drozdov, A.S.; Vinogradov, V.V.; Dudanov, I.P.; Shtil, A.A.; Bel’tyukov, P.P.; Shibeko, A.M.; Koltsova, E.M.; Nechipurenko, D.Y.; et al. Urokinase-conjugated magnetite nanoparticles as a promising drug delivery system for targeted thrombolysis: Synthesis and preclinical evaluation. ACS Appl. Mater. Interfaces 2018, 10, 36764–36775. [Google Scholar] [CrossRef] [PubMed]
- Osipov, V.V.; Kotov, Y.A.; Ivanov, M.G.; Samatov, O.M.; Lisenkov, V.V.; Platonov, V.V.; Murzakaev, A.M.; Medvedev, A.I.; Azarkevich, E.I. Laser synthesis of nanopowder. Laser Synth. Nanopowders 2006, 16, 116–125. [Google Scholar] [CrossRef]
- Khawja Ansari, S.A.M.; Ficiara, E.; Ruffinatti, F.A.; Stura, I.; Argenziano, M.; Abollino, O.; Cavalli, R.; Guiot, C.; D’Agata, F. Magnetic iron oxide nanoparticles: Synthesis, characterization and functionalization for biomedical applications in the central nervous system. Materials 2019, 12, 465. [Google Scholar] [CrossRef] [Green Version]
- Novoselova, I.P.; Safronov, A.P.; Samatov, O.M.; Beketov, I.V.; Medvedev, A.I.; Kurlyandskaya, G.V. Water based suspensions of iron oxide obtained by laser target evaporation for biomedical applications. J. Magn. Magn. Mater. 2016, 415, 35–38. [Google Scholar] [CrossRef]
- Safronov, A.P.; Beketov, I.V.; Komogortsev, S.V.; Kurlyandskaya, G.V.; Medvedev, A.I.; Leiman, D.V.; Larranaga, A.; Bhagat, S.M. Spherical magnetic nanoparticles fabricated by laser target evaporation. AIP Adv. 2013, 3, 052135. [Google Scholar] [CrossRef] [Green Version]
- Kurlyandskaya, G.V.; Safronov, A.P.; Shcherbinin, S.V.; Beketov, I.V.; Blyakhman, F.A.; Makarova, E.B.; Korch, M.A.; Svalov, A.V. Magnetic nanoparticles obtained by electrophysical technique: Focus on biomedical applications physics of the solid state. Phys. Solid State 2021, 63, 1447–1461. [Google Scholar] [CrossRef]
- Glaser, R. Biophysics; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1999. [Google Scholar]
- O’Handley, R.C. Modern Magnetic Materials; John Wiley & Sons: New York, NY, USA, 1972; p. 740. [Google Scholar]
- Coey, J.M.D. Magnetism and Magnetic Materials; Cambridge University Press: New York, NY, USA, 2010; p. 628. [Google Scholar]
- Roca, A.G.; Costo, R.; Rebolledo, A.F.; Veintemillas-Verdaguer, S.; Tartaj, P.; González-Carreño, T.; Morales, M.P.; Serna, C.J. Progress in the preparation of magnetic nanoparticles for applications in biomedicine. J. Phys. D Appl. Phys. 2009, 42, 224002. [Google Scholar] [CrossRef]
- Miyakoshi, J. Effects of static magnetic fields at the cellular level. Prog. Biophys. Mol. Biol. 2005, 87, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Blyakhman, F.A.; Melnikov, G.Y.; Makarova, E.B.; Fadeyev, F.A.; Sedneva-Lugovets, D.V.; Shabadrov, P.A.; Volchkov, S.O.; Mekhdieva, K.R.; Safronov, A.P.; Fernández Armas, S.; et al. Effects of constant magnetic field to the proliferation rate of human fibroblasts grown onto different substrates: Tissue culture polystyrene, polyacrylamide hydrogel and ferrogels γ-Fe2O3 magnetic nanoparticles. Nanomaterials 2020, 10, 1697. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From clinical significance to quantification. Adv. Sci. 2021, 8, 2004433. [Google Scholar] [CrossRef] [PubMed]
- Bull, E.; Madani, S.Y.; Sheth, R.; Seifalian, A.; Green, M.; Seifalian, A.M. Stem cell tracking using iron oxide nanoparticles. Int. J. Nanomed. 2014, 9, 1641–1653. [Google Scholar] [CrossRef] [Green Version]
- Buznikov, N.A.; Kurlyandskaya, G.V. A model for the magnetoimpedance effect in non-symmetric nanostructured multilayered films with ferrogel coverings. Sensors 2021, 21, 5151. [Google Scholar] [CrossRef]
