Ultrasensitive Diamond Microelectrode Application in the Detection of Ca2+ Transport by AnnexinA5-Containing Nanostructured Liposomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Solutions
2.2. Preparation of Liposomes and Proteoliposomes
2.3. Atomic Force Microscopy (AFM) Analyses of Liposome and Proteoliposome
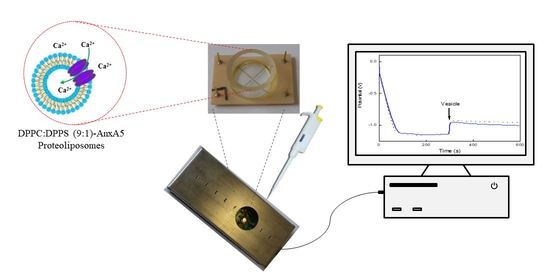
2.4. Potential Measurements Using Diamond Microelectrodes
3. Results
3.1. Characterization of Liposomes and Proteoliposomes
3.2. Potentiometric Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Denisenko, A.; Aleksov, A.; Kohn, E. PH Sensing by Surface-Doped Diamond Effect of the Diamond Surface Termination. Diam. Relat. Mater. 2001, 10, 667–672. [Google Scholar] [CrossRef]
- Denisenko, A.; Jamornmarn, G.; El-Hajj, H.; Kohn, E. PH Sensor on O-Terminated Diamond Using Boron-Doped Channel. Diam. Relat. Mater. 2007, 16, 905–910. [Google Scholar] [CrossRef]
- Du, X.; Mo, Z.; Li, Z.; Zhang, W.; Luo, Y.; Nie, J.; Wang, Z.; Liang, H. Boron-doped diamond (BDD) electro-oxidation coupled with nanofiltration for secondary wastewater treatment: Antibiotics degradation and biofouling. Environ. Int. 2021, 146, 106291. [Google Scholar] [CrossRef] [PubMed]
- Uwayezu, J.N.; Carabante, I.; Lejon, T.; van Hees, P.; Karlsson, P.; Hollman, P.; Kumpiene, J. Electrochemical degradation of per- and poly-fluoroalkyl substances using boron-doped diamond electrodes. J. Environ. Manag. 2021, 290, 112573. [Google Scholar] [CrossRef] [PubMed]
- Shivdasani, M.N.; Evans, M.; Burns, O.; Yeoh, J.; Allen, P.J.; Nayagam, D.A.X.; Villalobos, J.; Abbott, C.J.; Luu, C.D.; Opie, N.L.; et al. In vivo feasibility of epiretinal stimulation using ultrananocrystalline diamond electrodes. J. Neural Eng. 2020, 17, 045014. [Google Scholar] [CrossRef] [PubMed]
- Sikder, M.K.U.; Tong, W.; Pingle, H.; Kingshott, P.; Needham, K.; Shivdasani, M.N.; Fallon, J.B.; Seligman, P.; Ibbotson, M.R.; Prawer, S.; et al. Laminin coated diamond electrodes for neural stimulation. Mater. Sci. Eng. C 2021, 118, 111454. [Google Scholar] [CrossRef]
- Hejazi, M.A.; Tong, W.; Stacey, A.; Soto-Breceda, A.; Ibbotson, M.R.; Yunzab, M.; Maturana, M.I.; Almasi, A.; Jung, Y.J.; Sun, S.; et al. Hybrid diamond/ carbon fiber microelectrodes enable multimodal electrical/chemical neural interfacing. Biomaterials 2020, 230, 119648. [Google Scholar] [CrossRef]
- Lawrence, N.S.; Pagels, M.; Meredith, A.; Jones, T.G.J.; Hall, C.E.; Pickles, C.S.J.; Godfried, H.P.; Banks, C.E.; Compton, R.G.; Jiang, L. Electroanalytical applications of boron-doped diamond microelectrode arrays. Talanta 2006, 69, 829–834. [Google Scholar] [CrossRef]
- Abt, B.; Hartmann, A.; Pasquarelli, A.; Strehle, S.; Mizaikoff, B.; Kranz, C. Electrochemical Determination of Sulphur-containing Pharmaceuticals Using Boron-doped Diamond Electrodes. Electroanalysis 2016, 28, 1641–1646. [Google Scholar] [CrossRef]
- Hébert, C.; Scorsone, E.; Bendali, A.; Kiran, R.; Cottance, M.; Girard, H.A.; Degardin, J.; Dubus, E.; Lissorgues, G.; Rousseau, L.; et al. Boron doped diamond biotechnology: From sensors to neurointerfaces. Faraday Discuss. 2014, 172, 47–59. [Google Scholar] [CrossRef] [Green Version]
