Label-Free Cholesteric Liquid Crystal Biosensing Chips for Heme Oxygenase-1 Detection within Cerebrospinal Fluid as an Effective Outcome Indicator for Spontaneous Subarachnoid Hemorrhage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. CSF Sample Collection, Preparation, and Analysis
2.3. Study Design
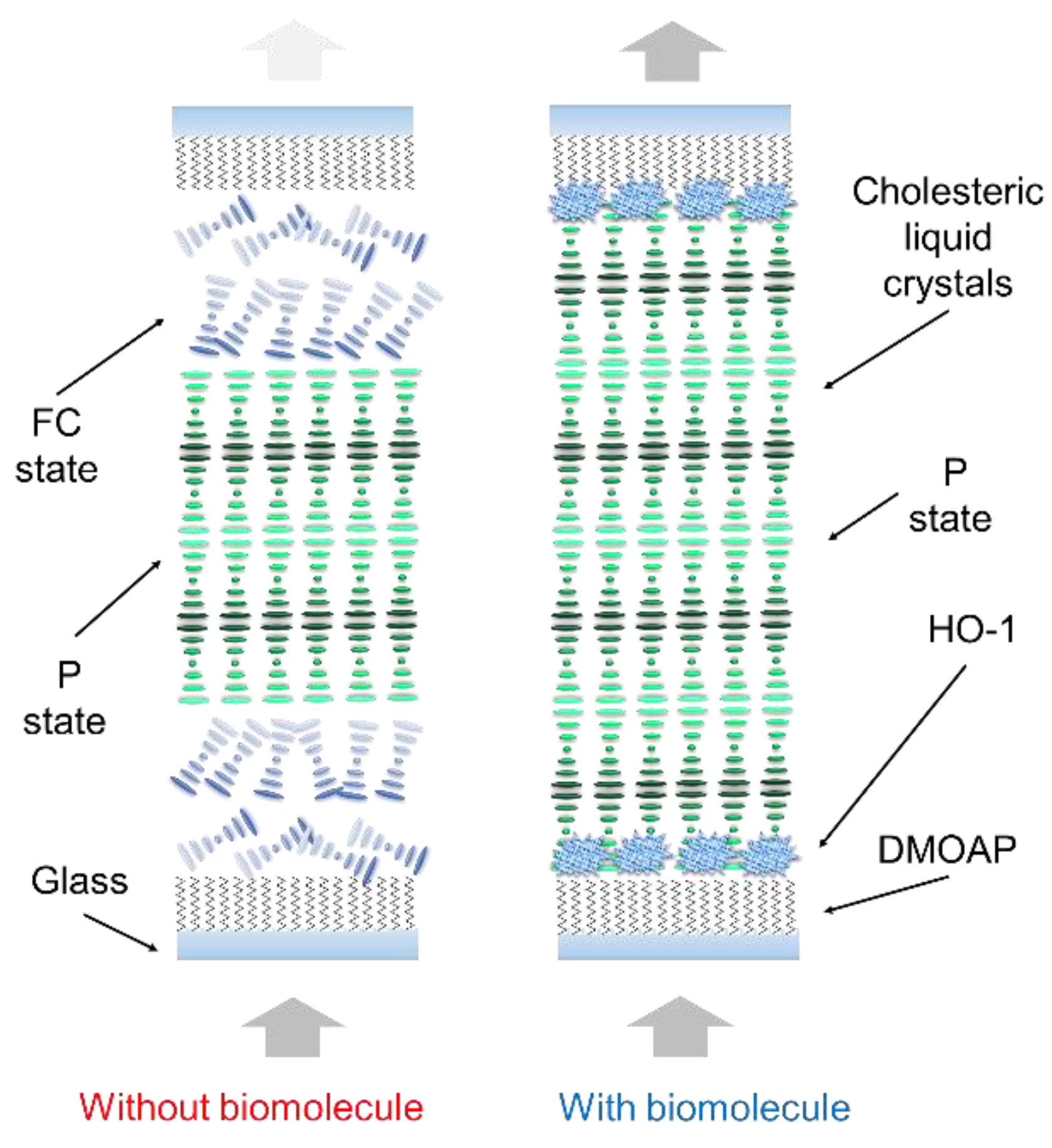
2.4. CLC Biosensor Preparation
3. Results and Discussion
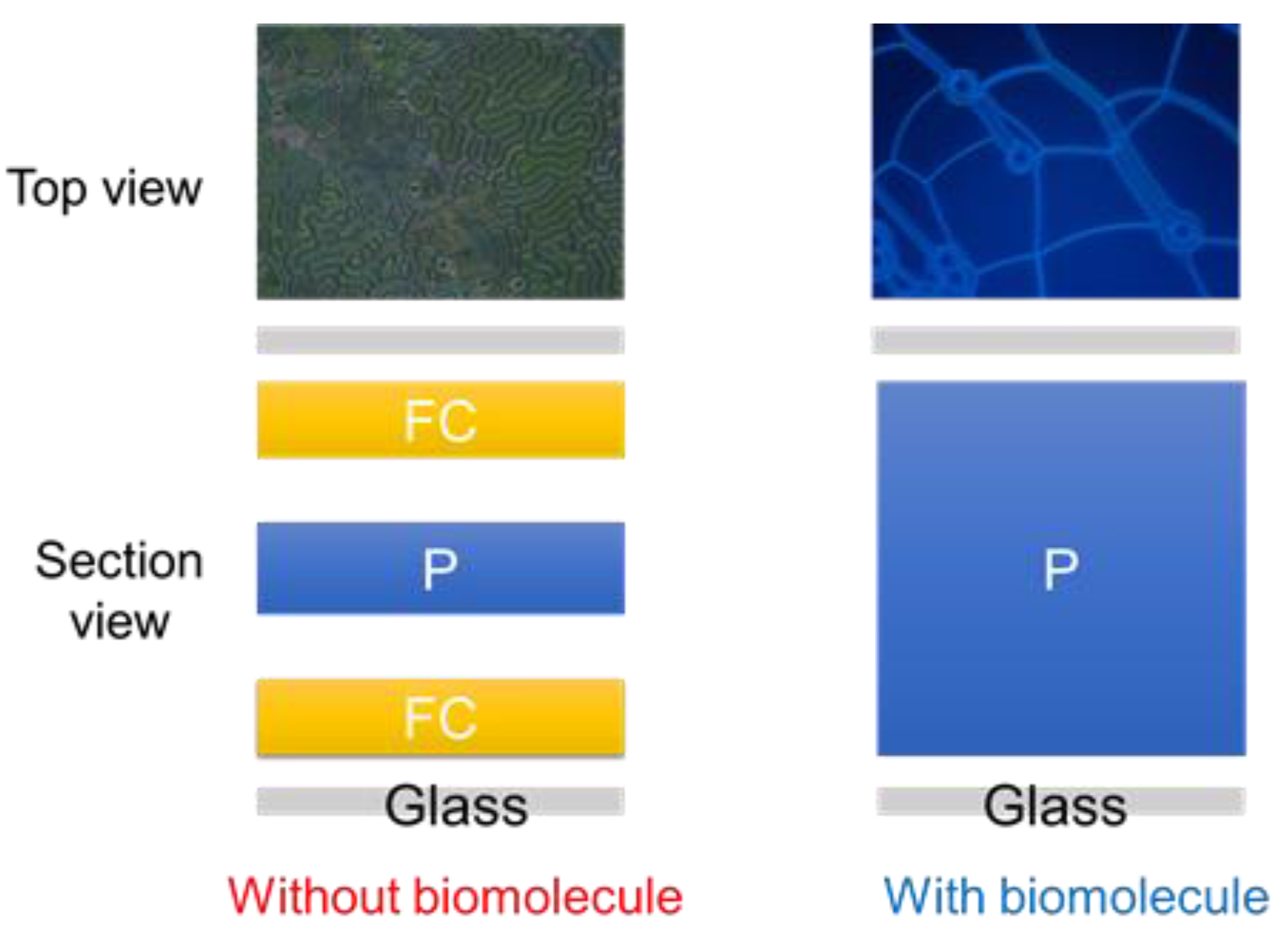
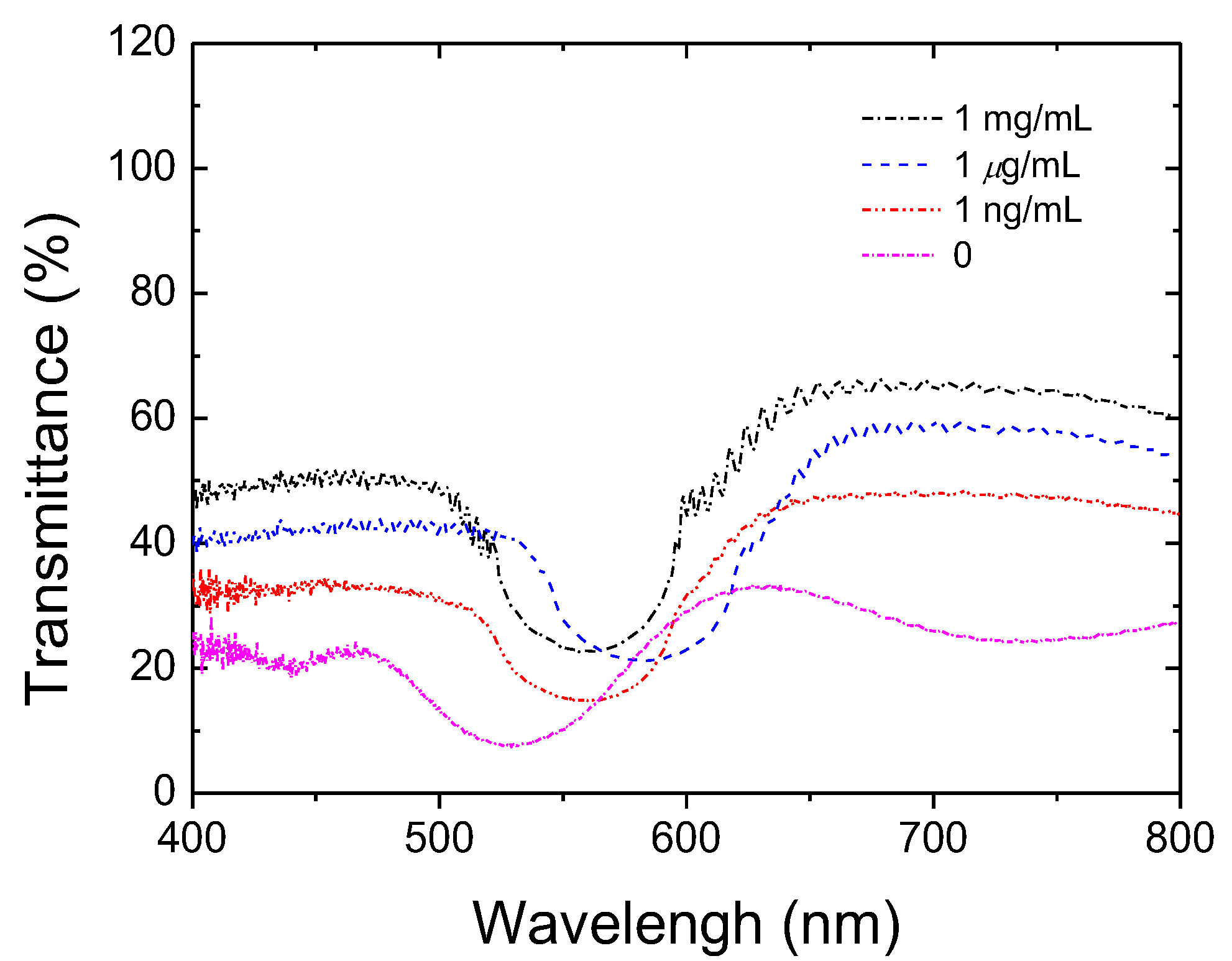
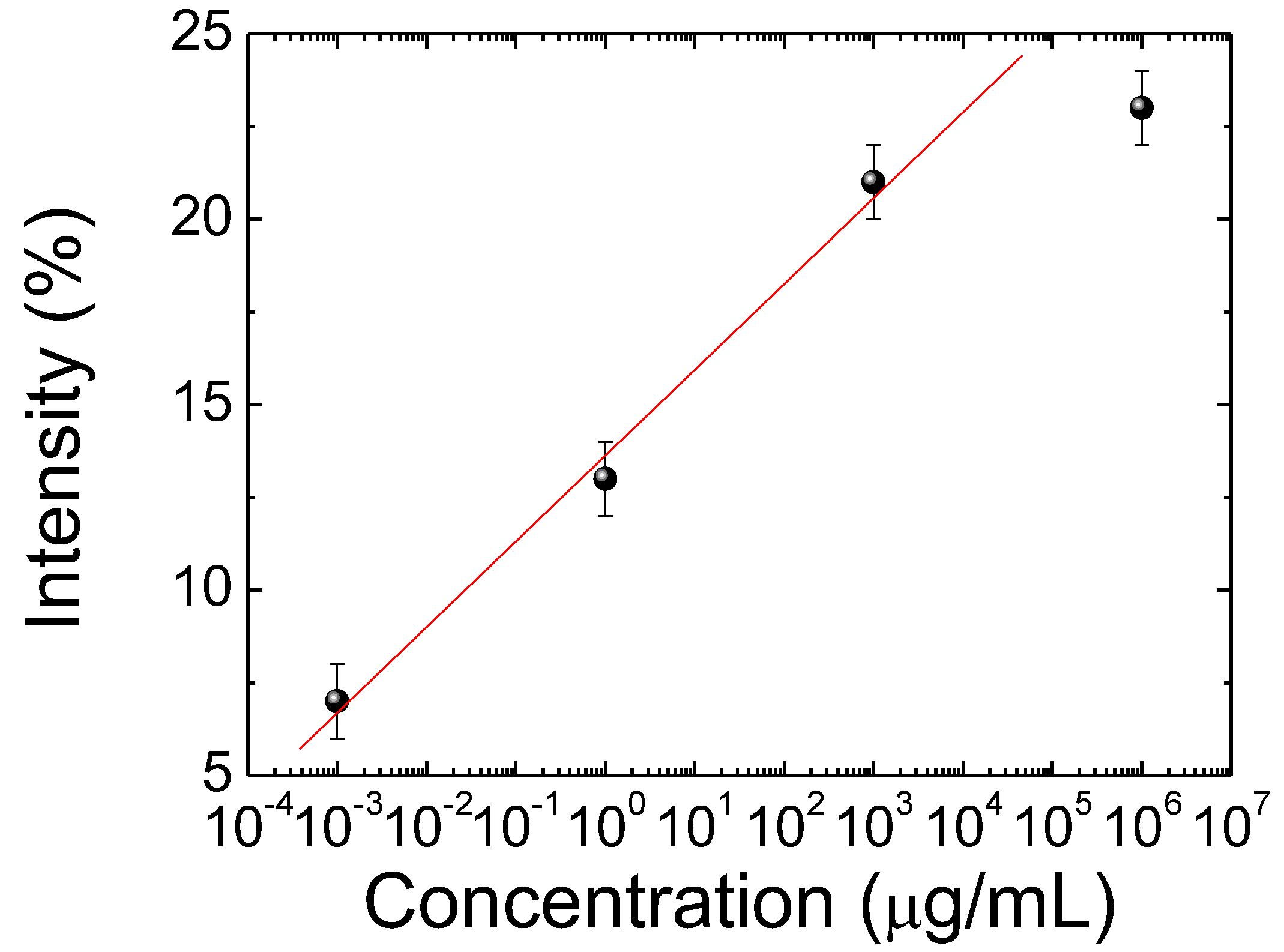
3.1. Detecting HO-1 by the CLC Biosensor
3.2. Clinical Study
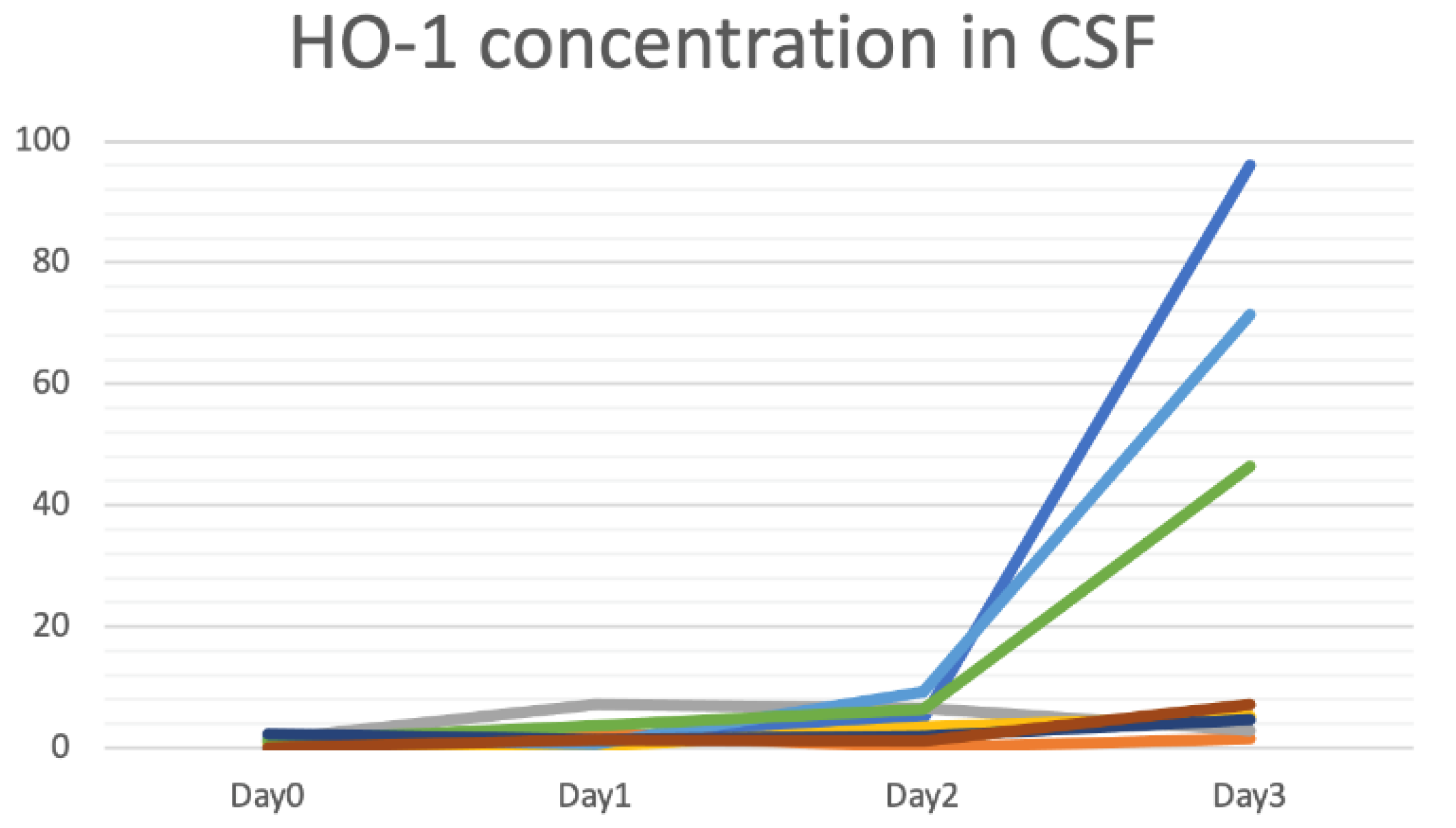
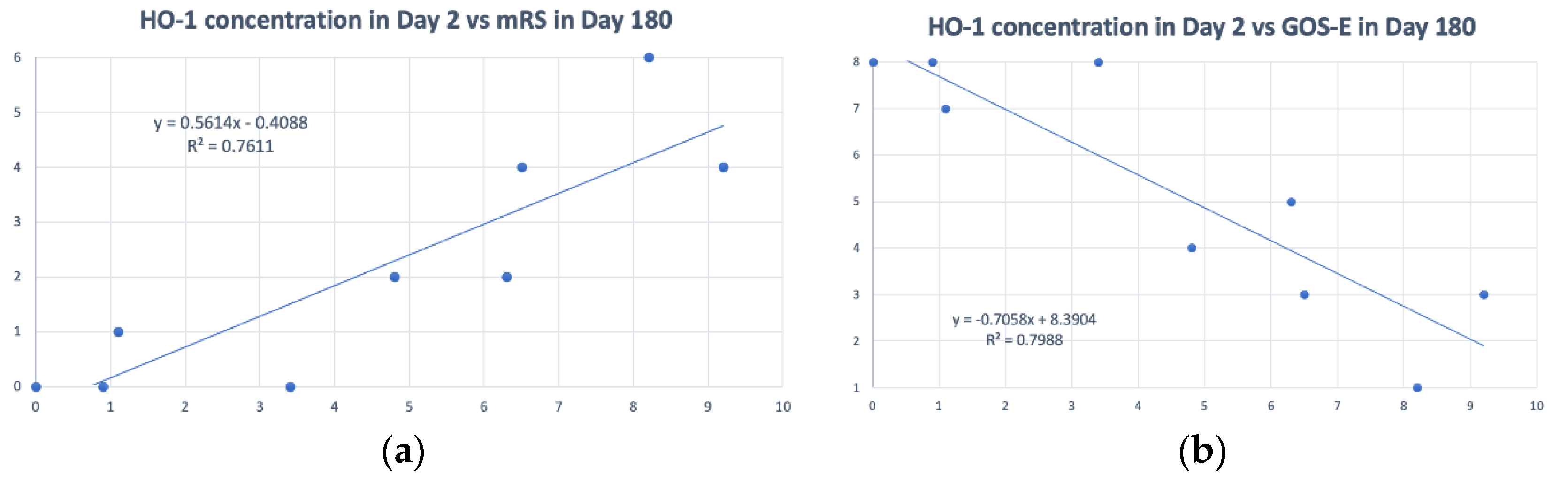
3.3. Detecting HO-1 by the CLC Biosensors from CSF
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Brisman, J.L.; Song, J.K.; Newell, D.W. Cerebral aneurysms. N. Engl. J. Med. 2006, 355, 928–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niskanen, M.M.; Hernesniemi, J.A.; Vapalahti, M.P.; Kari, A. One-year outcome in early aneurysm surgery: Prediction of outcome. Acta Neurochir. 1993, 123, 25–32. [Google Scholar] [CrossRef]
