Slippery Epidural ECoG Electrode for High-Performance Neural Recording and Interface
Abstract
:1. Introduction
2. Materials and Methods
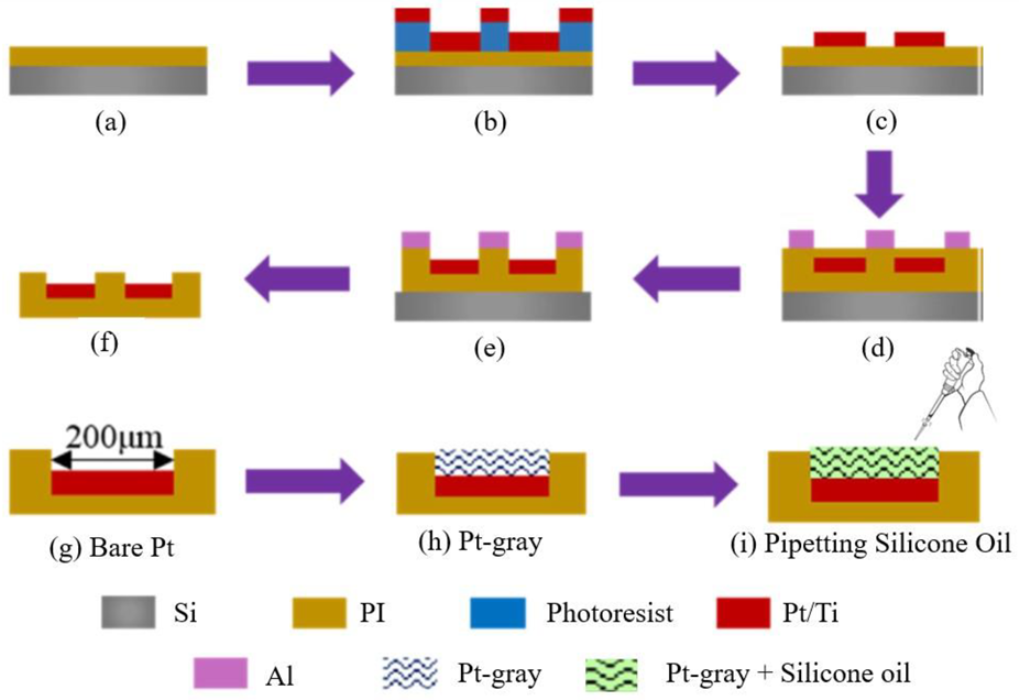
2.1. Fabrication Procedure of ECoG Electrode
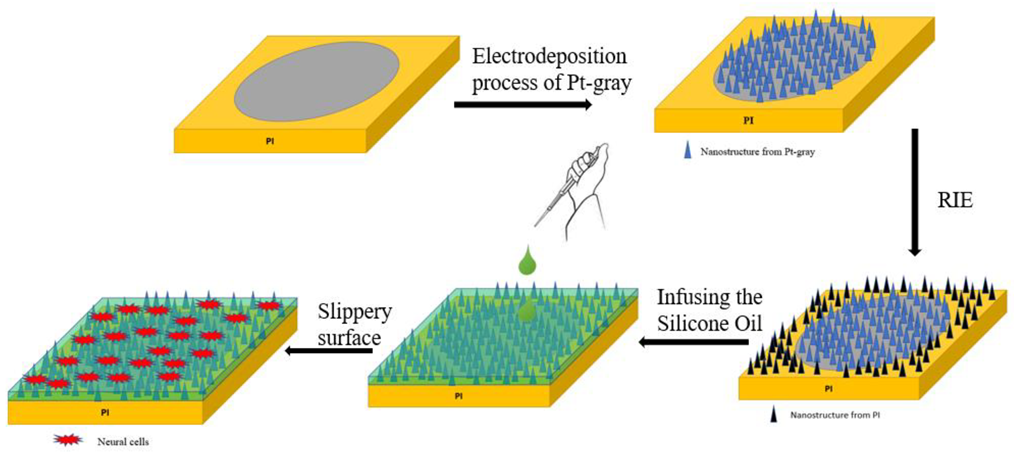
2.2. Electrodeposition and SLIPS Coating Procedure on ECoG Electrode
2.3. Electrochemical Characterization
2.4. Characterization
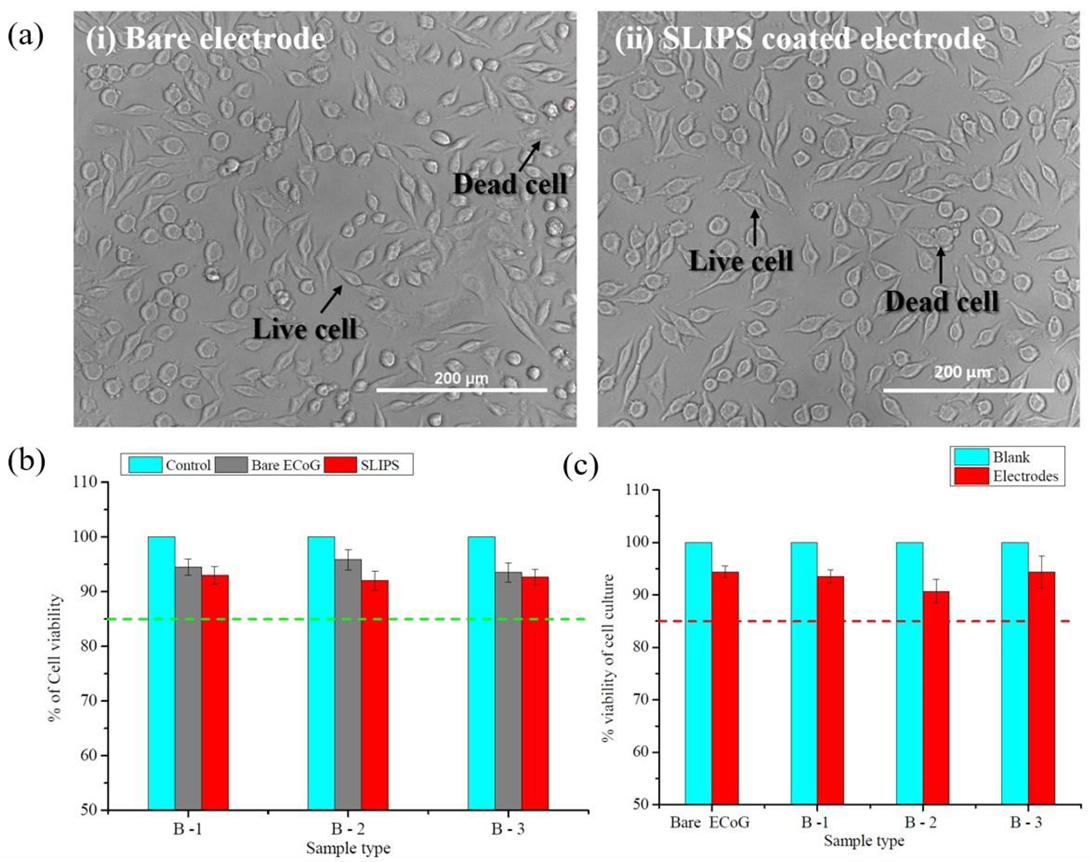
2.5. In Vitro Study (Cytotoxicity Study)
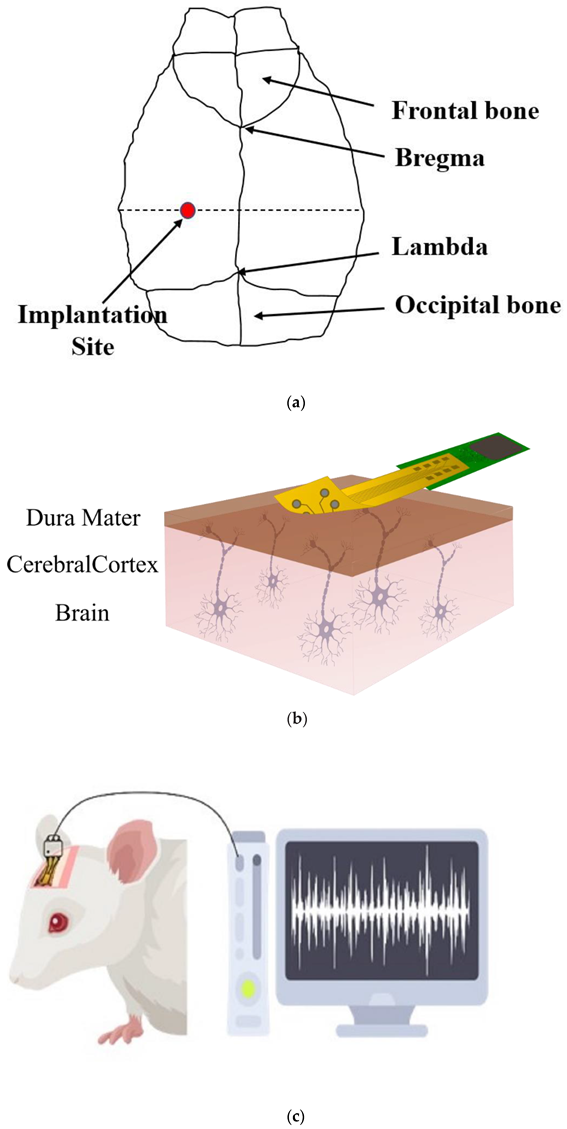
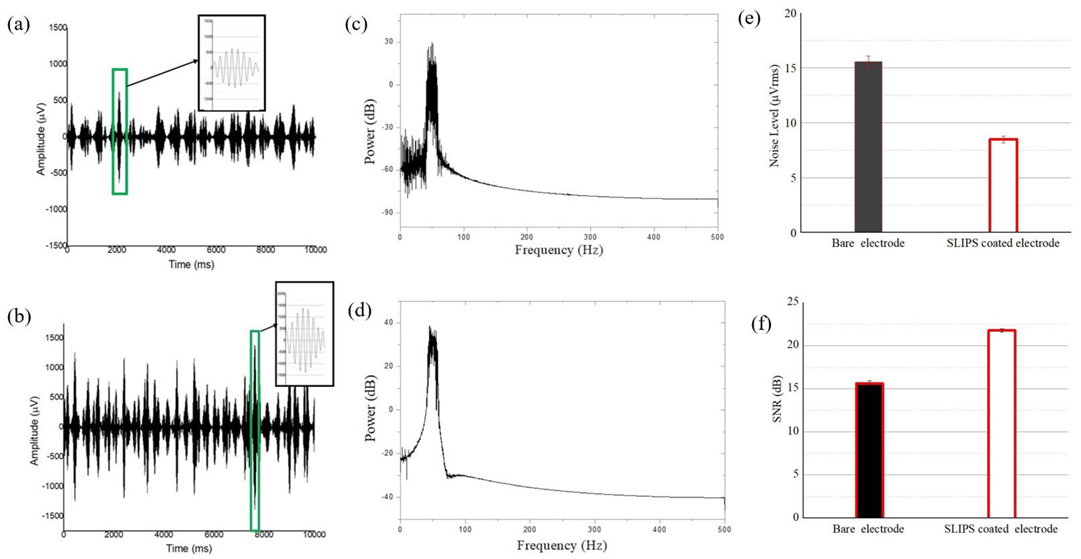
2.6. In Vivo Recording (Acute Signal Recording)
3. Results and Discussions
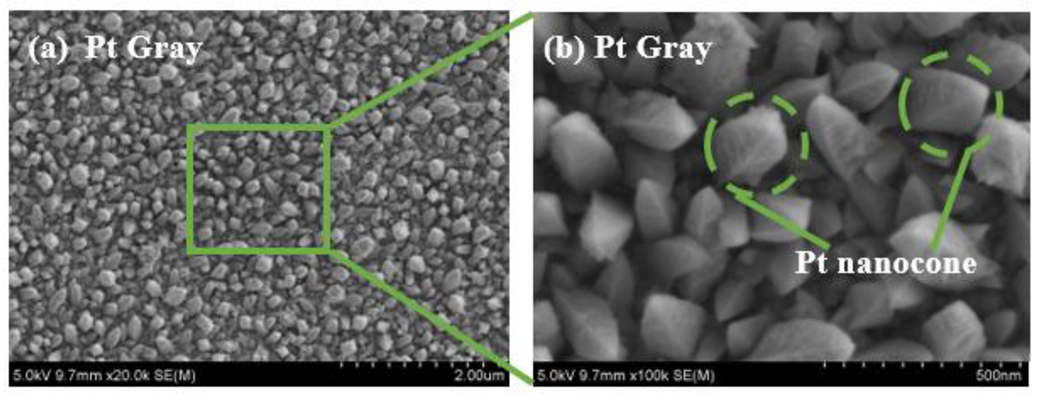
3.1. Electrodeposition Process and Morphology Study
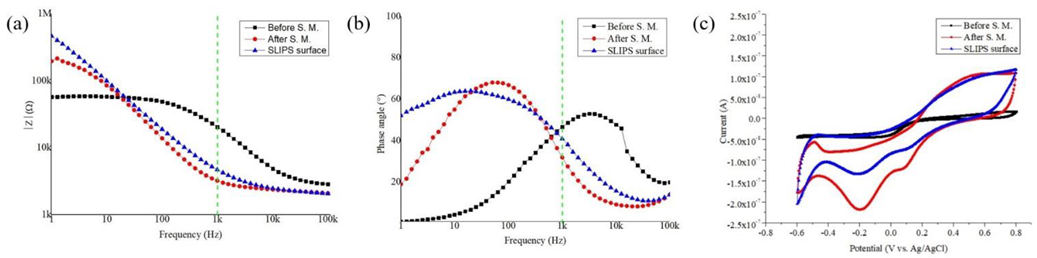
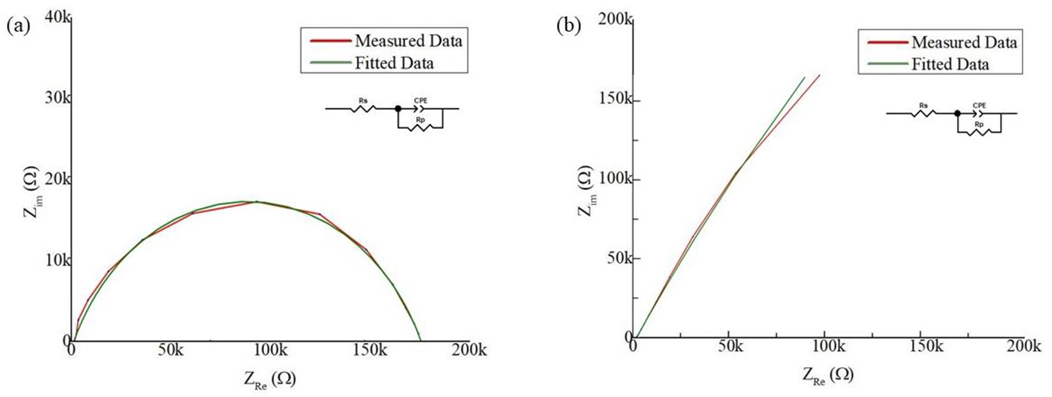
3.2. Evaluation of Electrochemical Performance
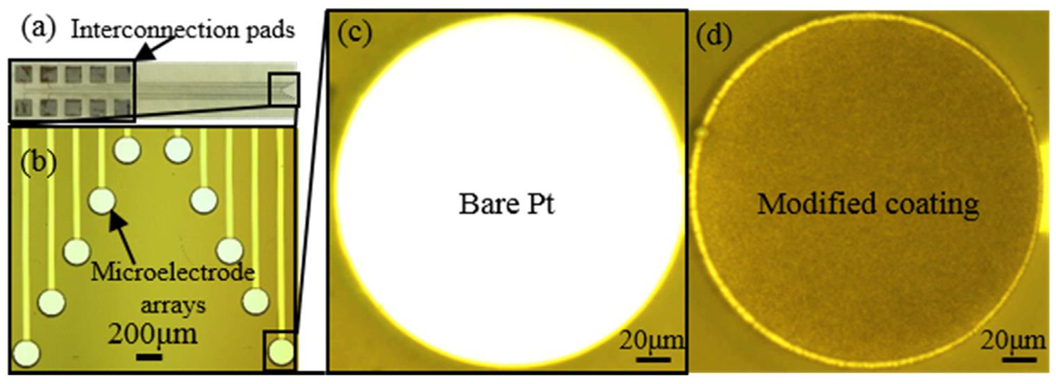
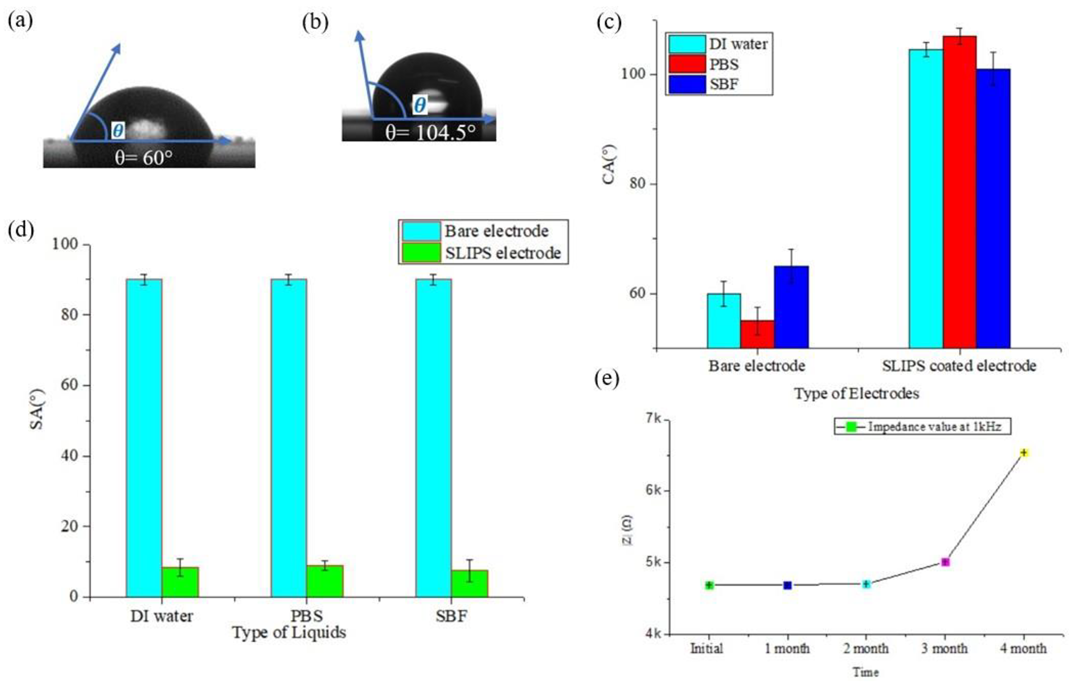
3.3. Characterization of SLIPS Coating
3.4. Evaluation of In Vitro Study (Cytotoxicity Study)
3.5. Evaluation of In Vivo Recording (Acute Signal Recording)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watts, R.L.; Koller, W.C. Movement Disorders: Neurologic Principles & Practice; McGraw-Hill Professional: New York, NY, USA, 2004. [Google Scholar]
- Hong, G.; Lieber, C.M. Novel electrode technologies for neural recordings. Nat. Rev. Neurosci. 2019, 20, 330–345. [Google Scholar] [CrossRef] [PubMed]
