Dye-Doped Fluorescent Silica Nanoparticles for Live Cell and In Vivo Bioimaging
Abstract
:1. Introduction
2. Properties and Synthetic Method of SiNPs
2.1. Properties of SiNPs
- SiNPs are extremely hydrophilic and convenient to prepare, synthesize and separate.
- Silica material is biocompatible and non-toxic and, thus, causes little damage to living cells.
- Silica material can let excitation light and emission pass through without changing their characteristics.
- SiNPs attach biomolecules effectively and can be easily modified with a variety of functional groups, such as poly(ethylene glycol) (PEG), hydroxyl (OH), carboxyl (COOH), phosphonate (CH3HPO2) and amine (NH2) on their surface.
- The silica matrix can encapsulate dyes and fluorescent nanoparticles without changing the optical character in convenient methods [25], and the stability of the dye can be improved after being doped in silica matrix.
- The controllable particle size of SiNPs makes them suitable to apply for in vivo bioimaging.
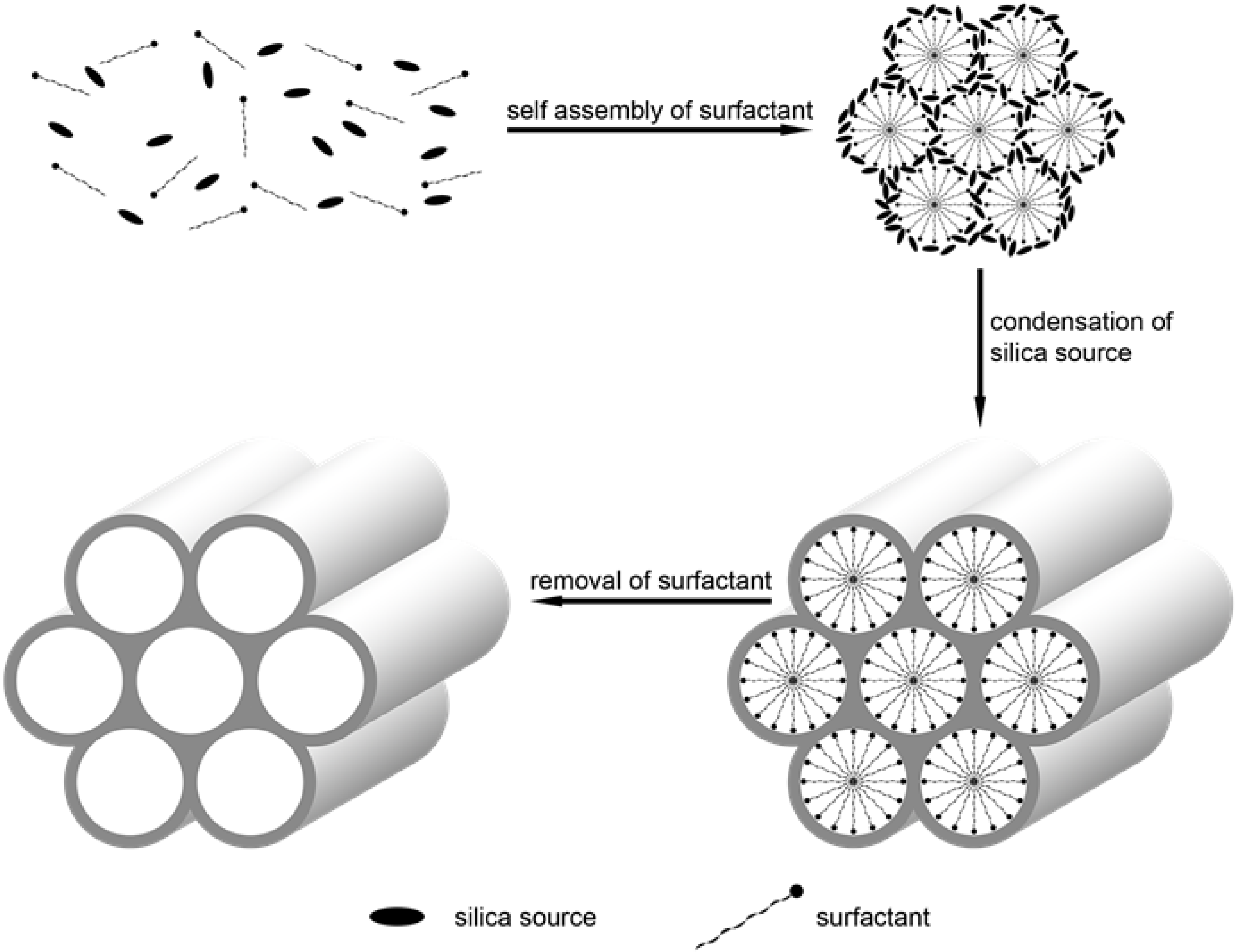
2.2. Synthetic Method of SiNPs
3. Bioimaging
3.1. Traditional Fluorescent Molecular
3.2. Near-Infrared Fluorescent Dye
3.3. Two-Photon Fluorescent Dye
3.4. Multiple Fluorescent Molecules
4. Challenges and Perspective
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Perkel, J.M. Cell signaling: In vivo veritas. Science 2007, 316, 1763–1768. [Google Scholar] [CrossRef]
- Koo, H.; Huh, M.S.; Ryu, J.H.; Lee, D.-E.; Sun, I.-C.; Choi, K.; Kim, K.; Kwon, I.C. Nanoprobes for biomedical imaging in living systems. Nano Today 2011, 6, 204–220. [Google Scholar] [CrossRef]
- Ng, K.K.; Lovell, J.F.; Zheng, G. Lipoprotein-inspired nanoparticles for cancer theranostics. Acc. Chem. Res. 2011, 44, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Cui, Y.; Levenson, R.M.; Chung, L.W.; Nie, S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 2004, 22, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Wolfbeis, O.S. An overview of nanoparticles commonly used in fluorescent bioimaging. Chem. Soc. Rev. 2015, 44, 4743–4768. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Chen, X. Nanoplatforms for targeted molecular imaging in living subjects. Small 2007, 3, 1840–1854. [Google Scholar] [CrossRef] [PubMed]
- Jun, B.H.; Hwang, D.W.; Jung, H.S.; Jang, J.; Kim, H.; Kang, H.; Kang, T.; Kyeong, S.; Lee, H.; Jeong, D.H. Ultrasensitive, biocompatible, quantum-dot-embedded silica nanoparticles for bioimaging. Adv. Funct. Mater. 2012, 22, 1843–1849. [Google Scholar] [CrossRef]