- Rosengart, A.J.; Schultheiss, K.E.; Tolentino, J.; Macdonald, R.L. Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke 2007, 38, 2315–2321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berlin, N.I.; Berk, P.D. Quantitative aspects of bilirubin metabolism for hematologists. Blood 1981, 57, 983–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Everse, J.; Hsia, N. The toxicities of native and modified hemoglobins. Free Radic. Biol. Med. 1997, 22, 1075–1099. [Google Scholar] [CrossRef]
- Macdonald, R.L.; Weir, B.K. A review of hemoglobin and the pathogenesis of cerebral vasospasm. Stroke 1991, 22, 971–982. [Google Scholar] [CrossRef] [Green Version]
- Otterbein, L.E.; Choi, A.M. Heme oxygenase: Colors of defense against cellular stress. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 279, L1029–L1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryter, S.W.; Tyrrell, R.M. The heme synthesis and degradation pathways: Role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic. Biol. Med. 2000, 28, 289–309. [Google Scholar] [CrossRef]
- Clark, J.F.; Sharp, F.R. Bilirubin oxidation products (BOXes) and their role in cerebral vasospasm after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2006, 26, 1223–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.Y.; Keep, R.F.; He, Y.; Sagher, O.; Hua, Y.; Xi, G. Hemoglobin and iron handling in brain after subarachnoid hemorrhage and the effect of deferoxamine on early brain injury. J. Cereb. Blood Flow Metab. 2010, 30, 1793–1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, S.; Zhang, Z.D.; Marton, L.S.; Yamini, B.; Windmeyer, E.; Johns, L.; Kowalczuk, A.; Lin, G.; Macdonald, R.L. Heme oxygenase-1 and ferritin are increased in cerebral arteries after subarachnoid hemorrhage in monkeys. J. Cereb. Blood Flow Metab. 2000, 20, 1066–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, H.; Kanamaru, K.; Tsunoda, H.; Inada, H.; Kuroki, M.; Sun, H.; Waga, S.; Tanaka, T. The functional significance of heme oxygenase-1 gene induction in a rat vasospasm model. Acta Neurochir. Suppl. 2001, 77, 89–91. [Google Scholar] [CrossRef]