- Anumanchipalli, G.K.; Chartier, J.; Chang, E.F. Speech synthesis from neural decoding of spoken sentences. Nature 2019, 568, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Aflalo, T.; Kellis, S.; Klaes, C.; Lee, B.; Shi, Y.; Pejsa, K.; Shanfield, K.; Hayes-Jackson, S.; Aisen, M.; Heck, C. Decoding motor imagery from the posterior parietal cortex of a tetraplegic human. Science 2015, 348, 906–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, A.B.; Cui, X.T.; Weber, D.J.; Moran, D.W. Brain-controlled interfaces: Movement restoration with neural prosthetics. Neuron 2006, 52, 205–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seymour, J.P.; Wu, F.; Wise, K.D.; Yoon, E. State-of-the-art MEMS and microsystem tools for brain research. Microsyst. Nanoeng. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Lee, Y.; Shin, H.; Lee, D.; Choi, S.; Cho, I.J.; Seo, J. A Lubricated Nonimmunogenic Neural Probe for Acute Insertion Trauma Minimization and Long-Term Signal Recording. Adv. Sci. 2021, 8, e2100231. [Google Scholar] [CrossRef]
- Schander, A.; Strokov, S.; Stemmann, H.; Teßmann, T.; Kreiter, A.K.; Lang, W. A flexible 202-channel epidural ECoG array with PEDOT: PSS coated electrodes for chronic recording of the visual cortex. IEEE Sens. J. 2018, 19, 820–825. [Google Scholar] [CrossRef]
- Yan, T.; Kameda, S.; Suzuki, K.; Kaiju, T.; Inoue, M.; Suzuki, T.; Hirata, M. Minimal Tissue Reaction after Chronic Subdural Electrode Implantation for Fully Implantable Brain–Machine Interfaces. Sensors 2021, 21, 178. [Google Scholar] [CrossRef]
- Polikov, V.S.; Tresco, P.A.; Reichert, W.M. Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Methods 2005, 148, 1–18. [Google Scholar] [CrossRef]
- Salatino, J.W.; Ludwig, K.A.; Kozai, T.D.; Purcell, E.K. Glial responses to implanted electrodes in the brain. Nat. Biomed. Eng. 2017, 1, 862–877. [Google Scholar] [CrossRef]
