MXene-Based Nucleic Acid Biosensors for Agricultural and Food Systems
Abstract
:1. Introduction
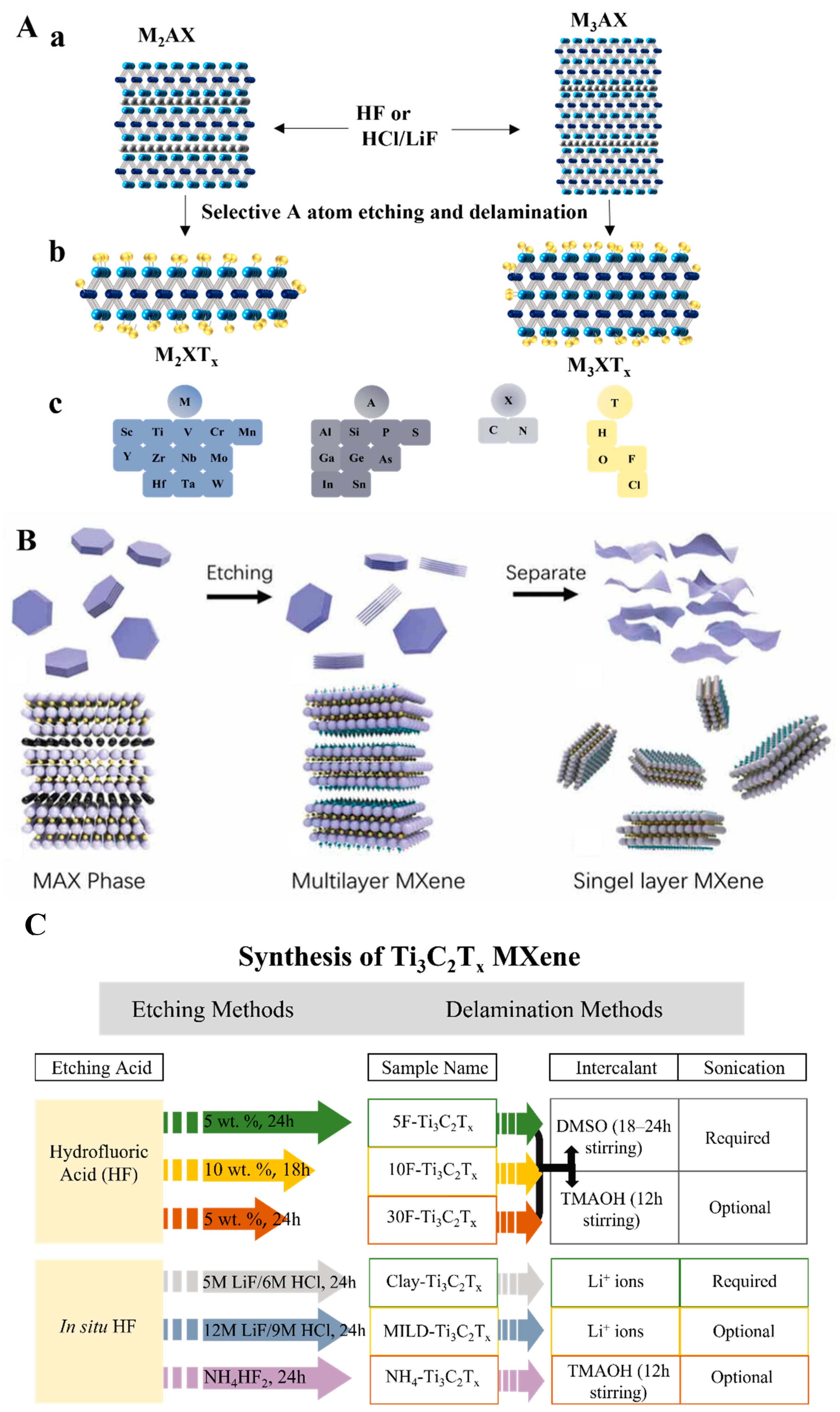
2. MXene Synthesis and Characterization
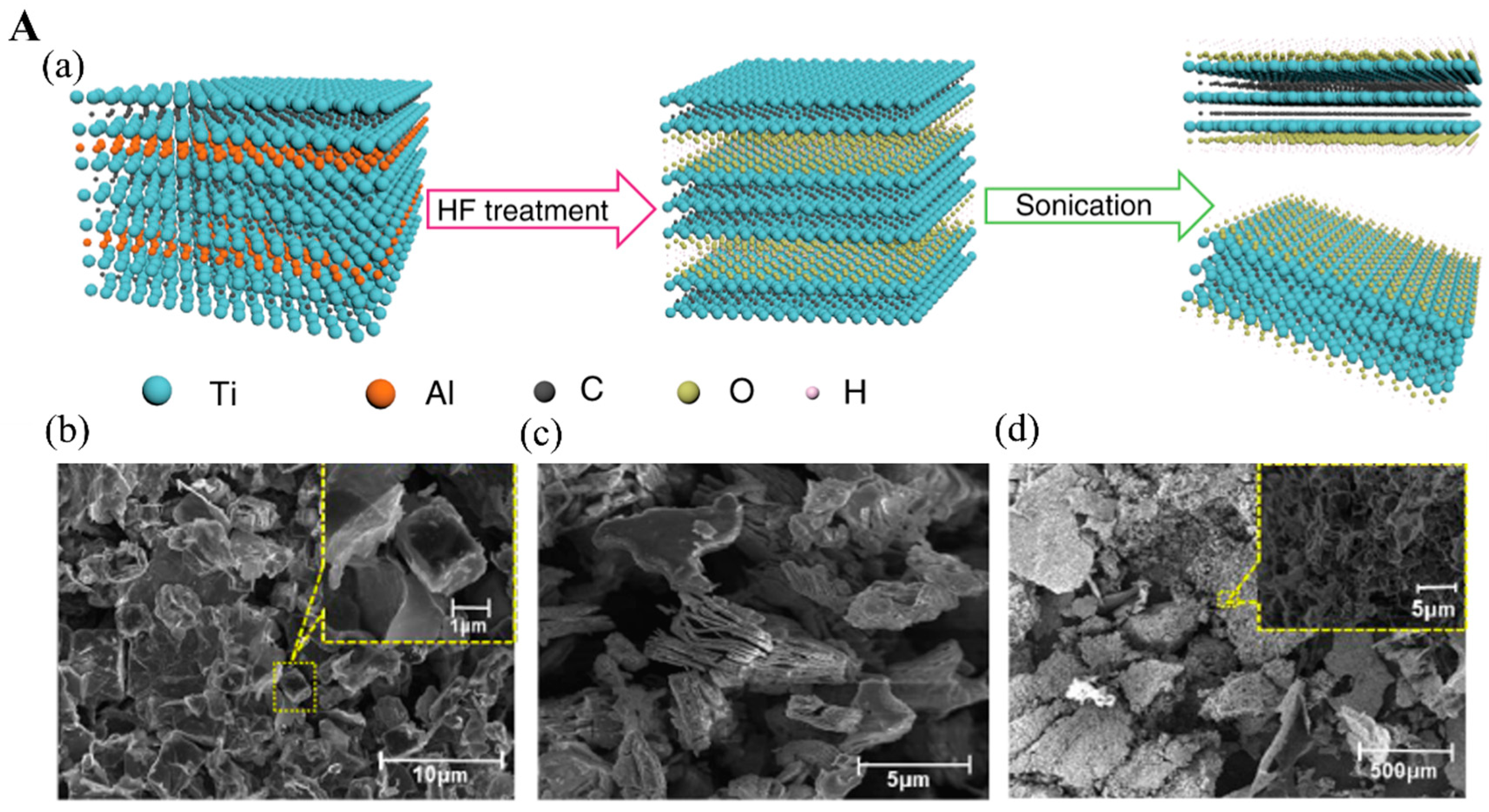
2.1. Top-Down Etching of MAX Phase Precursors
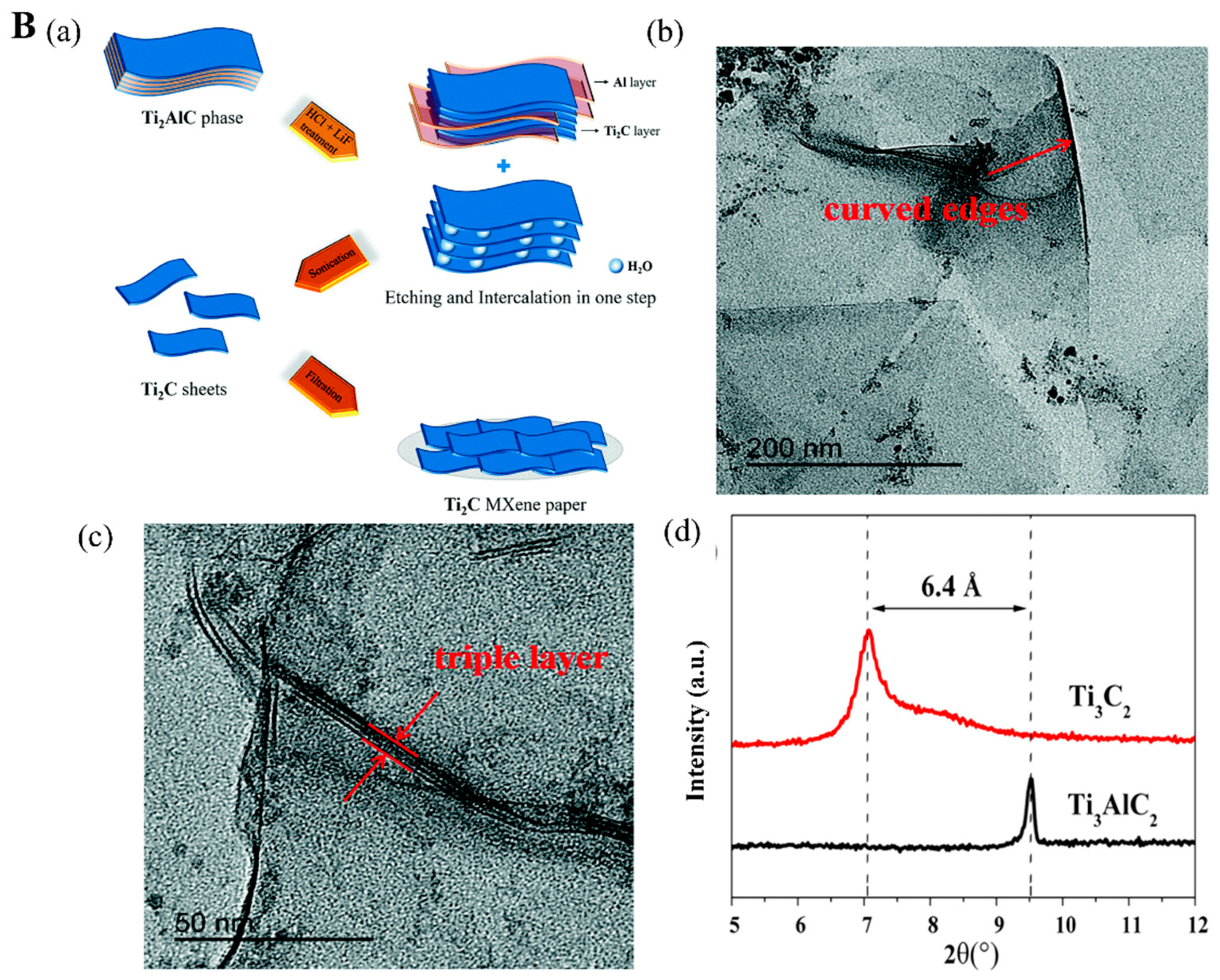
2.1.1. HF Etching
2.1.2. In Situ HF Etching
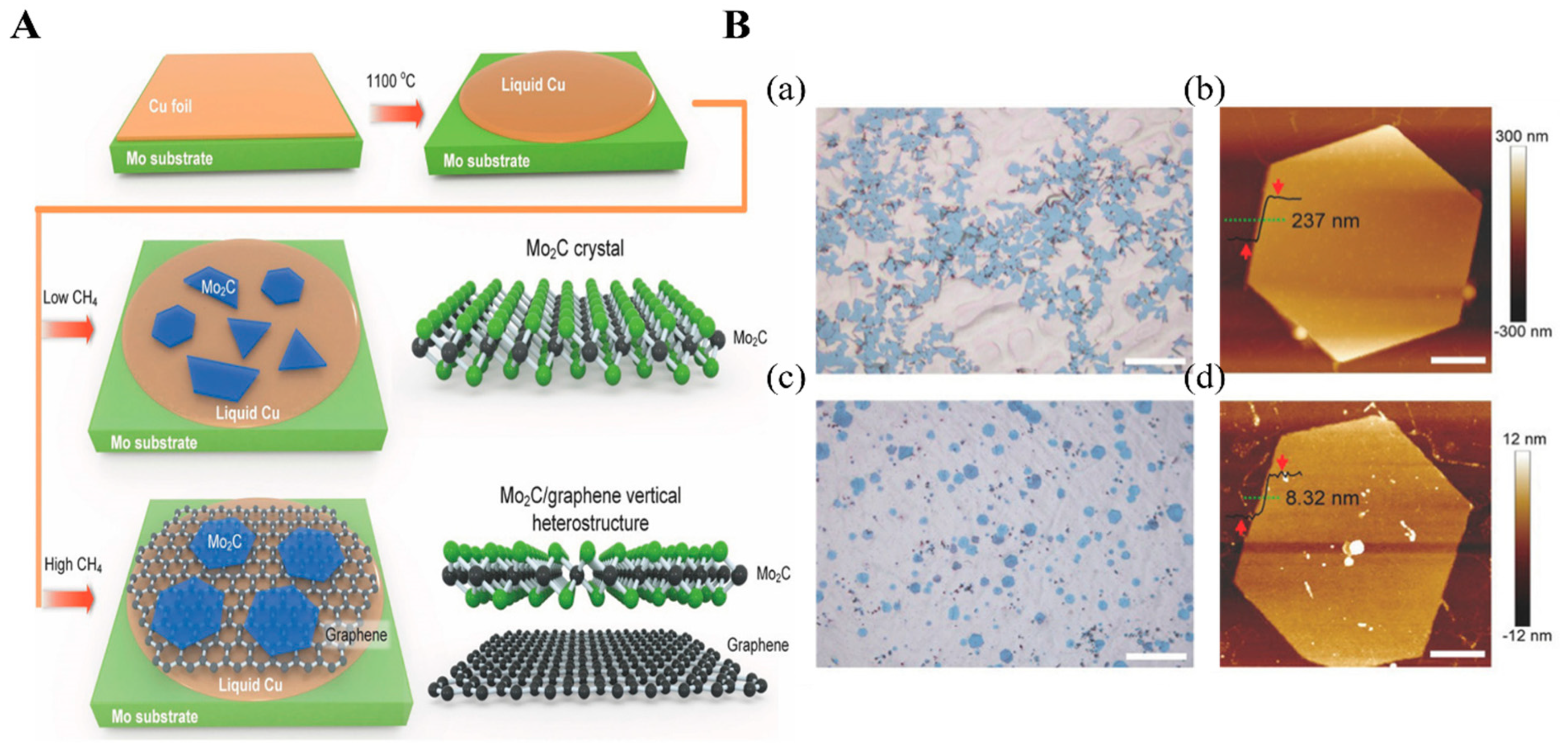
2.2. Bottom-up Synthesis of MXene
3. MXene in NA Biosensors
3.1. NA-Based Biosensor
3.1.1. Genosensor
3.1.2. Aptasensor
3.1.3. NA Enzyme (NAzyme) Biosensor
3.2. Role of MXene in NA-Based Biosensors
4. Application of MXene-Based NA Biosensors in the Agricultural Food System
4.1. Pathogen Detection
4.2. Mycotoxins Detection
4.3. Antibiotics Detection
4.4. Other Targets
5. Summary and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, W.; You, Y.; Gunasekaran, S. LSPR-based colorimetric biosensing for food quality and safety. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5829–5855. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, L.; Wichers, J.; Xu, M.; Van Hoof, R.; Van Dooremalen, C.; Van Amerongen, A.; Peters, J. Biosensing Chlorpyrifos in Environmental Water Samples by a Newly Developed Carbon Nanoparticle-Based Indirect Lateral Flow Assay. Biosensors 2022, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Lubin, A.A.; Plaxco, K.W. Folding-based electrochemical biosensors: The case for responsive nucleic acid architectures. Acc. Chem. Res. 2010, 43, 496–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McConnell, E.M.; Cozma, I.; Morrison, D.; Li, Y. Biosensors made of synthetic functional nucleic acids toward better human health. Anal. Chem. 2019, 92, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Manzanares-Palenzuela, C.L.; Martín-Fernández, B.; López, M.S.-P.; López-Ruiz, B. Electrochemical genosensors as innovative tools for detection of genetically modified organisms. TrAC Trends Anal. Chem. 2015, 66, 19–31. [Google Scholar] [CrossRef]
- Tian, C.; Zhao, L.; Zhu, J.; Zhang, S. Ultrasensitive detection of trace Hg2+ by SERS aptasensor based on dual recycling amplification in water environment. J. Hazard. Mater. 2021, 416, 126251. [Google Scholar] [CrossRef]
- Ullah, S.; Shahzad, F.; Qiu, B.; Fang, X.; Ammar, A.; Luo, Z.; Zaidi, S.A. MXene-Based Aptasensors: Advances, Challenges, and Prospects. Prog. Mater. Sci. 2022, 129, 100967. [Google Scholar]
- Zhu, C.; Du, D.; Lin, Y. Graphene-like 2D nanomaterial-based biointerfaces for biosensing applications. Biosens. Bioelectron. 2017, 89, 43–55. [Google Scholar] [CrossRef]
- Kurra, N.; Ahmed, B.; Gogotsi, Y.; Alshareef, H.N. MXene-on-paper coplanar microsupercapacitors. Adv. Energy Mater. 2016, 6, 1601372. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Q.; Huang, H.; Mao, L.; Liu, M.; Zhang, X.; Wei, Y. Recent progress and advances in the environmental applications of MXene related materials. Nanoscale 2020, 12, 3574–3592. [Google Scholar] [CrossRef]
- Hong, W.; Wyatt, B.C.; Nemani, S.K.; Anasori, B. Double transition-metal MXenes: Atomistic design of two-dimensional carbides and nitrides. MRS Bull. 2020, 45, 850–861. [Google Scholar] [CrossRef]
- Kalambate, P.K.; Gadhari, N.S.; Li, X.; Rao, Z.; Navale, S.T.; Shen, Y.; Patil, V.R.; Huang, Y. Recent advances in MXene–based electrochemical sensors and biosensors. TrAC Trends Anal. Chem. 2019, 120, 115643. [Google Scholar] [CrossRef]
- Wang, W.; Yin, Y.; Gunasekaran, S. Oxygen-terminated few-layered Ti3C2Tx MXene nanosheets as peroxidase-mimic nanozyme for colorimetric detection of kanamycin. Biosens. Bioelectron. 2022, 218, 114774. [Google Scholar] [CrossRef] [PubMed]
- Morales-García, A.N.; Calle-Vallejo, F.; Illas, F. MXenes: New horizons in catalysis. ACS Catal. 2020, 10, 13487–13503. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, D.; Yuan, Y.; Wang, Y.; Cheng, Z.; Liu, Y.; Xie, Z. A lightweight and conductive MXene/graphene hybrid foam for superior electromagnetic interference shielding. Chem. Eng. J. 2020, 381, 122696. [Google Scholar] [CrossRef]
- Yoon, J.; Shin, M.; Lim, J.; Lee, J.-Y.; Choi, J.-W. Recent advances in MXene nanocomposite-based biosensors. Biosensors 2020, 10, 185. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, P.; Soomro, R.A.; Zhu, Q.; Xu, B. Advances in the synthesis of 2D MXenes. Adv. Mater. 2021, 33, 2103148. [Google Scholar] [CrossRef]