- Carabelli, V.; Gosso, S.; Marcantoni, A.; Xu, Y.; Colombo, E.; Gao, Z.; Vittone, E.; Kohn, E.; Pasquarelli, A.; Carbone, E. Nanocrystalline Diamond Microelectrode Arrays Fabricated on Sapphire Technology for High-Time Resolution of Quantal Catecholamine Secretion from Chromaffin Cells. Biosens. Bioelectron. 2010, 26, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Gosso, S.; Turturici, M.; Franchino, C.; Colombo, E.; Pasquarelli, A.; Carbone, E.; Carabelli, V. Heterogeneous Distribution of Exocytotic Microdomains in Adrenal Chromaffin Cells Resolved by High-Density Diamond Ultra-Microelectrode Arrays. J. Physiol. 2014, 592, 3215–3230. [Google Scholar] [CrossRef] [PubMed]
- Pasquarelli, A.; Marcantoni, A.; Gavello, D.; Battiato, A.; Picollo, F.; Olivero, P.; Carbone, E.; Carabelli, V. Simultaneous Fluorescent and Amperometric Detection of Catecholamine Release from Neuroendocrine Cells with Transparent Diamond MEAs. Front. Neurosci. 2016, 10, 1. [Google Scholar] [CrossRef]
- Hébert, C.; Cottance, M.; Degardin, J.; Scorsone, E.; Rousseau, L.; Lissorgues, G.; Bergonzo, P.; Picaud, S. Monitoring the Evolution of Boron Doped Porous Diamond Electrode on Flexible Retinal Implant by OCT and in Vivo Impedance Spectroscopy. Mater. Sci. Eng. C 2016, 69, 77–84. [Google Scholar] [CrossRef]
- Piret, G.; Hébert, C.; Mazellier, J.P.; Rousseau, L.; Scorsone, E.; Cottance, M.; Lissorgues, G.; Heuschkel, M.O.; Picaud, S.; Bergonzo, P.; et al. 3D-Nanostructured Boron-Doped Diamond for Microelectrode Array Neural Interfacing. Biomaterials 2015, 53, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Pasquarelli, A.; Gao, Z.; Alsawafi, S.; Izadi, I.; Colombo, E.; Gosso, S.; Marcantoni, A.; Ullmann, M.; Carabelli, V.; Carbone, E. Diamond microelectrode arrays: A versatile tool for in vitro measurements. In Proceedings of the 8th International Meeting on Substrate-Integrated Microelectrode Arrays, Reutlingen, Germany, 10–13 July 2012; pp. 317–318. [Google Scholar]
- Gosso, S.; Marcantoni, A.; Vandael, D.; Turturici, M.; Alsawafi, S.; Izadi, I.; Colombo, E.; Pasquarelli, A.; Carbone, E.; Carabelli, V. Multi-purpose nanocrystalline boron-doped diamond MEAs for amperometric, potentiometric and pH recordings from excitable cells. In Proceedings of the 8th International Meeting on Substrate-Integrated Microelectrode Arrays, Reutlingen, Germany, 10–13 July 2012; pp. 323–324. [Google Scholar]
- Maybeck, V.; Edgington, R.; Bongrain, A.; Welch, J.O.; Scorsone, E.; Bergonzo, P.; Jackman, R.B.; Offenhäusser, A. Boron-Doped Nanocrystalline Diamond Microelectrode Arrays Monitor Cardiac Action Potentials. Adv. Healthc. Mater. 2014, 2, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Hauptmann, R.; Maurer, I.Y.; Krystek, E.; Bodo, G.; Andree, H.; Reutelingsperger, C.P.M. Vascular Anticoagulant Beta: A Novel Human Ca2+/Phospholipid Binding Protein That Inhibits Coagulation and Phospholipase A2 Activity. Its Molecular Cloning, Expression and Comparison with VAC-Alpha. Eur. J. Biochem. 1989, 185, 63–71. [Google Scholar] [CrossRef]
- Andree, H.A.M.; Stuart, M.C.A.; Hermens, W.T.; Reutelingsperger, C.P.M.; Hemker, H.C.; Frederik, P.M.; Willems, G.M. Clustering of Lipid-Bound Annexin V May Explain Its Anticoagulant Effect. J. Biol. Chem. 1992, 267, 17907–17912. [Google Scholar] [CrossRef]