- Kozai, T.D.Y.; Jaquins-Gerstl, A.S.; Vazquez, A.L.; Michael, A.C.; X Tracy, C. Brain tissue responses to neural implants impact signal sensitivity and intervention strategies. ACS Chem. Neurosci. 2015, 6, 48–67. [Google Scholar] [CrossRef] [Green Version]
- Heiduschka, P.; Thanos, S. Implantable bioelectronic interfaces for lost nerve functions. Prog. Neurobiol. 1998, 55, 433–461. [Google Scholar] [CrossRef]
- Kozai, T.D.Y.; Vazquez, A.L.; Weaver, C.L.; Kim, S.G.; Cui, X.T. In vivo two-photon microscopy reveals immediate microglial reaction to implantation of microelectrode through extension of processes. J. Neural Eng. 2012, 9, 066001. [Google Scholar] [CrossRef] [Green Version]
- Kozai, T.D.Y.; Langhals, N.B.; Patel, P.R.; Deng, X.; Zhang, H.; Smith, K.L.; Lahann, J.; Kotov, N.A.; Kipke, D.R. Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces. Nat. Mater. 2012, 11, 1065–1073. [Google Scholar] [CrossRef] [Green Version]
- Burda, J.E.; Bernstein, A.M.; Sofroniew, M.V. Astrocyte roles in traumatic brain injury. Exp. Neurol. 2016, 275 Pt 3, 305–315. [Google Scholar] [CrossRef] [Green Version]
- Castagnola, E.; Ansaldo, A.; Maggiolini, E.; Ius, T.; Skrap, M.; Ricci, D.; Fadiga, L. Smaller, softer, lower-impedance electrodes for human neuroprosthesis: A pragmatic approach. Front. Neuroeng. 2014, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Lee, J.; Son, D.; Choi, M.K.; Kim, D.H. Deformable devices with integrated functional nanomaterials for wearable electronics. Nano Converg. 2016, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Lacour, S.P.; Courtine, G.; Guck, J. Materials and technologies for soft implantable neuroprostheses. Nat. Rev. Mater. 2016, 1, 16063. [Google Scholar] [CrossRef] [Green Version]