- Chen, N.; Yang, W.; Zhang, C. Perspectives on preparation of two-dimensional MXenes. Sci. Technol. Adv. Mater. 2021, 22, 917–930. [Google Scholar] [CrossRef]
- Ronchi, R.M.; Arantes, J.T.; Santos, S.F. Synthesis, structure, properties and applications of MXenes: Current status and perspectives. Ceram. Int. 2019, 45, 18167–18188. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, D.; Yang, J.; Zhou, S.; Wang, H.; Yuan, X.; Liang, J.; Li, X.; Chen, Y.; Li, H. 2D Single-and Few-Layered MXene: Synthesis, applications and perspectives. J. Mater. Chem. A 2022, 10, 13651–13672. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for synthesis and processing of two-dimensional titanium carbide (Ti3C2T x MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, B.; Zou, X.; Cheng, H.-M. Chemical vapor deposition growth and applications of two-dimensional materials and their heterostructures. Chem. Rev. 2018, 118, 6091–6133. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional transition metal carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, L.; Milligan, C.; Ma, T.; Zhou, L.; Cui, Y.; Qi, Z.; Libretto, N.; Xu, B.; Luo, J. Two-dimensional transition metal carbides as supports for tuning the chemistry of catalytic nanoparticles. Nat. Commun. 2018, 9, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Wang, Z.; Wang, J.; Yang, Y.; Wu, S.; You, C.; Tian, N.; Li, Y. Structural Evolution of MXenes and Their Composites for Electromagnetic Interference Shielding Applications. Nanoscale 2022, 14, 9218–9247. [Google Scholar] [CrossRef]
- Zhou, J.; Zha, X.; Zhou, X.; Chen, F.; Gao, G.; Wang, S.; Shen, C.; Chen, T.; Zhi, C.; Eklund, P. Synthesis and electrochemical properties of two-dimensional hafnium carbide. ACS Nano 2017, 11, 3841–3850. [Google Scholar] [CrossRef] [Green Version]
- Boota, M.; Pasini, M.; Galeotti, F.; Porzio, W.; Zhao, M.-Q.; Halim, J.; Gogotsi, Y. Interaction of polar and nonpolar polyfluorenes with layers of two-dimensional titanium carbide (MXene): Intercalation and pseudocapacitance. Chem. Mater. 2017, 29, 2731–2738. [Google Scholar] [CrossRef]
- Vaghasiya, J.V.; Mayorga-Martinez, C.C.; Sofer, Z.; Pumera, M. MXene-based flexible supercapacitors: Influence of an organic ionic conductor electrolyte on the performance. ACS Appl. Mater. Interfaces 2020, 12, 53039–53048. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Feng, M.; Xia, J.; Zhang, F.; Wang, Z. An electrochemical biosensor based on AuNPs/Ti3C2 MXene three-dimensional nanocomposite for microRNA-155 detection by exonuclease III-aided cascade target recycling. J. Electroanal. Chem. 2020, 878, 114669. [Google Scholar] [CrossRef]
- Li, L.; Wang, F.; Zhu, J.; Wu, W. The facile synthesis of layered Ti 2 C MXene/carbon nanotube composite paper with enhanced electrochemical properties. Dalton Trans. 2017, 46, 14880–14887. [Google Scholar] [CrossRef]
- Feng, A.; Yu, Y.; Jiang, F.; Wang, Y.; Mi, L.; Yu, Y.; Song, L. Fabrication and thermal stability of NH4HF2-etched Ti3C2 MXene. Ceram. Int. 2017, 43, 6322–6328. [Google Scholar] [CrossRef]
- Ihsanullah, I. MXenes as next-generation materials for the photocatalytic degradation of pharmaceuticals in water. J. Environ. Chem. Eng. 2022, 10, 107381. [Google Scholar] [CrossRef]
- Zhu, X.; Cao, Z.; Li, X.-L.; Pei, L.; Jones, J.; Zhou, Y.-N.; Dong, P.; Wang, L.; Ye, M.; Shen, J. Ion-intercalation regulation of MXene-derived hydrated vanadates for high-rate and long-life Zn-Ion batteries. Energy Storage Mater. 2022, 45, 568–577. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Kim, S.J.; Seo, D.; Chae, Y.; Anayee, M.; Lee, Y.; Gogotsi, Y.; Ahn, C.W.; Jung, H.-T. Etching mechanism of monoatomic aluminum layers during MXene synthesis. Chem. Mater. 2021, 33, 6346–6355. [Google Scholar] [CrossRef]
- Shekhirev, M.; Shuck, C.E.; Sarycheva, A.; Gogotsi, Y. Characterization of MXenes at every step, from their precursors to single flakes and assembled films. Prog. Mater. Sci. 2021, 120, 100757. [Google Scholar] [CrossRef]