- Schlaepfer, D.D.; Jones, J.; Haigler, H.T. Inhibition of Protein Kinase C by Annexin, V. Biochemistry 1992, 31, 1886–1891. [Google Scholar] [CrossRef]
- Davidson, F.F.; Lister, M.D.; Dennis, E.A. Binding and Inhibition Studies on Lipocortins Using Phosphatidylcholine Vesicles and Phospholipase A2 from Snake Venom, Pancreas, and a Macrophage-like Cell Line. J. Biol. Chem. 1990, 265, 5602–5609. [Google Scholar] [CrossRef]
- Genge, B.R.; Cao, X.; Wu, L.N.Y.; Buzzi, W.R.; Showman, R.W.; Arsenault, A.L.; Ishikawa, Y.; Wuthier, R.E. Establishment of the Primary Structure of the Major Lipid-dependent Ca2+ Binding Proteins of Chicken Growth Plate Cartilage Matrix Vesicles: Identity with Anchorin Cii (Annexin V) and Annexin II. J. Bone Miner. Res. 1992, 7, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Genge, B.R.; Wu, L.N.Y.; Buzzi, W.R.; Showman, R.W.; Wuthier, R.E. Characterization, Cloning and Expression of 67-KDa Annexin from Chicken Growth Plate Cartilage Matrix Vesicles. Biochem. Biophys. Res. Commun. 1993, 197, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.N.Y.; Genge, B.R.; Dunkelberger, D.G.; Legeros, R.Z.; Concannon, B.; Wuthier, R.E. Physicochemical Characterization of the Nucleational Core of Matrix Vesicles. J. Biol. Chem. 1997, 272, 4404–4411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolean, M.; Simão, A.M.S.; Kiffer-Moreira, T.; Hoylaerts, M.F.; Millán, J.L.; Itri, R.; Ciancaglini, P. Proteoliposomes with the Ability to Transport Ca2+ into the Vesicles and Hydrolyze Phosphosubstrates on Their Surface. Arch. Biochem. Biophys. 2015, 584, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Bolean, M.; Izzi, B.; van kerckhoven, S.; Bottini, M.; Ramos, A.P.; Millán, J.L.; Hoylaerts, M.F.; Ciancaglini, P. Matrix Vesicle Biomimetics Harboring Annexin A5 and Alkaline Phosphatase Bind to the Native Collagen Matrix Produced by Mineralizing Vascular Smooth Muscle Cells. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129629. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, T.; Nah, H.-D.; Demuth, D.R.; Harrison, G.; Golub, E.E.; Adams, S.L.; Pacifici, M. Annexin V-Mediated Calcium Flux across Membranes Is Dependent on the Lipid Composition: Implications for Cartilage Mineralization. Biochemistry 1997, 36, 3359–3367. [Google Scholar] [CrossRef] [PubMed]
- Wuthier, R.E.; Lipscomb, G.F. Matrix Vesicles: Structure, Composition, Formation and Function in Calcification. Front. Biosci. 2011, 16, 2812–2902. [Google Scholar] [CrossRef]
- Boye, T.L.; Jeppesen, J.C.; Maeda, K.; Pezeshkian, W.; Solovyeva, V.; Nylandsted, J.; Simonsen, A.C. Annexins Induce Curvature on Free-Edge Membranes Displaying Distinct Morphologies. Sci. Rep. 2018, 8, 10309, 1–14. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chipot, C.; Scheuring, S. Annexin-V Stabilizes Membrane Defects by Inducing Lipid Phase Transition. Nat. Commun. 2020, 11, 230. [Google Scholar] [CrossRef]
- Mularski, A.; Sønder, S.L.; Heitmann, A.S.B.; Nylandsted, J.; Simonsen, A.C. Simultaneous Membrane Binding of Annexin A4 and A5 Suppresses 2D Lattice Formation While Maintaining Curvature Induction. J. Colloid Interface Sci. 2021, 600, 854–864. [Google Scholar] [CrossRef]
