A Self-Powered Biosensor for the Detection of Glutathione
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrode Fabrication
2.3. Electrochemical Experiments
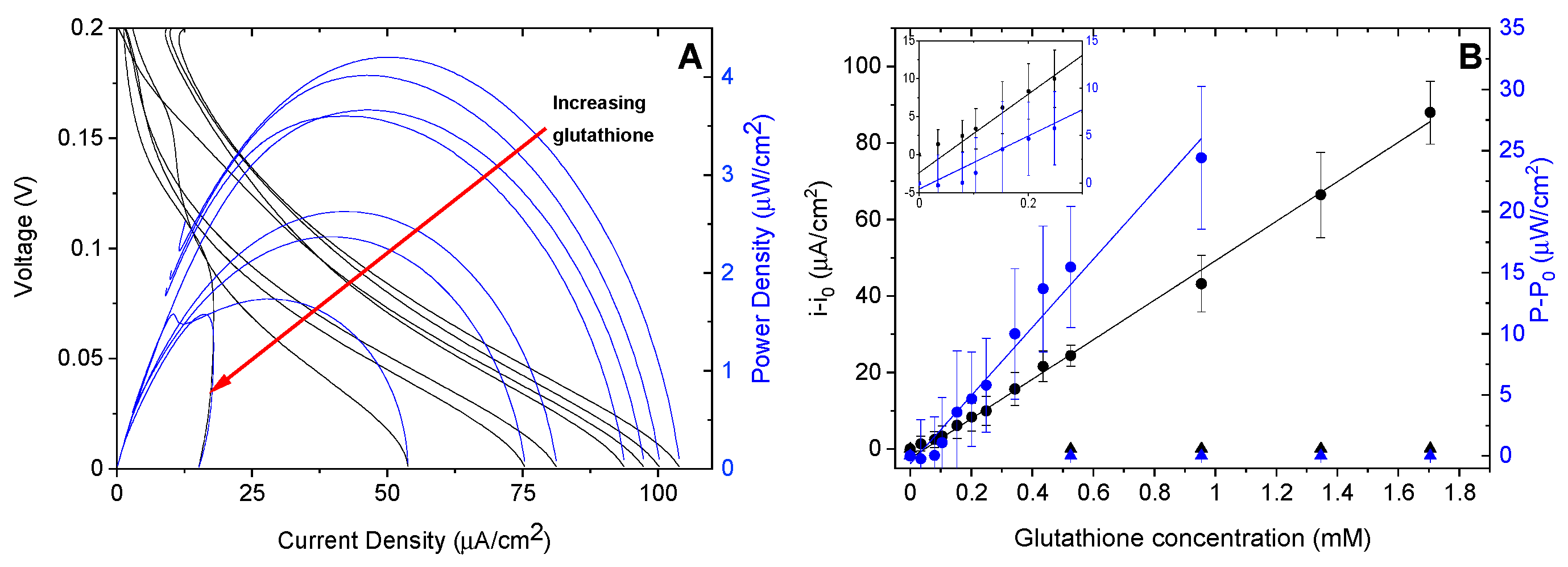
3. Results and Discussion
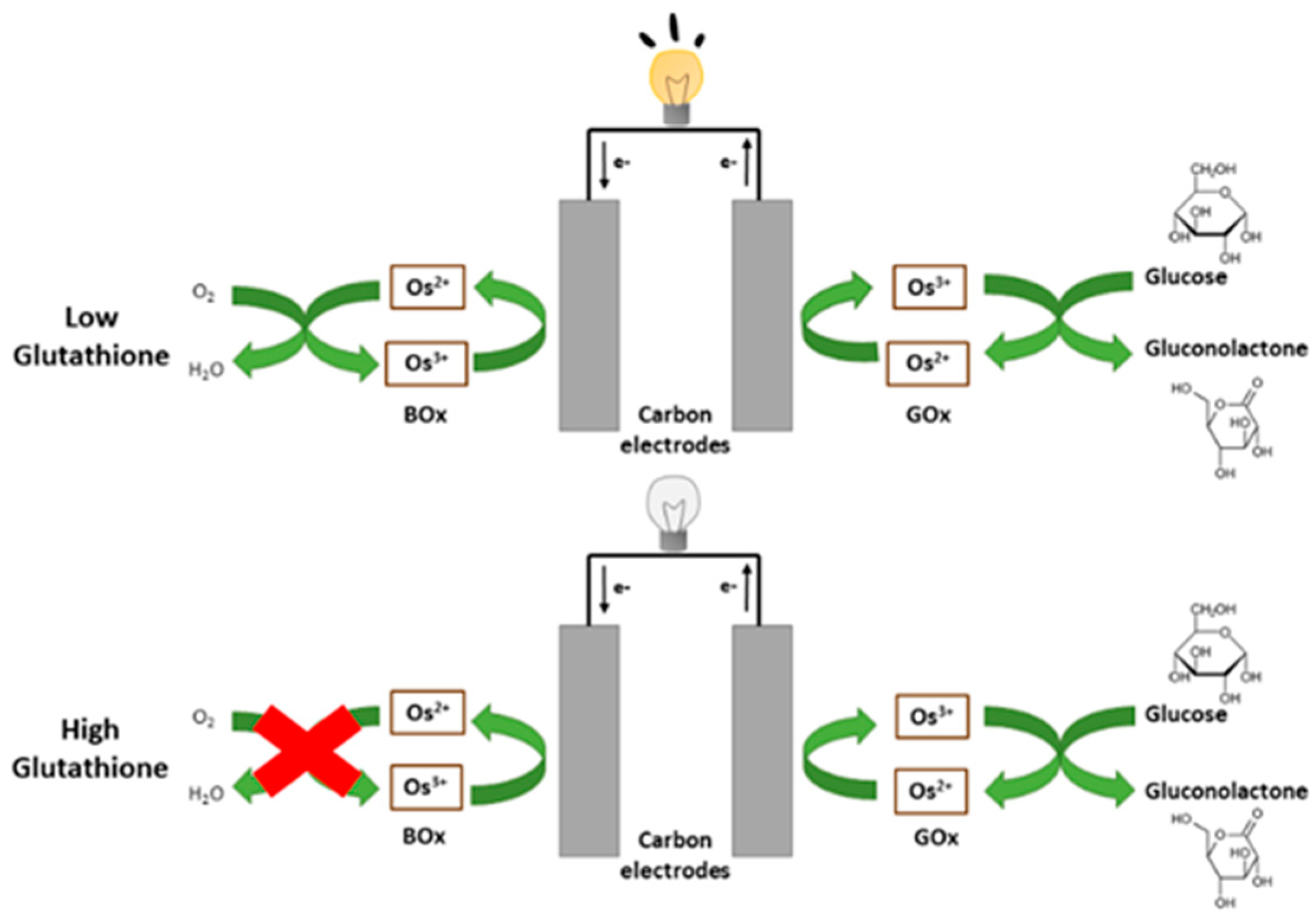
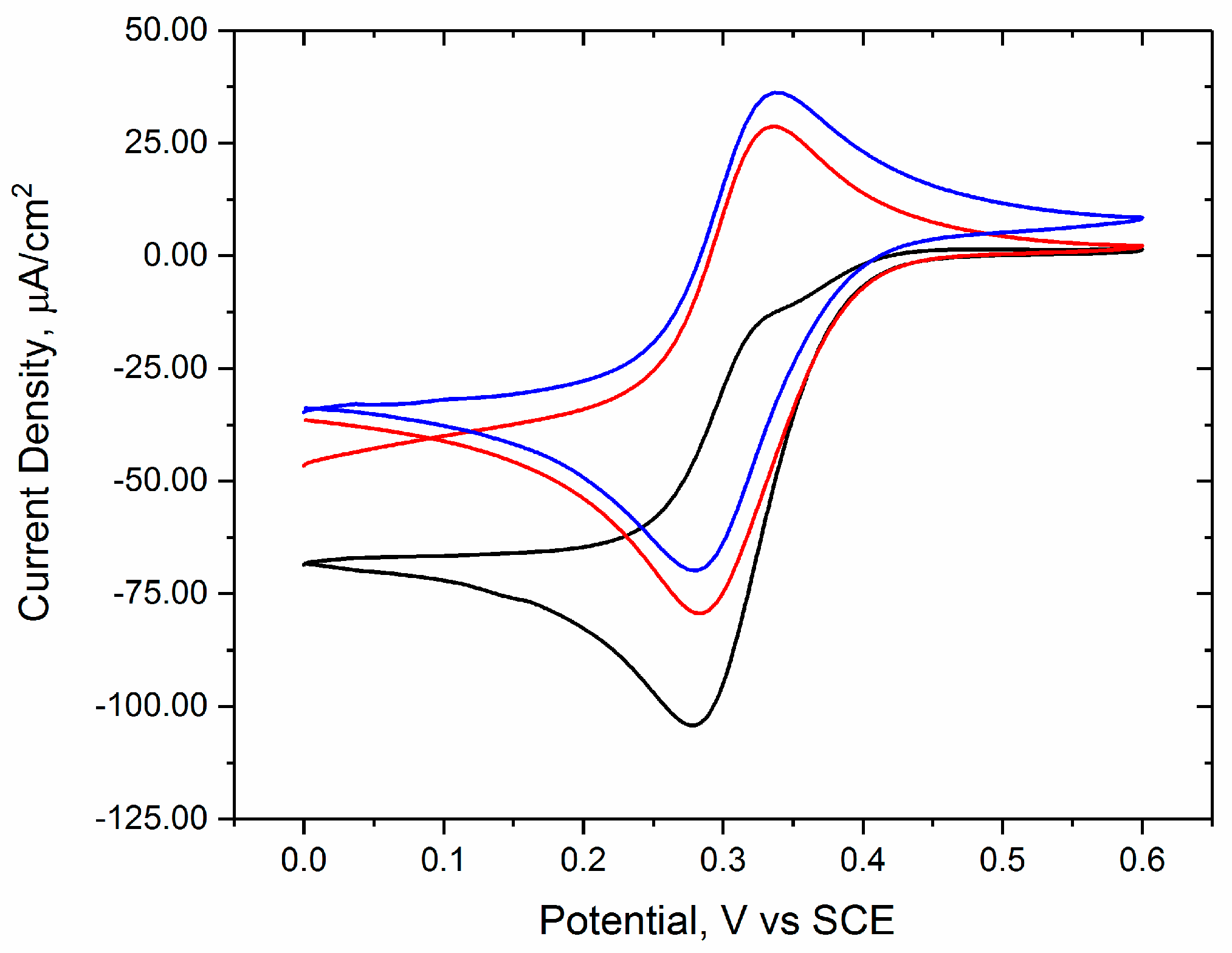
3.1. Confirmation of Bioelectrocatalytic Response to Glutathione
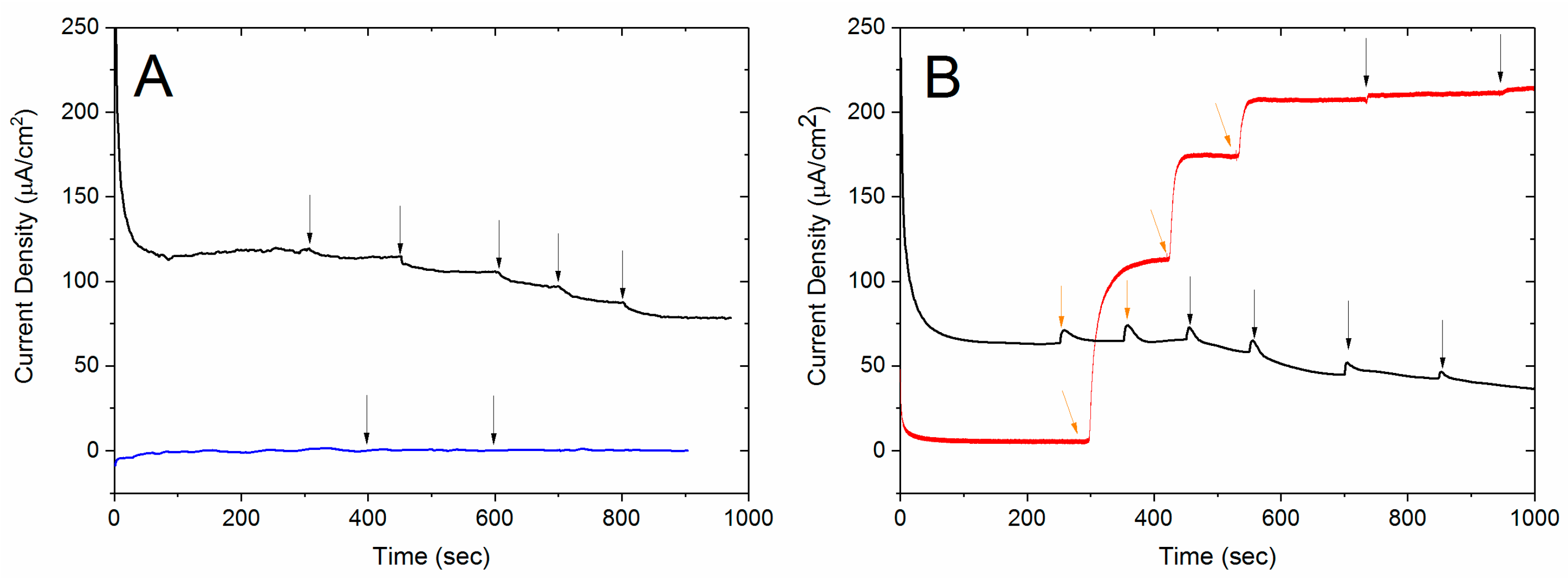
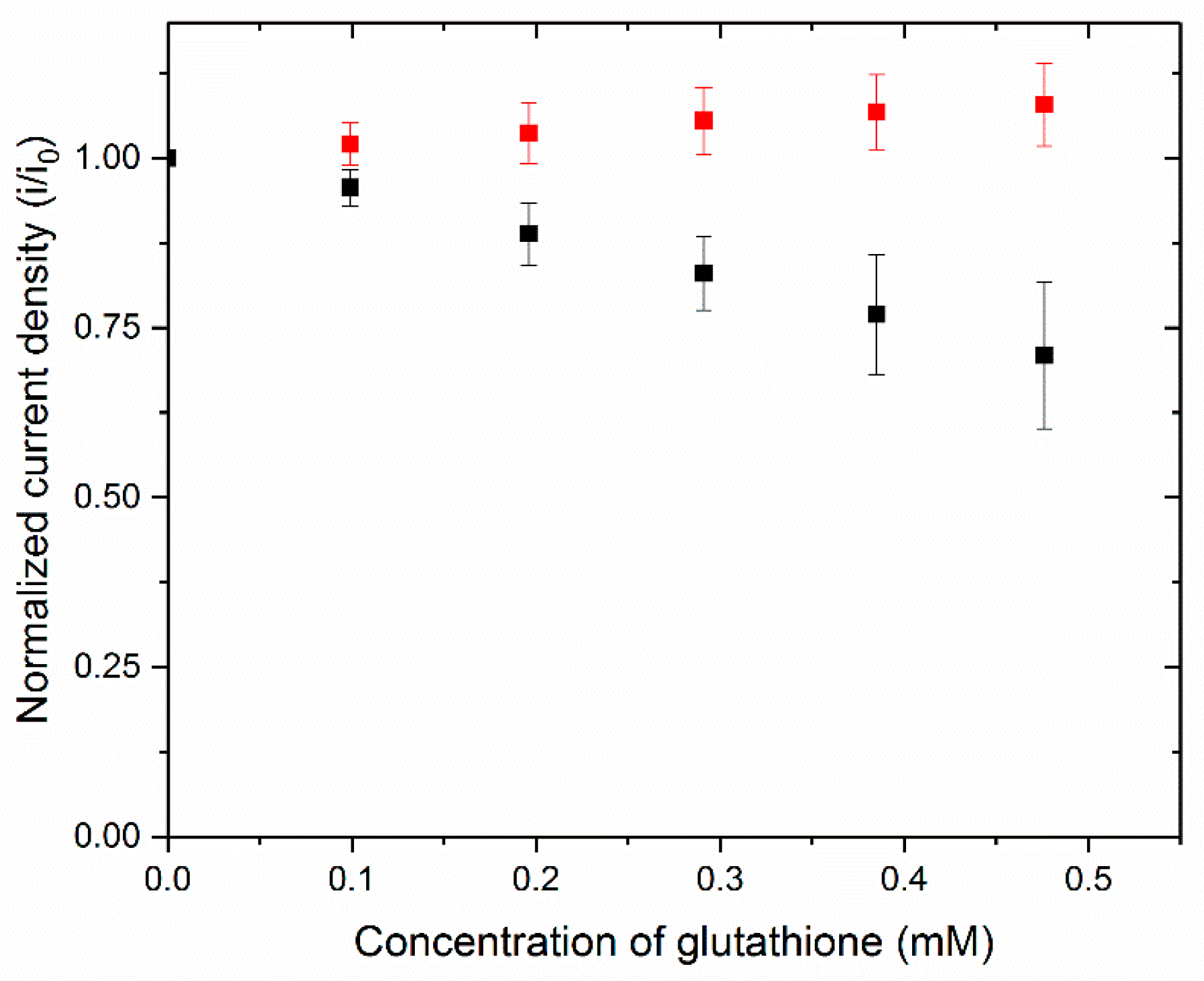
3.2. Testing the Complete Biosensor
3.3. Application of the Biosensor to Serum Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pompella, A.; Visvikis, A.; Paolicchi, A.; Tata, V.D.; Casini, A.F. The changing faces of glutathione, a cellular protagonist. Biochem. Pharmacol. 2003, 66, 1499–1503. [Google Scholar] [CrossRef]
- Harfield, J.C.; Batchelor-McAuley, C.; Compton, R.G. Electrochemical determination of glutathione: A review. Analyst 2012, 137, 2285. [Google Scholar] [CrossRef] [PubMed]