- Suzuki, H.; Kanamaru, K.; Tsunoda, H.; Inada, H.; Kuroki, M.; Sun, H.; Waga, S.; Tanaka, T. Heme oxygenase-1 gene induction as an intrinsic regulation against delayed cerebral vasospasm in rats. J. Clin. Investig. 1999, 104, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Petzold, A.; Worthington, V.; Appleby, I.; Kerr, M.E.; Kitchen, N.; Smith, M. Cerebrospinal fluid ferritin level, a sensitive diagnostic test in late-presenting subarachnoid hemorrhage. J. Stroke Cerebrovasc. Dis. 2011, 20, 489–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petzold, A.; Worthington, V.; Pritchard, C.; Appleby, I.; Kitchen, N.; Smith, M. The longitudinal profile of bilirubin and ferritin in the cerebrospinal fluid following a subarachnoid hemorrhage: Diagnostic implications. Neurocrit. Care 2009, 11, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Pyne-Geithman, G.J.; Morgan, C.J.; Wagner, K.; Dulaney, E.M.; Carrozzella, J.; Kanter, D.S.; Zuccarello, M.; Clark, J.F. Bilirubin production and oxidation in CSF of patients with cerebral vasospasm after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2005, 25, 1070–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, H.; Muramatsu, M.; Kojima, T.; Taki, W. Intracranial heme metabolism and cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke 2003, 34, 2796–2800. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, H.; Muramatsu, M.; Tanaka, K.; Fujiwara, H.; Kojima, T.; Taki, W. Cerebrospinal fluid ferritin in chronic hydrocephalus after aneurysmal subarachnoid hemorrhage. J. Neurol. 2006, 253, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.C.; Tang, S.C.; Lee, J.E.; Lai, D.M.; Huang, S.J.; Hsieh, S.T.; Jeng, J.S.; Tu, Y.K. Prognostic value of intrathecal heme oxygenase-1 concentration in patients with Fisher Grade III aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2014, 121, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, E.F.; Higashida, R.; Hmissi, A.; Le, E.J.; Macdonald, R.L.; Marr, A.; Mayer, S.A.; Roux, S.; Bruder, N. Thick and diffuse cisternal clot independently predicts vasospasm-related morbidity and poor outcome after aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2020, 134, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, N.M.; Elkarim, G.A.; Samuel, N.; Ayling, O.G.S.; Guha, D.; Fallah, A.; Aldakkan, A.; Jaja, B.N.R.; de Oliveira Manoel, A.L.; Ibrahim, G.M.; et al. Effects of decompressive craniectomy on functional outcomes and death in poor-grade aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis. J. Neurosurg. 2017, 127, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Aliño, V.J.; Yang, K.L. Using liquid crystals as a readout system in urinary albumin assays. Analyst 2011, 136, 3307–3313. [Google Scholar] [CrossRef]
- Chen, C.H.; Yang, K.L. Liquid crystal-based immunoassays for detecting hepatitis B antibody. Anal. Biochem. 2012, 421, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Abbott, N.L. Rubbed Films of Functionalized Bovine Serum Albumin as Substrates for the Imaging of Protein-Receptor Interactions Using Liquid Crystals. Adv. Mater. 2001, 13, 1445–1449. [Google Scholar] [CrossRef]
- Clare, B.H.; Abbott, N.L. Orientations of nematic liquid crystals on surfaces presenting controlled densities of peptides: Amplification of protein-peptide binding events. Langmuir 2005, 21, 6451–6461. [Google Scholar] [CrossRef] [PubMed]