- Mono, H.G.; Stokes, K.B. The electrode-tissue interface: The revolutionary role of steroid elution. Pacing Clin. Electrophysiol. 1992, 15, 95–107. [Google Scholar] [CrossRef]
- Rousche, P.J.; Normann, R.A. Chronic recording capability of the Utah Intracortical Electrode Array in cat sensory cortex. J. Neurosci. Methods 1998, 82, 1–15. [Google Scholar] [CrossRef]
- Nicolelis, M.A.; Dimitrov, D.; Carmena, J.M.; Crist, R.; Lehew, G.; Kralik, J.D.; Wise, S.P. Chronic, multisite, multielectrode recordings in macaque monkeys. Proc. Natl. Acad. Sci. USA 2003, 100, 11041–11046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suner, S.; Fellows, M.R.; Vargas-Irwin, C.; Nakata, G.K.; Donoghue, J.P. Reliability of signals from a chronically implanted, silicon-based electrode array in non-human primate primary motor cortex. IEEE Trans. Neural Syst. Rehabil. Eng. 2005, 13, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.K.; Jones, K.E.; Huber, R.J.; Horch, K.W.; Normann, R.A. A silicon-based, three-dimensional neural interface: Manufacturing processes for an intracortical electrode array. IEEE Trans. Biomed. Eng. 1991, 38, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Berdondini, L.; Bosca, A.; Nieus, T.; Maccione, A. Active Pixel Sensor Multielectrode Array for High Spatiotemporal Resolution; Springer: New York, NY, USA, 2014. [Google Scholar]