- Pinto, D.; Anasori, B.; Avireddy, H.; Shuck, C.E.; Hantanasirisakul, K.; Deysher, G.; Morante, J.R.; Porzio, W.; Alshareef, H.N.; Gogotsi, Y. Synthesis and electrochemical properties of 2D molybdenum vanadium carbides–solid solution MXenes. J. Mater. Chem. A 2020, 8, 8957–8968. [Google Scholar] [CrossRef]
- Lipatov, A.; Alhabeb, M.; Lukatskaya, M.R.; Boson, A.; Gogotsi, Y.; Sinitskii, A. Effect of synthesis on quality, electronic properties and environmental stability of individual monolayer Ti3C2 MXene flakes. Adv. Electron. Mater. 2016, 2, 1600255. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Zhu, M.; Zhang, W.; Zhen, X.; Pei, Z.; Xue, Q.; Zhi, C.; Shi, P. Ultrathin MXene-micropattern-based field-effect transistor for probing neural activity. Adv. Mater. 2016, 28, 3333–3339. [Google Scholar] [CrossRef]
- Zhai, X.; Xu, X.; Peng, J.; Jing, F.; Zhang, Q.; Liu, H.; Hu, Z. Enhanced optoelectronic performance of CVD-grown metal–semiconductor NiTe2/MoS2 heterostructures. ACS Appl. Mater. Interfaces 2020, 12, 24093–24101. [Google Scholar] [CrossRef]
- Jiang, Y.; Leyden, M.R.; Qiu, L.; Wang, S.; Ono, L.K.; Wu, Z.; Juarez-Perez, E.J.; Qi, Y. Combination of Hybrid CVD and Cation Exchange for Upscaling Cs-Substituted Mixed Cation Perovskite Solar Cells with High Efficiency and Stability. Adv. Funct. Mater. 2018, 28, 1703835. [Google Scholar] [CrossRef]
- Sonawane, S.L.; Labhane, P.K.; Sonawane, G.H. Carbon-based nanocomposite membranes for water purification. In Handbook of Nanomaterials for Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2021; pp. 555–574. [Google Scholar]
- Geng, D.; Zhao, X.; Chen, Z.; Sun, W.; Fu, W.; Chen, J.; Liu, W.; Zhou, W.; Loh, K.P. Direct synthesis of large-area 2D Mo2C on in situ grown graphene. Adv. Mater. 2017, 29, 1700072. [Google Scholar] [CrossRef] [PubMed]
- Umapathi, R.; Park, B.; Sonwal, S.; Rani, G.M.; Cho, Y.; Huh, Y.S. Advances in optical-sensing strategies for the on-site detection of pesticides in agricultural foods. Trends Food Sci. Technol. 2022, 119, 69–89. [Google Scholar] [CrossRef]
- Yen, T.M.; Zhang, T.; Chen, P.-W.; Ku, T.-H.; Chiu, Y.-J.; Lian, I.; Lo, Y.-H. Self-Assembled Pico-Liter Droplet Microarray for Ultrasensitive Nucleic Acid Quantification. ACS Nano 2015, 9, 10655–10663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.C.; Svedlund, J.; Darai, E.; Lee, Y.; Lee, D.; Lee, H.-B.; Kim, S.-M.; Kim, O.; Bae, H.J.; Choi, A. OPENchip: An on-chip in situ molecular profiling platform for gene expression analysis and oncogenic mutation detection in single circulating tumour cells. Lab Chip 2020, 20, 912–922. [Google Scholar] [CrossRef] [PubMed]
- Darmostuk, M.; Rimpelova, S.; Gbelcova, H.; Ruml, T. Current approaches in SELEX: An update to aptamer selection technology. Biotechnol. Adv. 2015, 33, 1141–1161. [Google Scholar] [CrossRef]
- Zhuo, Z.; Yu, Y.; Wang, M.; Li, J.; Zhang, Z.; Liu, J.; Wu, X.; Lu, A.; Zhang, G.; Zhang, B. Recent advances in SELEX technology and aptamer applications in biomedicine. Int. J. Mol. Sci. 2017, 18, 2142. [Google Scholar] [CrossRef] [Green Version]
- Lan, Y.; He, B.; Tan, C.S.; Ming, D. Applications of Smartphone-Based Aptasensor for Diverse Targets Detection. Biosensors 2022, 12, 477. [Google Scholar] [CrossRef]
- Kruger, K.; Grabowski, P.J.; Zaug, A.J.; Sands, J.; Gottschling, D.E.; Cech, T.R. Self-splicing RNA: Autoexcision and autocyclization of the ribosomal RNA intervening sequence of Tetrahymena. Cell 1982, 31, 147–157. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Bahrani, S.; Yousefi, K.; Behbudi, G.; Babapoor, A.; Omidifar, N.; Lai, C.W.; Gholami, A.; Chiang, W.-H. Recent advancements in polythiophene-based materials and their biomedical, geno sensor and DNA detection. Int. J. Mol. Sci. 2021, 22, 6850. [Google Scholar] [CrossRef]
- Singhal, C.; Pundir, C.; Narang, J. A genosensor for detection of consensus DNA sequence of Dengue virus using ZnO/Pt-Pd nanocomposites. Biosens. Bioelectron. 2017, 97, 75–82. [Google Scholar] [CrossRef]