- Younes-Mhenni, S.; Frih-Ayed, M.; Kerkeni, A.; Bost, M.; Chazot, G. Peripheral Blood Markers of Oxidative Stress in Parkinson’s Disease. Eur. Neurol. 2007, 58, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.G.; Patil, P.O. A comprehensive review on carbon dots and graphene quantum dots based fluorescent sensor for biothiols. Microchem. J. 2020, 157, 105011. [Google Scholar] [CrossRef]
- Chen, X.; Han, S.; Li, N.; Lian, J.; Zhang, Y.; Liu, Q.; Zhang, X.; Zhang, X. N,N-dicarboxymethyl Perylene-diimide modified CeCoO3: Enhanced peroxidase activity, synergetic catalytic mechanism and glutathione colorimetric sensing. Talanta 2020, 218, 121142. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, L.; Wang, X.; Dong, C.; Shuang, S. Graphene quantum dots wrapped square-plate-like MnO2 nanocomposite as a fluorescent turn-on sensor for glutathione. Talanta 2020, 219, 121180. [Google Scholar] [CrossRef]
- Chu, S.; Wang, H.; Du, Y.; Yang, F.; Yang, L.; Jiang, C. Portable Smartphone Platform Integrated with a Nanoprobe-Based Fluorescent Paper Strip: Visual Monitoring of Glutathione in Human Serum for Health Prognosis. ACS Sustain. Chem. Eng. 2020, 8, 8175–8183. [Google Scholar] [CrossRef]
- Katz, E.; Bückmann, A.F.; Willner, I. Self-Powered Enzyme-Based Biosensors. J. Am. Chem. Soc. 2001, 123, 10752–10753. [Google Scholar] [CrossRef] [PubMed]
- Germain, M.N.; Arechederra, R.L.; Minteer, S.D. Nitroaromatic Actuation of Mitochondrial Bioelectrocatalysis for Self-Powered Explosive Sensors. J. Am. Chem. Soc. 2008, 130, 15272–15273. [Google Scholar] [CrossRef] [PubMed]
- Meredith, M.T.; Minteer, S.D. Inhibition and Activation of Glucose Oxidase Bioanodes for Use in a Self-Powered EDTA Sensor. Anal. Chem. 2011, 83, 5436–5441. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.; Minteer, S.D. Long-term arsenic monitoring with an Enterobacter cloacae microbial fuel cell. Bioelectrochem. Amst. Neth. 2015, 106, 207–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Zhou, M.; Dong, S. A Self-Powered Acetaldehyde Sensor Based on Biofuel Cell. Anal. Chem. 2012, 84, 10345–10349. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.; Minteer, S.D. Self-powered herbicide biosensor utilizing thylakoid membranes. Anal. Methods 2013, 5, 1140–1144. [Google Scholar] [CrossRef]
- Baldrian, P. Purification and characterization of laccase from the white-rot fungus Daedalea quercina and decolorization of synthetic dyes by the enzyme. Appl. Microbiol. Biotechnol. 2004, 63, 560–563. [Google Scholar] [CrossRef] [PubMed]
- VandeZande, G.R.; Olvany, J.M.; Rutherford, J.L.; Rasmussen, M. Enzyme Immobilization and Mediation with Osmium Redox Polymers. In Enzyme Stabilization and Immobilization; Minteer, S.D., Ed.; Springer: New York, NY, USA, 2017; Volume 1504, pp. 165–179. ISBN 978-1-4939-6497-0. [Google Scholar]
- Forster, R.J.; Vos, J.G. Synthesis, characterization, and properties of a series of osmium- and ruthenium-containing metallopolymers. Macromolecules 1990, 23, 4372–4377. [Google Scholar] [CrossRef]
- Rasmussen, M.; Abdellaoui, S.; Minteer, S.D. Enzymatic biofuel cells: 30 years of critical advancements. Biosens. Bioelectron. 2016, 76, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Tasca, F.; Farias, D.; Castro, C.; Acuna-Rougier, C.; Antiochia, R. Bilirubin oxidase from Myrothecium verrucaria physically absorbed on graphite electrodes. Insights into the alternative resting form and the sources of activity loss. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Fitri, L.E.; Iskandar, A.; Sardjono, T.W.; Erliana, U.D.; Rahmawati, W.; Candradikusuma, D.; Saputra, U.B.; Suhartono, E.; Setiawan, B.; Sulistyaningsih, E. Plasma glutathione and oxidized glutathione level, glutathione/oxidized glutathione ratio, and albumin concentration in complicated and uncomplicated falciparum malaria. Asian Pac. J. Trop. Biomed. 2016, 6, 646–650. [Google Scholar] [CrossRef] [Green Version]

| Enzyme | Sensitivity (A/mM Glutathione) |
|---|---|
| Bilirubin oxidase | 0.685 |
| Laccase | 1.044 |
| Tyrosinase | 0.039 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, B.G.; Rutherford, J.L.; Weaver, A.E.; Beaver, K.; Rasmussen, M. A Self-Powered Biosensor for the Detection of Glutathione. Biosensors 2020, 10, 114. https://doi.org/10.3390/bios10090114
Roy BG, Rutherford JL, Weaver AE, Beaver K, Rasmussen M. A Self-Powered Biosensor for the Detection of Glutathione. Biosensors. 2020; 10(9):114. https://doi.org/10.3390/bios10090114
Chicago/Turabian StyleRoy, Brandon G., Julia L. Rutherford, Anna E. Weaver, Kevin Beaver, and Michelle Rasmussen. 2020. "A Self-Powered Biosensor for the Detection of Glutathione" Biosensors 10, no. 9: 114. https://doi.org/10.3390/bios10090114