- Wong, T.-S.; Kang, S.H.; Tang, S.K.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Tuo, Y.; Zhang, H.; Chen, W.; Liu, X. Corrosion protection application of slippery liquid-infused porous surface based on aluminum foil. Appl. Surf. Sci. 2017, 423, 365–374. [Google Scholar] [CrossRef]
- Shi, Z.; Xiao, Y.; Qiu, R.; Niu, S.; Wang, P. A facile and mild route for fabricating slippery liquid-infused porous surface (SLIPS) on CuZn with corrosion resistance and self-healing properties. Surf. Coat. Technol. 2017, 330, 102–112. [Google Scholar] [CrossRef]
- Xiang, T.; Zhang, M.; Sadig, H.R.; Li, Z.; Zhang, M.; Dong, C.; Yang, L.; Chan, W.; Li, C. Slippery liquid-infused porous surface for corrosion protection with self-healing property. Chem. Eng. J. 2018, 345, 147–155. [Google Scholar] [CrossRef]
- Glavan, A.C.; Martinez, R.V.; Subramaniam, A.B.; Yoon, H.J.; Nunes, R.M.; Lange, H.; Thuo, M.M.; Whitesides, G.M. Omniphobic “RF paper” produced by silanization of paper with fluoroalkyltrichlorosilanes. Adv. Funct. Mater. 2014, 24, 60–70. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.; Schlaich, C.; Prévost, S.; Schulz, A.; Böttcher, C.; Gradzielski, M.; Qi, Z.; Haag, R.; Schalley, C.A. Supramolecular polymers as surface coatings: Rapid fabrication of healable superhydrophobic and slippery surfaces. Adv. Mater. 2014, 26, 7358–7364. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, A.; Seeger, S. Nepenthes pitcher inspired anti-wetting silicone nanofilaments coatings: Preparation, unique anti-wetting and self-cleaning behaviors. Adv. Funct. Mater. 2014, 24, 1074–1080. [Google Scholar] [CrossRef]