- Ye, Y.; Mao, S.; He, S.; Xu, X.; Cao, X.; Wei, Z.; Gunasekaran, S. Ultrasensitive electrochemical genosensor for detection of CaMV35S gene with Fe3O4-Au@ Ag nanoprobe. Talanta 2020, 206, 120205. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Xu, J.; Yao, B.; Yang, L.; Yao, L.; Liu, G.; Chen, W. Facile design of multifunction-integrated linear oligonucleotide probe with multiplex amplification effect for label-free and highly sensitive GMO biosensing. Talanta 2022, 236, 122821. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.; Li, Y.; Qiao, Z.; Song, W.; Bi, S. Rolling circle replication for biosensing, bioimaging, and biomedicine. Trends Biotechnol. 2021, 39, 1160–1172. [Google Scholar] [CrossRef] [PubMed]
- Abolhasan, R.; Mehdizadeh, A.; Rashidi, M.R.; Aghebati-Maleki, L.; Yousefi, M. Application of hairpin DNA-based biosensors with various signal amplification strategies in clinical diagnosis. Biosens. Bioelectron. 2019, 129, 164–174. [Google Scholar] [CrossRef]
- Chen, C.; Feng, Y.; Xia, X.; La, M.; Zhou, B. Recent Advances in Nanomaterials-Based Electrochemical Biosensors for MicroRNAs Detection. Int. J. Electrochem. Sci. 2019, 14, 5174–5187. [Google Scholar] [CrossRef]
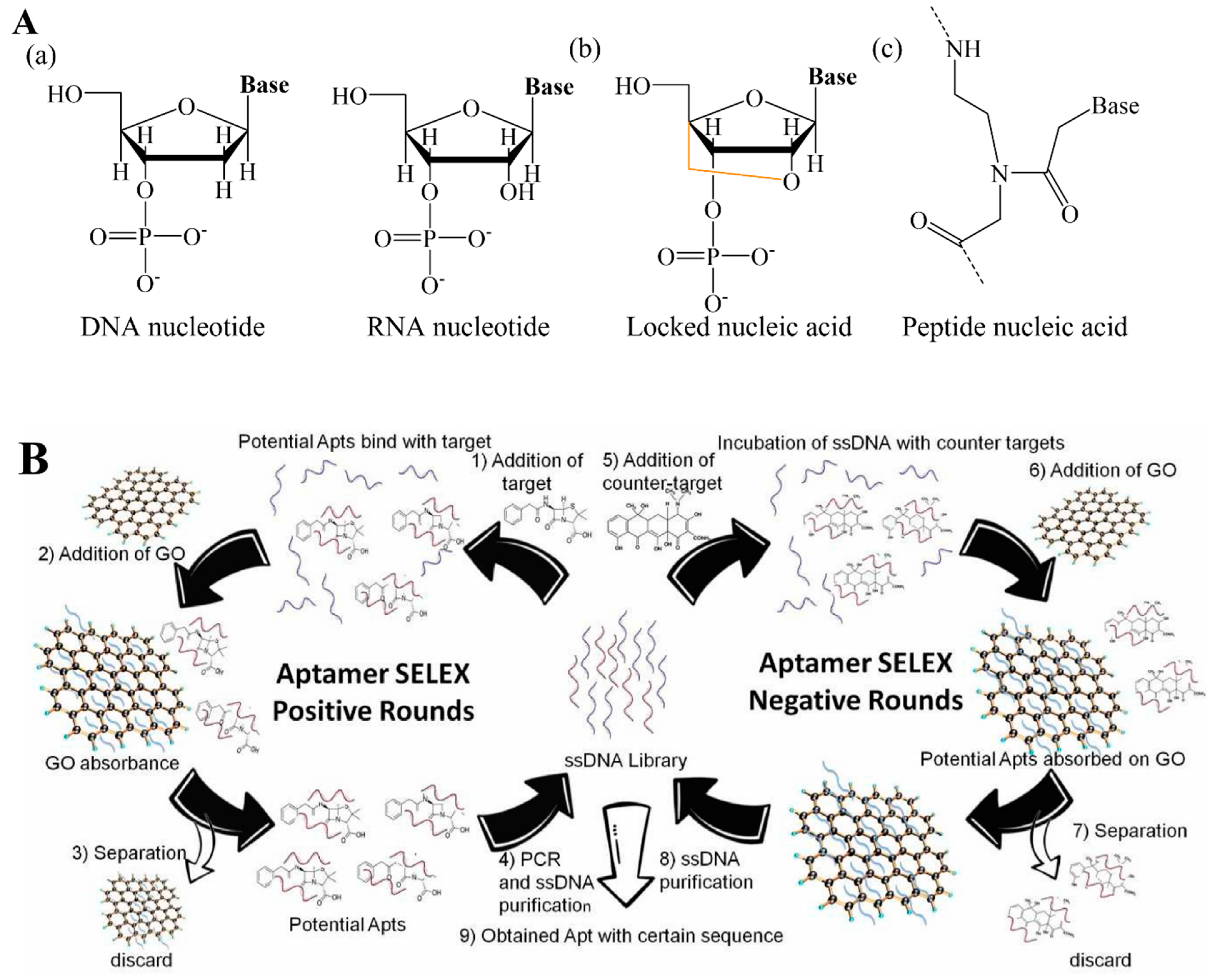
- Wang, F.; Li, P.; Chu, H.C.; Lo, P.K. Nucleic acids and their analogues for biomedical applications. Biosensors 2022, 12, 93. [Google Scholar] [CrossRef]
- Hagedorn, P.H.; Persson, R.; Funder, E.D.; Albæk, N.; Diemer, S.L.; Hansen, D.J.; Møller, M.R.; Papargyri, N.; Christiansen, H.; Hansen, B.R. Locked nucleic acid: Modality, diversity, and drug discovery. Drug Discov. Today 2018, 23, 101–114. [Google Scholar] [CrossRef] [Green Version]
- Siddiquee, S.; Rovina, K.; Azriah, A. A review of peptide nucleic acid. Adv. Tech. Biol. Med. 2015, 3, 1000131. [Google Scholar] [CrossRef]
- Guan, J.; He, K.; Gunasekaran, S. Selection of ssDNA aptamer using GO-SELEX and development of DNA nanostructure-based electrochemical aptasensor for penicillin. Biosens. Bioelectron. X 2022, 12, 100220. [Google Scholar] [CrossRef]
- Umapathi, R.; Ghoreishian, S.M.; Sonwal, S.; Rani, G.M.; Huh, Y.S. Portable electrochemical sensing methodologies for on-site detection of pesticide residues in fruits and vegetables. Coord. Chem. Rev. 2022, 453, 214305. [Google Scholar] [CrossRef]
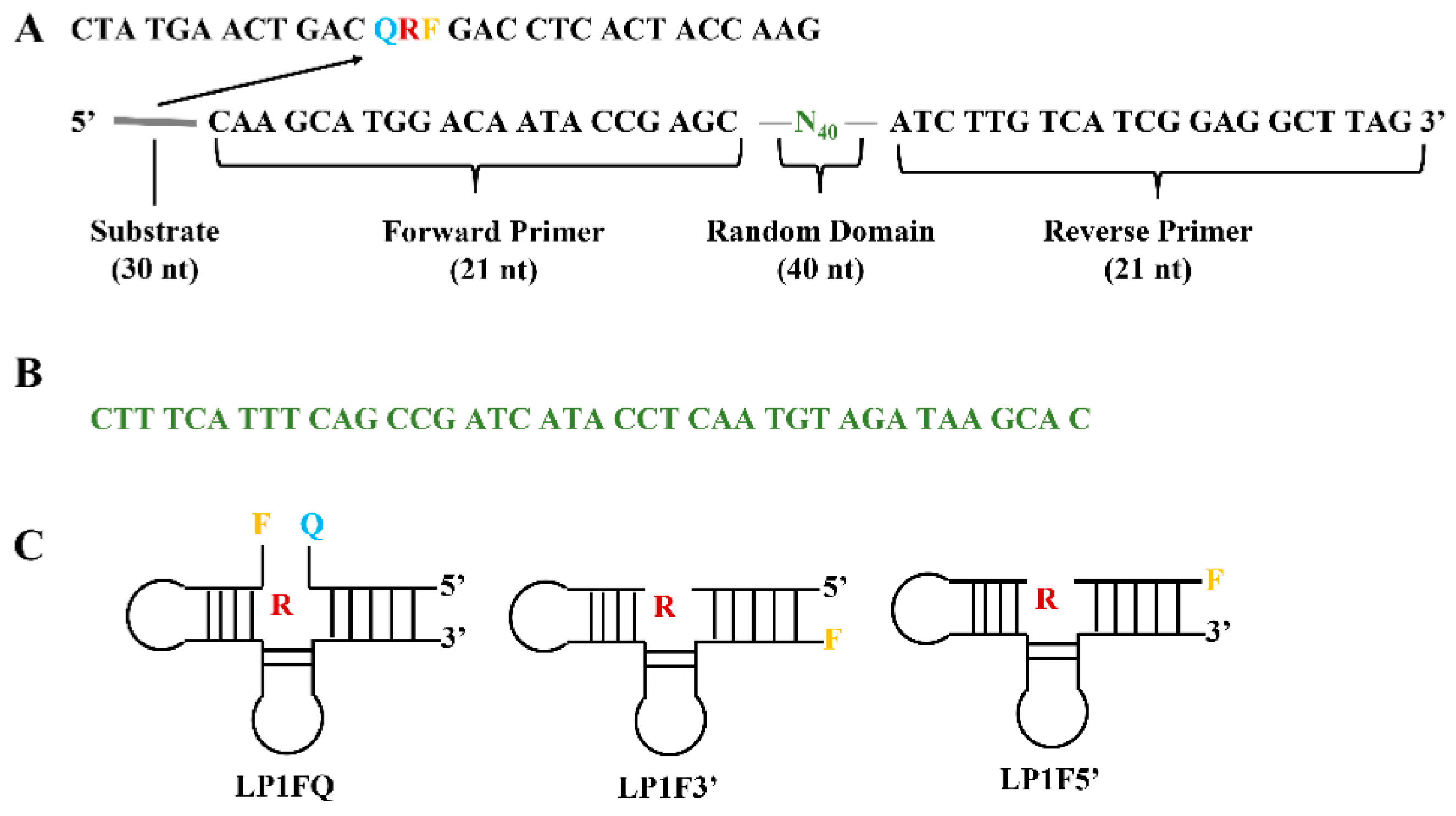
- Zhu, C.; Li, L.; Yang, G.; Qu, F. Investigating the Influences of Random-Region Length on Aptamer Selection Efficiency Based on Capillary Electrophoresis–SELEX and High-Throughput Sequencing. Anal. Chem. 2021, 93, 17030–17035. [Google Scholar] [CrossRef] [PubMed]
- Rosch, J.C.; Neal, E.H.; Balikov, D.A.; Rahim, M.; Lippmann, E.S. CRISPR-mediated isogenic cell-SELEX approach for generating highly specific aptamers against native membrane proteins. Cell. Mol. Bioeng. 2020, 13, 559–574. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; He, K.; Gunasekaran, S. Self-assembled tetrahedral DNA nanostructures-based ultrasensitive label-free detection of ampicillin. Talanta 2022, 243, 123292. [Google Scholar] [CrossRef] [PubMed]
- Cech, T.R. The ribosome is a ribozyme. Science 2000, 289, 878–879. [Google Scholar] [CrossRef]
- Guerrier-Takada, C.; Gardiner, K.; Marsh, T.; Pace, N.; Altman, S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell 1983, 35, 849–857. [Google Scholar] [CrossRef]
- Maurel, M.-C.; Leclerc, F.; Hervé, G. Ribozyme Chemistry: To be or not to be under high pressure. Chem. Rev. 2019, 120, 4898–4918. [Google Scholar] [CrossRef] [Green Version]
- Ishida, S.; Terasaka, N.; Katoh, T.; Suga, H. An aminoacylation ribozyme evolved from a natural tRNA-sensing T-box riboswitch. Nat. Chem. Biol. 2020, 16, 702–709. [Google Scholar] [CrossRef]
- Walton, T.; DasGupta, S.; Duzdevich, D.; Oh, S.S.; Szostak, J.W. In vitro selection of ribozyme ligases that use prebiotically plausible 2-aminoimidazole–activated substrates. Proc. Natl. Acad. Sci. USA 2020, 117, 5741–5748. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Yang, M.; Yang, T.; Chang, Y.; Peng, S.; Xu, Q.; Wang, D.; Zhou, X.; Shao, Y. A catalytic triplex DNAzyme for porphyrin metalation. Chem. Commun. 2021, 57, 6499–6502. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, J.; Yang, X.; Quan, K.; Wang, H.; Ying, L.; Xie, N.; Ou, M.; Wang, K. Aptazyme–gold nanoparticle sensor for amplified molecular probing in living cells. Anal. Chem. 2016, 88, 5981–5987. [Google Scholar] [CrossRef]
- Liu, J.; Cao, Z.; Lu, Y. Functional nucleic acid sensors. Chem. Rev. 2009, 109, 1948–1998. [Google Scholar] [CrossRef] [PubMed]