- Matz, P.G.; Massa, S.M.; Weinstein, P.R.; Turner, C.; Panter, S.S.; Sharp, F.R. Focal hyperexpression of hemeoxygenase-1 protein and messenger RNA in rat brain caused by cellular stress following subarachnoid injections of lysed blood. J. Neurosurg. 1996, 85, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.C.; Tang, C.Y.; Lee, W. Fast-switching bistable cholesteric intensity modulator. Opt. Express 2011, 19, 9744–29749. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.C.; Wu, C.Y.; Chen, C.H.; Zyryanov, V.Y.; Lee, W. Electro-optical device based on photonic structure with a dual-frequency cholesteric liquid crystal. Opt. Lett. 2011, 36, 2632–2634. [Google Scholar] [CrossRef]
- Sun, S.-H.; Lee, M.-J.; Lee, Y.-H.; Lee, W.; Song, X.; Chen, C.-Y. Immunoassays for the cancer biomarker CA125 based on a large-birefringence nematic liquid-crystal mixture. Biomed. Opt. Express 2015, 6, 245–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsiao, Y.C.; Sung, Y.C.; Lee, M.J.; Lee, W. Highly sensitive color-indicating and quantitative biosensor based on cholesteric liquid crystal. Biomed. Opt. Express 2015, 6, 5033–5038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, H.-W.; Lee, Y.-H.; Lee, M.-J.; Hsu, Y.