- Bautista-Hernández, L.A.; Gómez-Olivares, J.L.; Buentello-Volante, B.; Bautista-de Lucio, V.M. Fibroblasts: The unknown sentinels eliciting immune responses against microorganisms. Eur. J. Microbiol. Immunol. 2017, 7, 151–157. [Google Scholar] [CrossRef]
- Amin, R.M.; Abdelmonem, A.; Verwanger, T.; Elsherbini, E.; Krammer, B. Cytotoxicity of magnetic nanoparticles on normal and malignant human skin cells. Nano LIFE 2014, 4, 1440002. [Google Scholar] [CrossRef]
- Shibata, M.; Kanetaka, H.; Furuya, M.; Yokota, K.; Ogawa, T.; Kawashita, M. Cytotoxicity evaluation of iron nitride nanoparticles for biomedical applications. J. Biomed. Mater. Res. 2021, 109, 1784–1791. [Google Scholar] [CrossRef]
- Chen, L.; Chen, C.; Wang, P.; Song, T. Mechanisms of cellular effects directly induced by magnetic nanoparticles under magnetic fields. J. Nanomater. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Marycz, K.; Marędziak, M.; Lewandowski, D.; Zachanowicz, E.; Zięcina, A.; Wiglusz, R.J.; Pązik, R. The effect of Co0.2Mn0.8Fe2O4 ferrite nanoparticles on the C2 canine mastocytoma cell line and adipose-derived mesenchymal stromal stem cells (ASCs) cultured under a static magnetic field: Possible implications in the treatment of dog mastocytoma. Cell. Mol. Bioeng. 2017, 10, 209–222. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wei, Z.; Lv, H.; Wu, L.; Cui, Y.; Yao, H.; Li, J.; Zhang, H.; Yang, B.; Jiang, J. Iron oxide nanoparticles promote the migration of mesenchymal stem cells to injury sites. IJN 2019, 14, 573–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.-K.; Liu, Y.-P.; Ho, J.H.; Hsu, S.-C.; Lee, O.K. Amine-surface-modified superparamagnetic iron oxide nanoparticles interfere with differentiation of human mesenchymal stem cells. J. Orthop. Res. 2012, 30, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Ohta, K.; Ishida, Y.; Fukui, A.; Mizuta, K.; Nishi, H.; Takechi, M.; Kamata, N. Toll-like receptor (TLR) expression and TLR-mediated interleukin-8 production by human submandibular gland epithelial cells. Mol. Med. Rep. 2014, 10, 2377–2382. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. CSH Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Oh, J.-H.; Lee, D.H.; Bae, J.-S.; Jin, C.L.; Park, C.-H.; Chung, J.H. Toll-like receptor family members in skin fibroblasts are functional and have a higher expression compared to skin keratinocytes. Int. J. Mol. Med. 2015, 35, 1443–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masur, S.K.; Dewal, H.S.; Dinh, T.T.; Erenburg, I.; Petridou, S. Myofibroblasts differentiate from fibroblasts when plated at low density. Proc. Natl. Acad. Sci. USA 1996, 93, 4219–4223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javani Jouni, F.; Abdolmaleki, P.; Movahedin, M. Investigation on the effect of static magnetic field up to 15 mT on the viability and proliferation rate of rat bone marrow stem cells. Vitro Cell. Dev. Biol.-Anim. 2013, 49, 212–219. [Google Scholar] [CrossRef]
- Zafari, J.; Vazini, H.; Javani Jouni, F.; Abdolmaleki, P.; Monajemi, R.; Shams, E.; Satari, M. Anticancer effects of moderate static magnetic field on cancer cells in vitro. Res. Mol. Med. 2019, 6, 72–84. [Google Scholar] [CrossRef]
- Raylman, R.R.; Clavo, A.C.; Wahl, R.L. Exposure to strong static magnetic field slows the growth of human cancer cells in vitro. Bioelectromagnetics 1996, 17, 358–363. [Google Scholar] [CrossRef] [Green Version]
- Fan, Z.; Hu, P.; Xiang, L.; Liu, Y.; He, R.; Lu, T. A Static magnetic field inhibits the migration and telomerase function of mouse breast cancer cells. BioMed Res. Int. 2020, 2020, 7472618. [Google Scholar] [CrossRef]