- McConnell, E.M.; Cozma, I.; Mou, Q.; Brennan, J.D.; Lu, Y.; Li, Y. Biosensing with DNAzymes. Chem. Soc. Rev. 2021, 50, 8954–8994. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Li, G.; Xu, L.; Yu, Q.; Yue, X.; Wu, Y.; Ye, B. Peptide-conjugated hemin/G-quadruplex as a versatile probe for “signal-on” electrochemical peptide biosensor. Talanta 2020, 209, 120611. [Google Scholar] [CrossRef] [PubMed]
- Rothenbroker, M.; McConnell, E.M.; Gu, J.; Urbanus, M.L.; Samani, S.E.; Ensminger, A.W.; Filipe, C.D.; Li, Y. Selection and Characterization of an RNA-Cleaving DNAzyme Activated by Legionella pneumophila. Angew. Chem. Int. Ed. 2021, 60, 4782–4788. [Google Scholar] [CrossRef]
- Chang, D.; Chang, T.; Salena, B.; Li, Y. An Unintentional Discovery of a Fluorogenic DNA Probe for Ribonuclease I. ChemBioChem. 2020, 21, 464–468. [Google Scholar] [CrossRef]
- Mathew, M.; Rout, C.S. Electrochemical biosensors based on Ti3C2Tx MXene: Future perspectives for on-site analysis. Curr. Opin. Electrochem. 2021, 30, 100782. [Google Scholar] [CrossRef]
- Feng, W.; Wang, R.; Zhou, Y.; Ding, L.; Gao, X.; Zhou, B.; Hu, P.; Chen, Y. Ultrathin molybdenum carbide MXene with fast biodegradability for highly efficient theory-oriented photonic tumor hyperthermia. Adv. Funct. Mater. 2019, 29, 1901942. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, J.; Yi, S.; Wan, X.; Tang, J. Biodegradable and photostable Nb2C MXene quantum dots as promising nanofluorophores for metal ions sensing and fluorescence imaging. Sens. Actuators B Chem. 2020, 309, 127735. [Google Scholar] [CrossRef]
- Kashefi-Kheyrabadi, L.; Koyappayil, A.; Kim, T.; Cheon, Y.-P.; Lee, M.-H. A MoS2@Ti3C2Tx MXene hybrid-based electrochemical aptasensor (MEA) for sensitive and rapid detection of Thyroxine. Bioelectrochemistry 2021, 137, 107674. [Google Scholar] [CrossRef]
- Liu, L.; Hong, J.; Wang, W.; Xiao, S.; Xie, H.; Wang, Q.; Gan, N. Fluorescent aptasensor for detection of live foodborne pathogens based on multicolor perovskite-quantum-dot-encoded DNA probes and dual-stirring-bar-assisted signal amplification. J. Pharm. Anal. 2022, in press. [Google Scholar] [CrossRef]
- Wang, W.; Xiao, S.; Jia, Z.; Xie, H.; Li, T.; Wang, Q.; Gan, N. A dual-mode aptasensor for foodborne pathogens detection using Pt, phenylboric acid and ferrocene modified Ti3C2 MXenes nanoprobe. Sens. Actuators B Chem. 2022, 351, 130839. [Google Scholar] [CrossRef]
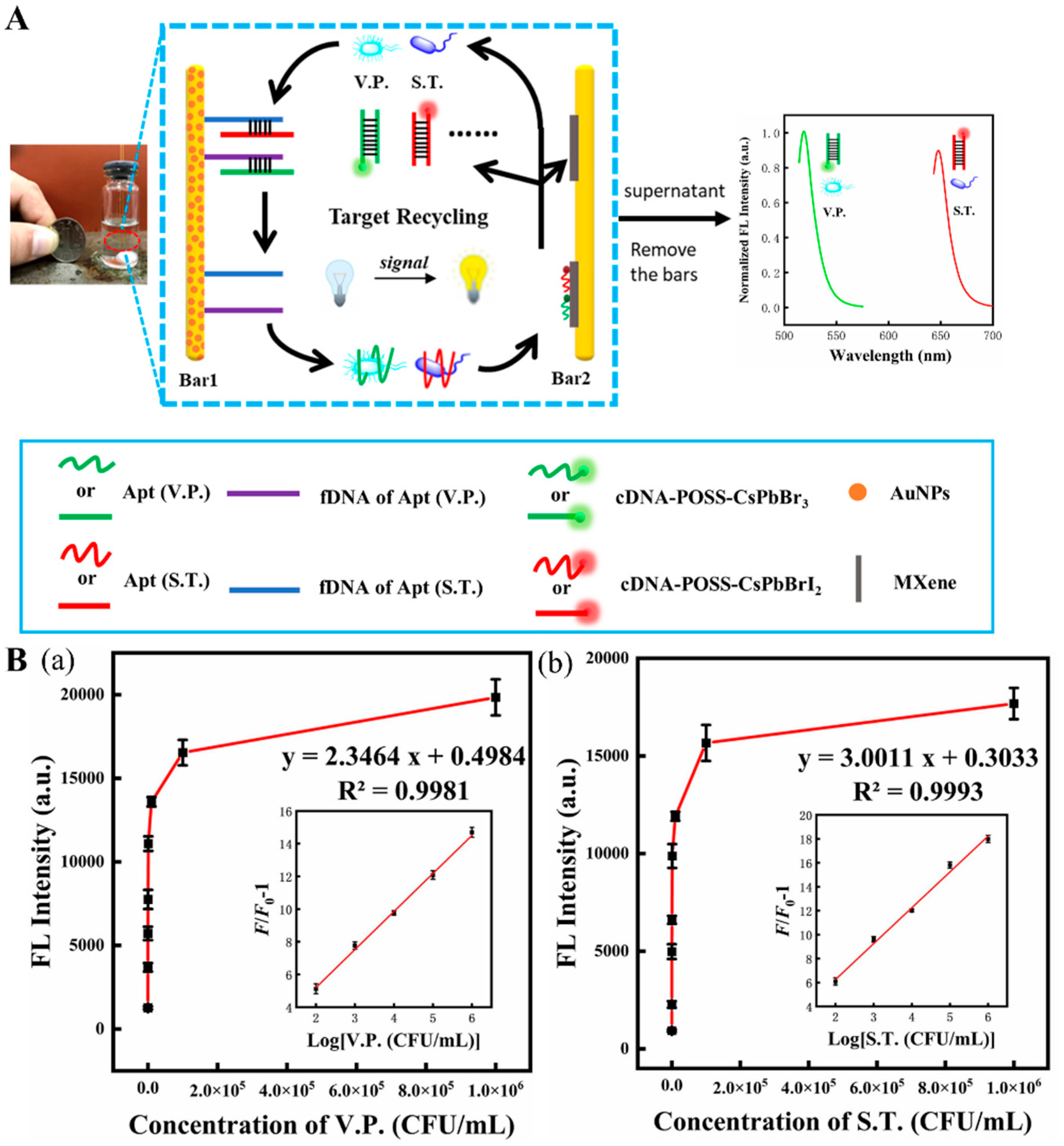
- Hong, J.; Wang, W.; Wang, J.; Wang, X.; Xie, H.; Li, T.; Gan, N. A turn-on–type fluorescence resonance energy transfer aptasensor for vibrio detection using aptamer-modified polyhedral oligomeric silsesquioxane-perovskite quantum dots/Ti3C2 MXenes composite probes. Microchim. Acta 2021, 188, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Duan, S.; He, F. Highly electrically conductive two-dimensional Ti3C2 Mxenes-based 16S rDNA electrochemical sensor for detecting Mycobacterium tuberculosis. Anal. Chim. Acta 2020, 1123, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Rizi, K.S.; Hatamluyi, B.; Darroudi, M.; Meshkat, Z.; Aryan, E.; Soleimanpour, S.; Rezayi, M. PCR-free electrochemical genosensor for Mycobacterium tuberculosis complex detection based on two-dimensional Ti3C2 Mxene-polypyrrole signal amplification. Microchem. J. 2022, 179, 107467. [Google Scholar] [CrossRef]
- Sheng, A.; Wang, P.; Yang, J.; Tang, L.; Chen, F.; Zhang, J. MXene Coupled with CRISPR-Cas12a for analysis of endotoxin and bacteria. Anal. Chem. 2021, 93, 4676–4681. [Google Scholar] [CrossRef]
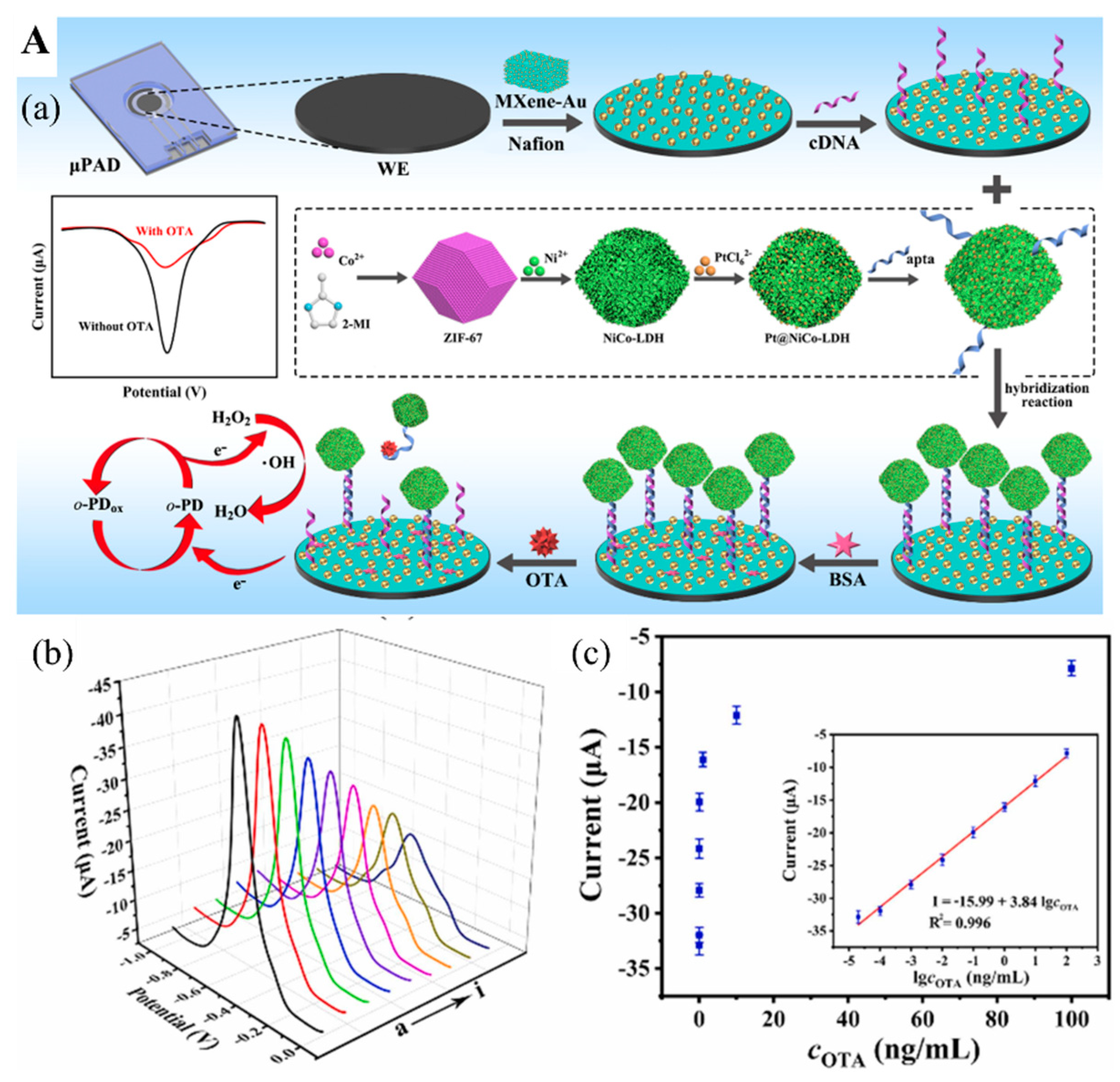
- Zhang, X.; Wang, F.; Zhi, H.; Zhao, J.; Wan, P.; Feng, L. Electrochemical “signal on/off” paper-based aptasensor for ochratoxin A detection based on MXene-Au and Pt@ NiCo-LDH-catalyzed signal amplification. Sens. Actuators B Chem. 2022, 368, 132161. [Google Scholar] [CrossRef]
- Zheng, F.; Ke, W.; Shi, L.; Liu, H.; Zhao, Y. Plasmonic Au–Ag janus nanoparticle engineered ratiometric surface-enhanced raman scattering aptasensor for Ochratoxin A detection. Anal. Chem. 2019, 91, 11812–11820. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, X.; Wang, Z.; Han, J.; Chen, S. Core–shell Au@ PtAg modified TiO2–Ti3C2 heterostructure and target-triggered DNAzyme cascade amplification for photoelectrochemical detection of ochratoxin A. Anal. Chim. Acta 2022, 1216, 339943. [Google Scholar] [CrossRef]