- Park, K.; Kim, S.; Jo, Y.; Park, J.; Kim, I.; Hwang, S.; Lee, Y.; Kim, S.Y.; Seo, J. Lubricant skin on diverse biomaterials with complex shapes via polydopamine-mediated surface functionalization for biomedical applications. Bioact. Mater. 2022. [Google Scholar] [CrossRef]
- Park, J.; Kim, Y.; Chun, B.; Seo, J. Rational engineering and applications of functional bioadhesives in biomedical engineering. Biotechnol. J. 2021, 16, 2100231. [Google Scholar] [CrossRef]
- Zhou, D.M.; Ok, J.; Talbot, N.H.; Mech, B.V.; Little, J.S.; Greenberg, R.J. Electrode with Increased Stability and Method of Manufacturing the Same. U.S. Patent No 7,937,153, 3 May 2011. [Google Scholar]
- Zeng, Q.; Xia, K.; Sun, B.; Yin, Y.; Wu, T.; Humayun, M.S. Electrodeposited iridium oxide on platinum nanocones for improving neural stimulation microelectrodes. Electrochim. Acta 2017, 237, 152–159. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, T.; Cai, Z.; Cao, Y.; Yang, H.; Duan, Y.Y. Anodically electrodeposited iridium oxide films microelectrodes for neural microstimulation and recording. Sens. Actuators B Chem. 2009, 137, 334–339. [Google Scholar] [CrossRef]
- Yi, W.; Chen, C.; Feng, Z.; Xu, Y.; Zhou, C.; Masurkar, N.; Cavanaugh, J.; Cheng, M.M.-C. A flexible and implantable microelectrode arrays using high-temperature grown vertical carbon nanotubes and a biocompatible polymer substrate. Nanotechnology 2015, 26, 125301. [Google Scholar] [CrossRef]
- Carretero, N.M.; Lichtenstein, M.; Pérez, E.; Sandoval, S.; Tobias, G.; Suñol, C.; Casan-Pastor, N. Enhanced charge capacity in iridium oxide-graphene oxide hybrids. Electrochim. Acta 2015, 157, 369–377. [Google Scholar] [CrossRef]