- Huber, R.; Romisch, J.; Paques, E.P. The Crystal and Molecular Structure of Human Annexin V, an Anticoagulant Protein That Binds to Calcium and Membranes. EMBO J. 1990, 9, 3867–3874. [Google Scholar] [CrossRef] [PubMed]
- Liemann, S.; Huber, R. Three-Dimensional Structure of Annexins. C. Cell Mol. Life Sci. 1997, 53, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Arispe, N.; Rojas, E.; Genge, B.R.; Wu, L.N.Y.; Wuthier, R.E. Similarity in Calcium Channel Activity of Annexin V and Matrix Vesicles in Planar Lipid Bilayers. Biophys. J. 1996, 71, 1764–1775. [Google Scholar] [CrossRef] [Green Version]
- Bottini, M.; Mebarek, S.; Anderson, K.L.; Strzelecka-Kiliszek, A.; Bozycki, L.; Simão, A.M.S.; Bolean, M.; Ciancaglini, P.; Pikula, J.B.; Pikula, S.; et al. Matrix Vesicles from Chondrocytes and Osteoblasts: Their Biogenesis, Properties, Functions and Biomimetic Models. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Plaut, J.S.; Strzelecka-Kiliszek, A.; Bozycki, L.; Pikula, S.; Buchet, R.; Mebarek, S.; Chadli, M.; Bolean, M.; Simao, A.M.S.; Ciancaglini, P.; et al. Quantitative Atomic Force Microscopy Provides New Insight into Matrix Vesicle Mineralization. Arch. Biochem. Biophys. 2019, 667, 14–21. [Google Scholar] [CrossRef]
- Kaneko, N.; Ago, H.; Matsuda, R.; Inagaki, E.; Miyano, M. Crystal Structure of Annexin V with Its Ligand K-201 as a Calcium Channel Activity Inhibitor. J. Mol. Biol. 1997, 274, 16–20. [Google Scholar] [CrossRef]
- Kaneko, N.; Matsuda, R.; Toda, M.; Shimamoto, K. Inhibition of Annexin V-Dependent Ca2+ Movement in Large Unilamellar Vesicles by K201, a New 1,4-Benzothiazepine Derivative. Biochim. Biophys. Acta Biomembr. 1997, 1330, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, A.; Escherich, A.; Lewit-Bentley, A.; Benz, J.; Raguenes-Nicol, C.; Russo-Marie, F.; Gerke, V.; Moroder, L.; Huber, R. Interactions of Benzodiazepine Derivatives with Annexins. J. Biol. Chem. 1998, 273, 2885–2894. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Xu, J.; Kirsch, T. Annexin-Mediated Ca2+ Influx Regulates Growth Plate Chondrocyte Maturation and Apoptosis. J. Biol. Chem. 2003, 278, 3762–3769. [Google Scholar] [CrossRef] [Green Version]
- Kirsch, T.; Harrison, G.; Golub, E.E.; Nah, H.D. The Roles of Annexins and Types II and X Collagen in Matrix Vesicle-Mediated Mineralization of Growth Plate Cartilage. J. Biol. Chem. 2000, 275, 35577–35583. [Google Scholar] [CrossRef] [Green Version]
- Hartree, E.F. Determination of Protein: A Modification of the Lowry Method That Gives a Linear Photometric Response. Anal. Biochem. 1972, 48, 422–427. [Google Scholar] [CrossRef]
- Bolean, M.; Borin, I.A.; Simão, A.M.S.; Bottini, M.; Bagatolli, L.A.; Hoylaerts, M.F.; Millán, J.L.; Ciancaglini, P. Topographic Analysis by Atomic Force Microscopy of Proteoliposomes Matrix Vesicle Mimetics Harboring TNAP and AnxA5. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Carabelli, V.; Carbone, E.; Colombo, E.; Demaria, F.; Dipalo, M.; Gosso, S.; Manfredotti, C.; Pasquarelli, A.; Rossi, S.; et al. Transparent Diamond Microelectrodes for Biochemical Application. Diam. Relat. Mater. 2010, 19, 1021–1026. [Google Scholar] [CrossRef]
- Shirafuji, J.; Sugino, T. Electrical properties of diamond surfaces. Diam. Relat. Mater. 1996, 5, 706–713. [Google Scholar] [CrossRef]