- Li, Y.L.; Xie, F.-T.; Yao, C.; Zhang, G.; Guan, Y.; Yang, Y.; Yang, J.-M.; Hu, R. DNA tetrahedral-based dual-signal ratiometric electrochemical aptasensor for the detection of ochratoxin A in corn kernels samples. Analyst 2022, 147, 4578–4586. [Google Scholar] [CrossRef]
- Al-Dhahebi, A.M.; Jose, R.; Mustapha, M.; Saheed, M.S.M. Ultrasensitive aptasensor using electrospun MXene/polyvinylidene fluoride nanofiber composite for Ochratoxin A detection. Food Chem. 2022, 390, 133105. [Google Scholar] [CrossRef]
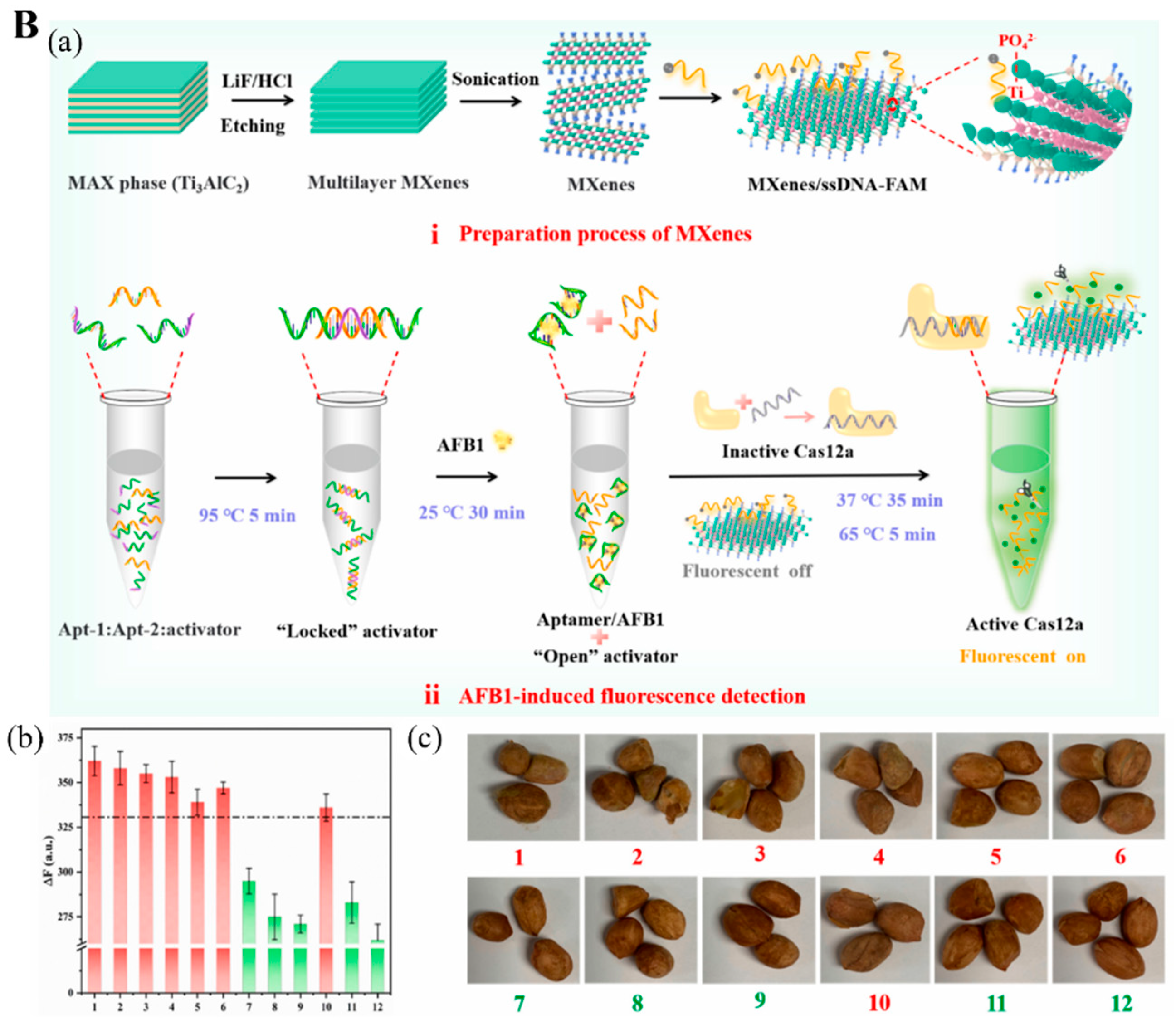
- Wu, Z.; Sun, D.-W.; Pu, H.; Wei, Q. A novel fluorescence biosensor based on CRISPR/Cas12a integrated MXenes for detecting Aflatoxin B1. Talanta 2022, 252, 123773. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Sun, D.-W.; Pu, H.; Wei, Q.; Lin, X. Ti3C2Tx MXenes loaded with Au nanoparticle dimers as a surface-enhanced Raman scattering aptasensor for AFB1 detection. Food Chem. 2022, 372, 131293. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Li, C.; Meng, X.; Yu, W.; Duan, N.; Wang, Z.; Wu, S. CRISPR-Cas12a-mediated luminescence resonance energy transfer aptasensing platform for deoxynivalenol using gold nanoparticle-decorated Ti3C2Tx MXene as the enhanced quencher. J. Hazard. Mater. 2022, 433, 128750. [Google Scholar] [CrossRef] [PubMed]
- Sangu, S.S.; Illias, N.M.; Ong, C.C.; Gopinath, S.C.B.; Saheed, M.S.M. MXene-based aptasensor: Characterization and high-performance voltammetry detection of deoxynivalenol. BioNanoScience 2021, 11, 314–323. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Huang, Y.; Xiong, M.; Wang, F.; Li, C. A label-free electrochemical biosensor for highly sensitive detection of gliotoxin based on DNA nanostructure/MXene nanocomplexes. Biosens. Bioelectron. 2019, 142, 111531. [Google Scholar] [CrossRef]
- Sangu, S.S.; Gopinath, S.C.B.; Shukur, M.F.A.; Saheed, M.S.M. An Electrochemical Approach for Ultrasensitive Detection of Zearalenone in Commodity Using Disposable Screen-Printed Electrode Coated with MXene/Chitosan Film. BioNanoScience 2022, 12, 814–823. [Google Scholar] [CrossRef]
- Fan, L.; Huang, J.J.; Liao, J. Competitive smartphone-based portable electrochemical aptasensor system based on an MXene/cDNA-MB probe for the determination of Microcystin-LR. Sens. Actuators B Chem. 2022, 369, 132164. [Google Scholar] [CrossRef]
- Ullah, N.; Chen, W.; Noureen, B.; Tian, Y.; Du, L.; Wu, C.; Ma, J. An electrochemical Ti3C2Tx aptasensor for sensitive and label-free detection of marine biological toxins. Sensors 2021, 21, 4938. [Google Scholar] [CrossRef]
- Yang, J.; Zhong, W.; Yu, Q.; Zou, J.; Gao, Y.; Liu, S.; Zhang, S.; Wang, X.; Lu, L. MXene–AuNP-Based Electrochemical Aptasensor for Ultra-Sensitive Detection of Chloramphenicol in Honey. Molecules 2022, 27, 1871. [Google Scholar] [CrossRef]
- Jiang, D.; Wei, M.; Du, X.; Qin, M.; Shan, X.; Chen, Z. One-pot synthesis of ZnO quantum dots/N-doped Ti3C2 MXene: Tunable nitrogen-doping properties and efficient electrochemiluminescence sensing. Chem. Eng. J. 2022, 430, 132771. [Google Scholar] [CrossRef]
- You, F.; Wei, J.; Cheng, Y.; Wen, Z.; Ding, C.; Hao, N.; Wang, K. Selective and sensitive photoelectrochemical aptasensor for streptomycin detection based on Bi4VO8Br/Ti3C2 nanohybrids. J. Hazard. Mater. 2021, 414, 125539. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Hui, Y.; Zhang, L.; Zhang, F.; Liu, Y.; Zheng, J.; Jia, R.; Song, Y.; Wang, B. A novel electrochemical aptasensor based on Ti3C2-MOFs nanocomposites for rapid streptomycin detection in milk samples. Sens. Actuators B Chem. 2022, 368, 132119. [Google Scholar] [CrossRef]
- Jiang, D.; Wei, M.; Du, X.; Qin, M.; Shan, X.; Wang, W.; Chen, Z. Ultrasensitive near-infrared aptasensor for enrofloxacin detection based on wavelength tunable AgBr nanocrystals electrochemiluminescence emission triggered by O-terminated Ti3C2 MXene. Biosens. Bioelectron. 2022, 200, 113917. [Google Scholar] [PubMed]