- Cogan, S.F. Neural stimulation and recording electrodes. Annu. Rev. Biomed. Eng. 2008, 10, 275–309. [Google Scholar] [CrossRef] [Green Version]
- Boehler, C.; Stieglitz, T.; Asplund, M. Nanostructured platinum grass enables superior impedance reduction for neural microelectrodes. Biomaterials 2015, 67, 346–353. [Google Scholar] [CrossRef]
- Aziz, T.; Fan, H.; Khan, F.U.; Haroon, M.; Cheng, L. Modified silicone oil types, mechanical properties and applications. Polym. Bull. 2019, 76, 2129–2145. [Google Scholar] [CrossRef]
- Bohn, H.F.; Federle, W. Insect aquaplaning: Nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface. Proc. Natl. Acad. Sci. USA 2004, 101, 14138–14143. [Google Scholar] [CrossRef]
- Federle, W.; Riehle, M.; Curtis, A.S.; Full, R.J. An integrative study of insect adhesion: Mechanics and wet adhesion of pretarsal pads in ants. Integr. Comp. Biol. 2002, 42, 1100–1106. [Google Scholar] [CrossRef]
- Srivastava, G.K.; Alonso-Alonso, M.L.; Fernandez-Bueno, I.; Garcia-Gutierrez, M.T.; Rull, F.; Medina, J.; Coco, R.M.; Pastor, J.C. Comparison between direct contact and extract exposure methods for PFO cytotoxicity evaluation. Sci. Rep. 2018, 8, 1425. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Franklin, K.B. Paxinos and Franklin's the Mouse Brain in Stereotaxic Coordinates; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Technologies, I. RHD Recording Headstages. Available online: https://intantech.com/RHD_headstages.html?tabSelect=RHD32ch&yPos=0 (accessed on 2 November 2021).
- Technologies, I. RHD USB Interface Board. Available online: https://intantech.com/RHD_USB_interface_board.html (accessed on 2 November 2021).