-C.; Lee, W. Label-free immunodetection of the cancer biomarker CA125 using high-Δn liquid crystals. J. Biomed. Opt. 2014, 19, 077006. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luh, H.-T.; Chung, Y.-W.; Cho, P.-Y.; Hsiao, Y.-C. Label-Free Cholesteric Liquid Crystal Biosensing Chips for Heme Oxygenase-1 Detection within Cerebrospinal Fluid as an Effective Outcome Indicator for Spontaneous Subarachnoid Hemorrhage. Biosensors 2022, 12, 204. https://doi.org/10.3390/bios12040204
Luh H-T, Chung Y-W, Cho P-Y, Hsiao Y-C. Label-Free Cholesteric Liquid Crystal Biosensing Chips for Heme Oxygenase-1 Detection within Cerebrospinal Fluid as an Effective Outcome Indicator for Spontaneous Subarachnoid Hemorrhage. Biosensors. 2022; 12(4):204. https://doi.org/10.3390/bios12040204
Chicago/Turabian StyleLuh, Hui-Tzung, Yi-Wei Chung, Po-Yi Cho, and Yu-Cheng Hsiao. 2022. "Label-Free Cholesteric Liquid Crystal Biosensing Chips for Heme Oxygenase-1 Detection within Cerebrospinal Fluid as an Effective Outcome Indicator for Spontaneous Subarachnoid Hemorrhage" Biosensors 12, no. 4: 204. https://doi.org/10.3390/bios12040204