- Bouter, A.; Gounou, C.; Bérat, R.; Tan, S.; Gallois, B.; Granier, T.; D’Estaintot, B.L.; Pöschl, E.; Brachvogel, B.; Brisson, A.R. Annexin-A5 Assembled into Two-Dimensional Arrays Promotes Cell Membrane Repair. Nat. Commun. 2011, 2, 270. [Google Scholar] [CrossRef] [Green Version]
- Park, H.C.; Quan, H.; Yang, H.C. Effects of phosphatidylserine-containing liposomes on odontogenic differentiation of human dental pulp cells. Dent. Mater. J. 2017, 36, 76–81. [Google Scholar] [CrossRef] [Green Version]
- Fezoua-Boubegtiten, Z.; Desbat, B.; Brisson, A.; Gounou, C.; Laguerre, M.; Lecomte, S. Effect of Mg2+ versus Ca2+ on the Behavior of Annexin A5 in a Membrane-Bound State. Eur. Biophys. J. 2011, 40, 641–649. [Google Scholar] [CrossRef]
- Genge, B.R.; Wu, L.N.; Wuthier, R.E. Identification of phospholipid-dependent calcium-binding proteins as constituents of matrix vesicles. J. Biol. Chem. 1989, 264, 10917–10921. [Google Scholar] [CrossRef]
- Genge, B.R.; Wu, L.N.; Wuthier, R.E. Differential fractionation of matrix vesicle proteins. Further characterization of the acidic phospholipid-dependent Ca2+-binding proteins. J. Biol. Chem. 1990, 265, 4703–4710. [Google Scholar] [CrossRef]
- Andersson, M.; Malmendal, A.; Linse, S.; Ivarsson, I.; Forsen, S.; Svensson, L.A. Structural basis for the negative allostery between Ca (2+)- and Mg (2+)-binding in the intracellular Ca (2+)-receptor calbindin D9k. Protein Sci. 1997, 6, 1139–1147. [Google Scholar] [CrossRef] [Green Version]
- Babu, A.; Su, H.; Ryu, Y.; Gulati, J. Determination of residue specificity in the EF-hand of troponin C for Ca2+ coordination, by genetic engineering. J. Biol. Chem. 1992, 267, 15469–15474. [Google Scholar] [CrossRef]
- Fahie, K.; Pitts, R.; Elkins, K.; Nelson, D.J. Molecular dynamics study of Ca (2+) binding loop variants of silver hake parvalbumin with aspartic acid at the "gateway" position. J. Biomol. Struct. Dyn. 2002, 19, 821–837. [Google Scholar] [CrossRef] [PubMed]
- Buchbinder, J.L.; Baraniak, J.; Frey, P.A.; Reed, G.H. Stereochemistry of metal ion coordination to the terminal thiophosphoryl group of adenosine 5’-O- (3- thiotriphosphate) at the active site of pyruvate kinase. Biochemistry 1993, 32, 14111–14116. [Google Scholar] [CrossRef] [PubMed]
- Soultanas, P.; Dillingham, M.S.; Velankar, S.S.; Wigley, D.B. DNA binding mediates conformational changes and metal ion coordination in the active site of PcrA helicase. J. Mol. Biol. 1999, 290, 137–148. [Google Scholar] [CrossRef]






| #Recording | Aim | Initial Solution | 1st Dispensing | 2nd Dispensing | Results |
|---|---|---|---|---|---|
| 1 | Test capacitive effects of volume changes | 1 mM Ca2+ in water | 1 mM Ca2+ in water | - | Stable potential |
| 2 | 1 mM Mg2+ in water | 1 mM Mg2+ in water | - | ||
| 3 | Test potential change by buffer dispensing | 1 mM Ca2+ in water | Tris (16 mM pH 7.5) | - | ~150 mV step with second equilibration |
| 4 | 1 mM Mg2+ in water | Tris(16 mM pH 7.5) | - | ||
| 5 | Test liposome response to Mg2+ | 1 mM Mg2+ in water | Tris + Lipo | - | ~150 mV step with second equilibration |
| 6 | Test proteoliposome response to Mg2+ | 1 mM Mg2+ in water | Tris + Proteo | - | ~130 mV step with small drop vs. time |
| 7 | Test liposome response to Ca2+ | 1 mM Ca2+ in water | Tris + Lipo | - | ~140 mV step with second equilibration |