- Aryan, N.P.; Hans, K.; Albrecht, R. Stimulation and Recording Electrodes for Neural Prostheses; Springer International Publishing: New York, NY, USA, 2015; Volume 1. [Google Scholar]
- Su, Z.; Yang, C.; Xie, B.; Lin, Z.; Zhang, Z.; Liu, J.; Li, B.; Kang, F.; Wong, C.P. Scalable fabrication of MnO2 nanostructure deposited on free-standing Ni nanocone arrays for ultrathin, flexible, high-performance micro-supercapacitor. Energy Environ. Sci. 2014, 7, 2652–2659. [Google Scholar] [CrossRef]
- Nam, Y.; Wheeler, B.C. In vitro microelectrode array technology and neural recordings. Crit. Rev. Biomed. Eng. 2011, 39, 45–61. [Google Scholar] [PubMed] [Green Version]
- Negi, S.; Bhandari, R.; Rieth, L.; Solzbacher, F. In vitro comparison of sputtered iridium oxide and platinum-coated neural implantable microelectrode arrays. Biomed. Mater. 2010, 5, 015007. [Google Scholar] [CrossRef]
- Whalen III, J.J.; Weiland, J.D.; Searson, P.C. Electrochemical deposition of platinum from aqueous ammonium hexachloroplatinate solution. J. Electrochem. Soc. 2005, 152, C738. [Google Scholar] [CrossRef]
- Zeng, Q.; Sun, J.; Emori, W.; Jiang, S. Corrosion behavior of thermally sprayed NiCrBSi coating on 16MnR low-alloy steel in KOH solution. J. Mater. Eng. Perform. 2016, 25, 1773–1780. [Google Scholar] [CrossRef]
- Mouanga, M.; Berçot, P.; Rauch, J. Comparison of corrosion behaviour of zinc in NaCl and in NaOH solutions. Part I: Corrosion layer characterization. Corros. Sci. 2010, 52, 3984–3992. [Google Scholar] [CrossRef]
- Alahi, M.E.E.; Liu, Y.; Xu, Z.; Wang, H.; Wu, T.; Mukhopadhyay, S.C. Recent Advancement of Electrocorticography (ECoG) Electrodes for Chronic Neural Recording/Stimulation. Mater. Today Commun. 2021, 29, 102853. [Google Scholar] [CrossRef]
- Chung, T.; Wang, J.; Wang, J.; Cao, B.; Li, Y.; Pang, S. Electrode modifications to lower electrode impedance and improve neural signal recording sensitivity. J. Neural Eng. 2015, 12, 056018. [Google Scholar] [CrossRef]
- Molina-Luna, K.; Buitrago, M.M.; Hertler, B.; Schubring, M.; Haiss, F.; Nisch, W.; Schulz, J.B.; Luft, A.R. Cortical stimulation mapping using epidurally implanted thin-film microelectrode arrays. J. Neurosci. Methods 2007, 161, 118–125. [Google Scholar] [CrossRef]
- Tsytsarev, V.; Taketani, M.; Schottler, F.; Tanaka, S.; Hara, M. A new planar multielectrode array: Recording from a rat auditory cortex. J. Neural Eng. 2006, 3, 293. [Google Scholar] [CrossRef]
- Rubehn, B.; Bosman, C.; Oostenveld, R.; Fries, P.; Stieglitz, T. A MEMS-based flexible multichannel ECoG-electrode array. J. Neural Eng. 2009, 6, 036003. [Google Scholar] [CrossRef]
| Sample Condition | Initial Impedance (at 1 kHz) | Reduction of Impedance (%) | CSC/mC cm2 |
|---|---|---|---|
| Before S. M. | 20.38 kΩ | -- | 2.07 |
| After S. M. | 3.21 kΩ | 84.24 | 10.77 |
| SLIPS coating | 4.68 kΩ | 77.03 | 10.8 |
| Type of Electrode | Rp (Charge Transfer Resistance), kΩ | A(F) | n | Error (R2) (%) |
|---|---|---|---|---|
| Before the surface modification | 5.73 | 5.18 × 10−8 | 0.75 | 3.18 |
| SLIPS coating | 2.80 | 5.01 × 10−7 | 0.86 | 1.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alahi, M.E.E.; Liu, Y.; Khademi, S.; Nag, A.; Wang, H.; Wu, T.; Mukhopadhyay, S.C. Slippery Epidural ECoG Electrode for High-Performance Neural Recording and Interface. Biosensors 2022, 12, 1044. https://doi.org/10.3390/bios12111044
Alahi MEE, Liu Y, Khademi S, Nag A, Wang H, Wu T, Mukhopadhyay SC. Slippery Epidural ECoG Electrode for High-Performance Neural Recording and Interface. Biosensors. 2022; 12(11):1044. https://doi.org/10.3390/bios12111044
Chicago/Turabian StyleAlahi, Md Eshrat E., Yonghong Liu, Sara Khademi, Anindya Nag, Hao Wang, Tianzhun Wu, and Subhas Chandra Mukhopadhyay. 2022. "Slippery Epidural ECoG Electrode for High-Performance Neural Recording and Interface" Biosensors 12, no. 11: 1044. https://doi.org/10.3390/bios12111044