- Nakamae, T.; Adachi, N.; Kobayashi, T.; Nagata, Y.; Nakasa, T.; Tanaka, N.; Ochi, M. The effect of an external magnetic force on cell adhesion and proliferation of magnetically labeled mesenchymal stem cells. BMC Sports Sci. Med. Rehabil. 2010, 2, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Xiang, B.; Deng, J.; Freed, D.H.; Arora, R.C.; Tian, G. Inhibition of viability, proliferation, cytokines secretion, surface antigen expression, and adipogenic and osteogenic differentiation of adipose-derived stem cells by seven-day exposure to 0.5 T static magnetic fields. Stem Cells Int. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gruchlik, A.; Turek, A.; Polechoński, J.; Dzierżewicz, Z. Effects of 300 mT static magnetic field on IL-8 secretion in normal human colon myofibroblasts. Acta Pol. Pharm. 2015, 72, 713–717. [Google Scholar]
- Manjua, A.C.; Cabral, J.M.S.; Portugal, C.A.M.; Ferreira, F.C. Magnetic stimulation of the angiogenic potential of mesenchymal stromal cells in vascular tissue engineering. STAM 2021, 22, 461–480. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, X. Magnetic fields and reactive oxygen species. Int. J. Mol. Sci. 2017, 18, 2175. [Google Scholar] [CrossRef] [Green Version]
- Lew, W.-Z.; Huang, Y.-C.; Huang, K.-Y.; Lin, C.-T.; Tsai, M.-T.; Huang, H.-M. Static magnetic fields enhance dental pulp stem cell proliferation by activating the p38 mitogen-activated protein kinase pathway as its putative mechanism: SMF enhances DPSC proliferation via p38 MAPK pathway. J. Tissue Eng. Regen. Med. 2018, 12, 19–29. [Google Scholar] [CrossRef]
- Yuvchenko, A.A.; Lepalovskii, V.N.; Vas’kovskii, V.O.; Safronov, A.P.; Volchkov, S.O.; Kurlyandskaya, G.V. Magnetic impedance of structured film meanders in the presence of magnetic microand nanoparticles. Tech. Phys. 2014, 59, 230–236. [Google Scholar] [CrossRef]
- Kurlyandskaya, G.V.; Litvinova, L.S.; Safronov, A.P.; Schupletsova, V.V.; Tyukova, I.S.; Khaziakhmatova, O.G.; Slepchenko, G.B.; Yurova, K.A.; Cherempey, E.G.; Kulesh, N.A.; et al. Water-based suspensions of iron oxide nanoparticles with electrostatic or steric stabilization by chitosan: Fabrication, characterization and biocompatibility. Sensors 2017, 17, 2605. [Google Scholar] [CrossRef] [Green Version]
- Kulesh, N.A.; Novoselova, I.P.; Safronov, A.P.; Beketov, I.V.; Samatov, O.M.; Kurlyandskaya, G.V.; Morozova, M.V.; Denisova, T.P. Total reflection X-ray fluorescence spectroscopy as a tool for evaluation of iron concentration in ferrofluids and yeast samples. J. Magn. Magn. Mater. 2016, 415, 39–44. [Google Scholar] [CrossRef]
- Kokorina, L.A.; Chernyavskaya, Y.V.; Denisova, T.P.; Simonova, E.V.; Safronov, A.P.; Kurlyandskaya, G.V. Variability of pathogenicity factors representatives of the human microbiome under the influence of γ-Fe2O3 iron oxide nanoparticles. Chim. Techno Acta 2022, 9, 20229401. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fadeyev, F.A.; Blyakhman, F.A.; Safronov, A.P.; Melnikov, G.Y.; Nikanorova, A.D.; Novoselova, I.P.; Kurlyandskaya, G.V. Biological Impact of γ-Fe2O3 Magnetic Nanoparticles Obtained by Laser Target Evaporation: Focus on Magnetic Biosensor Applications. Biosensors 2022, 12, 627. https://doi.org/10.3390/bios12080627
Fadeyev FA, Blyakhman FA, Safronov AP, Melnikov GY, Nikanorova AD, Novoselova IP, Kurlyandskaya GV. Biological Impact of γ-Fe2O3 Magnetic Nanoparticles Obtained by Laser Target Evaporation: Focus on Magnetic Biosensor Applications. Biosensors. 2022; 12(8):627. https://doi.org/10.3390/bios12080627
Chicago/Turabian StyleFadeyev, Fedor A., Felix A. Blyakhman, Alexander P. Safronov, Grigory Yu. Melnikov, Anastasia D. Nikanorova, Iuliia P. Novoselova, and Galina V. Kurlyandskaya. 2022. "Biological Impact of γ-Fe2O3 Magnetic Nanoparticles Obtained by Laser Target Evaporation: Focus on Magnetic Biosensor Applications" Biosensors 12, no. 8: 627. https://doi.org/10.3390/bios12080627