- You, F.; Wen, Z.; Yuan, R.; Ding, L.; Wei, J.; Qian, J.; Long, L.; Wang, K. Selective and ultrasensitive detection of ciprofloxacin in milk using a photoelectrochemical aptasensor based on Ti3C2/Bi4VO8Br/TiO2 nanocomposite. J. Electroanal. Chem. 2022, 914, 116285. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, R.; Zhang, Z.; Chen, D.; Gao, Y.; Liu, Z.; Li, H.; Wang, C. Label-free electrochemical biosensor based on GR5 DNAzyme/Ti3C2Tx Mxenes for Pb2+ detection. J. Electroanal. Chem. 2022, 905, 115979. [Google Scholar] [CrossRef]
- Zhai, H.; Wang, Y.; Yin, J.; Zhang, Y.; Guo, Q.; Sun, X.; Guo, Y.; Yang, Q.; Li, F.; Zhang, Y. Electrochemiluminescence biosensor for determination of lead (II) ions using signal amplification by Au@ SiO2 and tripropylamine-endonuclease assisted cycling process. Microchim. Acta 2022, 189, 1–11. [Google Scholar] [CrossRef]
- Li, Y.; Liu, K.; Wang, B.; Liu, Z.; Yang, C.; Wang, J.; Ma, X.; Li, H.; Sun, C. Engineering DNAzyme strategies for fluorescent detection of lead ions based on RNA cleavage-propelled signal amplification. J. Hazard. Mater. 2022, 440, 129712. [Google Scholar] [CrossRef]
- Rasheed, P.A.; Pandey, R.P.; Jabbar, K.A.; Mahmoud, K.A. Nb4C3Tx (MXene)/Au/DNA aptasensor for the ultraselective electrochemical detection of lead in water samples. Electroanalysis 2022, 34, 1540–1546. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Lin, J.; Zhang, Y.; Qiu, M.; Chen, Y.; Li, M.; Tang, D. Ultrasensitive fluorometric biosensor based on Ti3C2 MXenes with Hg2+-triggered exonuclease III-assisted recycling amplification. Analyst 2021, 146, 2664–2669. [Google Scholar] [CrossRef]
- Zhi, S.; Shi, J.; Liang, A.; Jiang, Z. MXene nanosheet loaded gold nanocluster catalytic amplification–aptamer SERS quantitative assay platform for isocarbophos. Talanta 2022, 251, 123771. [Google Scholar] [CrossRef]
- Matsuda, S.; Hiyoshi, H.; Tandhavanant, S.; Kodama, T. Advances on Vibrio parahaemolyticus research in the postgenomic era. Microbiol. Immunol. 2020, 64, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Galán, J.E. Salmonella Typhimurium and inflammation: A pathogen-centric affair. Nat. Rev. Microbiol. 2021, 19, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Gunasekaran, S. Nanozymes-based biosensors for food quality and safety. TrAC Trends Anal. Chem. 2020, 126, 115841. [Google Scholar] [CrossRef]
- Ye, W.; Liu, T.; Zhang, W.; Zhang, W. The Toxic Mechanism of Gliotoxins and Biosynthetic Strategies for Toxicity Prevention. Int. J. Mol. Sci. 2021, 22, 13510. [Google Scholar] [CrossRef]
- Chang, Q.; Wang, W.; Regev-Yochay, G.; Lipsitch, M.; Hanage, W.P. Antibiotics in agriculture and the risk to human health: How worried should we be? Evol. Appl. 2015, 8, 240–247. [Google Scholar] [CrossRef] [Green Version]
- Paige, J.C.; Tollefson, L.; Miller, M.A. Health implications of residues of veterinary drugs and chemicals in animal tissues. Vet. Clin. N. Am. Food Anim. Pract. 1999, 15, 31–43. [Google Scholar] [CrossRef]
- Dinos, G.P.; Athanassopoulos, C.M.; Missiri, D.A.; Giannopoulou, P.C.; Vlachogiannis, I.A.; Papadopoulos, G.E.; Papaioannou, D.; Kalpaxis, D.L. Chloramphenicol derivatives as antibacterial and anticancer agents: Historic problems and current solutions. Antibiotics 2016, 5, 20. [Google Scholar] [CrossRef]
- Krause, K.M.; Serio, A.W.; Kane, T.R.; Connolly, L.E. Aminoglycosides: An overview. Cold Spring Harb. Perspect. Med. 2016, 6, a027029. [Google Scholar] [CrossRef]

| Analytes | MXene Biosensor | Detection Methods | LOD | Linear Range | Real Sample | Refs. | |
|---|---|---|---|---|---|---|---|
| Foodborne pathogens | Salmonella typhimurium | Aptamer/cDNA- PQDs/Ti3C2 MXene nanosheets | Fluorescent | 30 CFU/mL | 102–106 CFU/mL | Real aquatic sample | [81] |
| Vibrio parahaemolyticus | 10 CFU/mL | ||||||
| Vibrio parahaemolyticus | Aptamers/PBA-Fc@Pt@MXene | Electrochemical | 5 CFU/mL | 10–108 CFU/mL | Shrimp and water | [82] | |
| Colorimetric | 30 CFU/mL | 102–108 CFU/mL | |||||
| MXene/POSS-PQD-Apt | Fluorescent | 30 CFU/mL | 102–106 CFU/mL | Seawater | [83] | ||
| Mycobacterium tuberculosis | PNA/Zr-MXene | Electrochemical | 20 CFU/mL | 102–108 CFU/mL | Simulated sputum sample | [84] | |
| Ti3C2 MXene/polypyrrole/methylene blue | Electrochemical | 11.24 fM | 100 fM–25 nM | Clinical human sputum | [85] | ||