| 8 | Test proteoliposome response to Ca2+ | 1 mM Ca2+ in water | Tris + Proteo | - | ~120 mV step, drops to baseline |
| 9 | Test liposome response to Mg2+ in buffer | 1 mM Mg2+ in Tris | Tris + Lipo | - | ~40 mV step with second equilibration |
| 10 | Test proteoliposome response to Mg2+ in buffer | 1 mM Mg2+ in Tris | Tris + Proteo | - | ~30 mV step with second equilibration |
| 11 | Test liposome response to Ca2+ in buffer | 1 mM Ca2+ in Tris | Tris + Lipo | - | ~40 mV step with second equilibration |
| 12 | Test proteoliposome response to Ca2+ in buffer | 1 mM Ca2+ in Tris | Tris + Proteo | - | ~30 mV potential step, drops to baseline |
| 13 | Test liposome response to Na+ | 1 mM Na+ in water | Tris + Lipo | - | ~220 mV step with second equilibration |
| 14 | Test proteoliposome response to Na+ | 1 mM Na+ in water | Tris + Proteo | - | ~200 mV step with second equilibration |
| 15 | Test liposome response to Na+ in buffer | 1 mM Na+ in Tris | Tris + Lipo | - | ~20 mV potential step |
| 16 | Test proteoliposome response to Na+ in buffer | 1 mM Na+ in Tris | Tris + Proteo | - | ~10 mV potential step |
| 17 | Test potential change by chelator dispensing | 1 mM Ca2+ in Tris | EDTA | ~20 mV negative step ~stable over time | |
| 18 | Test chelator effect on liposome response to Ca2+ | 1 mM Ca2+ in Tris | Tris + Lipo | EDTA | (1) ~20 mV steady step (2) ~130 mV steady step |
| 19 | Test chelator effect on proteoliposome response to Ca2+ | 1 mM Ca2+ in Tris | Tris + Proteo | EDTA | (1) ~30 mV dropping slope (2) ~150 mV step with second equilibration |
| 20 | Test chelator effect on liposome response to Ca2+ | 1 mM Ca2+ in Tris | EDTA | Tris + Lipo | (1) ~40 mV negative step (2) step ends ~30 mV above baseline |
| 21 | Test chelator effect on proteoliposome response to Ca2+ | 1 mM Ca2+ in Tris | EDTA | Tris + Proteo | (1) ~40 mV negative step (2) step ends ~30 mV above baseline |
| 22 | Test chelator effect on proteoliposome response to Ca2+ | 1 mM Ca2+ in Tris + EDTA | Tris + Proteo | ~30 mV steady step |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasquarelli, A.; Andrilli, L.H.S.; Bolean, M.; Reis Ferreira, C.; Cruz, M.A.E.; de Oliveira, F.A.; Ramos, A.P.; Millán, J.L.; Bottini, M.; Ciancaglini, P. Ultrasensitive Diamond Microelectrode Application in the Detection of Ca2+ Transport by AnnexinA5-Containing Nanostructured Liposomes. Biosensors 2022, 12, 525. https://doi.org/10.3390/bios12070525
Pasquarelli A, Andrilli LHS, Bolean M, Reis Ferreira C, Cruz MAE, de Oliveira FA, Ramos AP, Millán JL, Bottini M, Ciancaglini P. Ultrasensitive Diamond Microelectrode Application in the Detection of Ca2+ Transport by AnnexinA5-Containing Nanostructured Liposomes. Biosensors. 2022; 12(7):525. https://doi.org/10.3390/bios12070525
Chicago/Turabian StylePasquarelli, Alberto, Luiz Henrique Silva Andrilli, Maytê Bolean, Claudio Reis Ferreira, Marcos Antônio Eufrásio Cruz, Flavia Amadeu de Oliveira, Ana Paula Ramos, José Luis Millán, Massimo Bottini, and Pietro Ciancaglini. 2022. "Ultrasensitive Diamond Microelectrode Application in the Detection of Ca2+ Transport by AnnexinA5-Containing Nanostructured Liposomes" Biosensors 12, no. 7: 525. https://doi.org/10.3390/bios12070525