| Escherichia coli | MXene/CRSPR-Cas12a/ssDNA-fluorophore | Fluorescent | 23 CFU/mL | 3.2 × 10–3.2 × 107 CFU/mL | Purified water, milk, grapefruit juice, green tea | [86] | |
| Lipopolysaccharide | 11 pg/mL | 0.1–8000 ng/mL | |||||
| Mycotoxin | Ochratoxin A | Aptamer/cDNA-MXene-Au/Pt@NiCo-LDH | Electrochemical | 8.9 fg/mL | 20 fg/mL–100 ng/mL | Corn and wheat sample | [87] |
| MXene/Aptamer/Au-Ag Janus nanocomposites | Surface-Enhanced Raman Scattering | 1.28 pM | 0.01–50 nM | Red wine sample | [88] | ||
| DNAzyme cascade amplification/MXene-TiO2/Au@PtAg | Photoelectrochemical | 1.73 fg/mL | 5 fg/mL–10 ng/mL | - | [89] | ||
| Au@ MXene/tetrahedral DNA/signal probe DNA-Au@MOF (UIO-66) | Electrochemical | 330 fg/mL | 1 pg/mL–100 ng/mL | Corn sample | [90] | ||
| MXene/polyvinylidene fluoride nanofiber/ Aptamer | Electrochemical | 2.5 fg/mL | 1 fg/mL–1 ng/mL | Grape juice | [91] | ||
| Aflatoxin B1 | MXene/fluorophore-modified ssDNA/ Aptamer-CRISPR-Cas12 | Fluorescent | 0.92 pg/mL | 0.001–80 ng/mL | Peanut sample | [92] | |
| MXene/Au dimer-Ag/ aptamer | Surface-Enhanced Raman Scattering | 0.6 pg/mL | 0.001–100 ng/mL | Peanut sample | [93] | ||
| Deoxynivalenol | MXene-Au/luminophore-modified ssDNA/ Aptamer-CRISPR-Cas12 | Luminescent | 0.64 ng/mL | 1–500 ng/mL | Corn and lake water | [94] | |
| MXene/Aptamer/APTES-glutaraldehyde | Electrochemical | 1 fg/mL | 1 fg/mL–1 ng/mL | Paddy plant extractions | [95] | ||
| Gliotoxin | MXene/tetrahedral DNA nanostructure/cDNA-peroxidase polymer | Electrochemical | 5 pM | 5 pM–10 nM | Human serum sample | [96] | |
| Zearalenone | MXene/Chitosan/Aptamer/APTES-glutaraldehyde | Electrochemical | 0.4 pg/mL | 1 fg/mL–1 ng/mL | Corn and cow milk | [97] | |
| Microcystin-LR | MXene@Au nanocomposites/cDNA-methylene blue/reduced graphene oxide/Au/Aptamer | Electrochemical | 4 × 10−5 nM | 0.0001–5 nM | Tap water and surface water | [98] | |
| Saxitoxin | MXene/silane coupling agent/Aptamer | Electrochemical | 0.03 nM | 1.0–200 nM | Mussel tissue | [99] | |
| Antibiotics | Chloramphenicol | Aptamer/MXene | Electrochemical | 0.03 pM | 0.0001–10 nM | Honey sample | [100] |
| Aptamer/ZnO quantum dots-N doped MXene | Electrochemiluminescence | 0.019 ng/mL | 0.1–100 ng/mL | Pond water and milk | [101] | ||
| Streptomycin | Aptamer/Bi4VO8Br/Ti3C2 nanostructure | Photoelectrochemical | 0.3 nM | 1–1000 nM | Honey sample | [102] | |
| cDNA/Aptamer/MOF (UIO-66-NH2-PEI)/PANI-Ti3C2 nanostructure | Electrochemical | 0.0033 nM | 0.01–200 nM | Milk samples | [103] | ||
| Enrofloxacin | Aptamer/O-Ti3C2 MXene-AgBr nanocrystal | Electrochemiluminescence | 5.97 × 10−13 mol/L | 1.0 × 10−12–1.0 × 10−6 mol/L | Pond water and tap water | [104] | |
| Ciprofloxacin | Aptamer/Ti3C2-Bi4VO8Br-TiO2 | Photoelectrochemical | 0.3 nM | 1–1500 nM | Milk samples | [105] | |
| Metal ions | Pb2+ | Nafion/Ti3C2Tx MXene/GR5 DNAzyme | Electrochemical | 0.1 nM | 0.5–32 nM | Liver tissues of rat and chicken | [106] |
| Aptazyme/cDNA/Au@SiO2- Ru(bpy)32+/Ti3C2Tx MXene@Au | Electrochemiluminescence | 0.059 ng/L | 0.1 –106 ng/L | Tap, lake and industrial waste water | [107] | ||
| MXene/FAM-DNA substrate/GR5 DNAzyme | Fluorescent | 0.05 ng/mL | 0.2–10 ng/L | Tap and river water | [108] | ||
| Au@Nb4C3Tx MXene/ Aptamer | Electrochemical | 4 nM | 10 nM–5 µM | Tap and bottled water | [109] | ||
| Hg2+ | Aptazyme/fluorophore-cDNA/Exo III-assisted system Ti3C2Tx MXene | Fluorescent | 42.5 pM | 0.05–50 nM | Tap and river water | [110] | |
| Insecticides | - Isocarbophos (ICP) | MXene@Au/ICP aptamer/mandelic acid-HAuCl 4 | Surface-Enhanced Raman Scattering | 4.5 × 10−5 nmol/L | 1.0 × 10−3–2.5 × 10−2 nmol/L | Farm water | [111] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Gunasekaran, S. MXene-Based Nucleic Acid Biosensors for Agricultural and Food Systems. Biosensors 2022, 12, 982. https://doi.org/10.3390/bios12110982
Wang W, Gunasekaran S. MXene-Based Nucleic Acid Biosensors for Agricultural and Food Systems. Biosensors. 2022; 12(11):982. https://doi.org/10.3390/bios12110982
Chicago/Turabian StyleWang, Weizheng, and Sundaram Gunasekaran. 2022. "MXene-Based Nucleic Acid Biosensors for Agricultural and Food Systems" Biosensors 12, no. 11: 982. https://doi.org/10.3390/bios12110982