- Salata, O.V. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2004, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballou, B.; Lagerholm, B.C.; Ernst, L.A.; Bruchez, M.P.; Waggoner, A.S. Noninvasive imaging of quantum dots in mice. Bioconjug. Chem. 2004, 15, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Selvan, S.T. Silica-coated quantum dots and magnetic nanoparticles for bioimaging applications (mini-review). Biointerphases 2010, 5, FA110–FA115. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, W.; Tan, W. Bioconjugated silica nanoparticles: Development and applications. Nano Res. 2008, 1, 99–115. [Google Scholar] [CrossRef]
- Sung, M.H.; McNally, J.G. Live cell imaging and systems biology. Wiley Interdiscip. Rev. Syst. Biol. Med. 2011, 3, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Massoud, T.F.; Gambhir, S.S. Molecular imaging in living subjects: Seeing fundamental biological processes in a new light. Genes Dev. 2003, 17, 545–580. [Google Scholar] [CrossRef] [PubMed]
- Koo, V.; Hamilton, P.; Williamson, K. Non-invasive in vivo imaging in small animal research. Anal. Cell. Pathol. 2006, 28, 127–139. [Google Scholar]
- Rosi, N.L.; Mirkin, C.A. Nanostructures in biodiagnostics. Chem. Rev. 2005, 105, 1547–1562. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.W. Fluorescence emission-based detection and diagnosis of malignancy. J. Cell. Biochem. 2002, 87, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Velikov, K.P.; van Blaaderen, A. Synthesis and characterization of monodisperse core-shell colloidal spheres of zinc sulfide and silica. Langmuir 2001, 17, 4779–4786. [Google Scholar] [CrossRef]
- Tan, W.; Wang, K.; He, X.; Zhao, X.J.; Drake, T.; Wang, L.; Bagwe, R.P. Bionanotechnology based on silica nanoparticles. Med.Res. Rev. 2004, 24, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, K.; Santra, S.; Zhao, X.; Hilliard, L.R.; Smith, J.E.; Wu, Y.; Tan, W. Watching silica nanoparticles glow in the biological world. Anal. Chem. 2006, 78, 646–654. [Google Scholar] [CrossRef]
- Santra, S.; Wang, K.; Tapec, R.; Tan, W. Development of novel dye-doped silica nanoparticles for biomarker application. J. Biomed. Opt. 2001, 6, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Mu, Y.; Lu, J.; Wei, W.; Wan, Y.; Liu, S. Target-cell-specific fluorescence silica nanoprobes for imaging and theranostics of cancer cells. Anal. Chem. 2014, 86, 3602–3609. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Shen, X.; Chen, Y.; Zhang, C.; Yan, J.; Yang, H.; Wu, C.; Zeng, H.; Liu, Y. P Polyetherimide-grafted Fe3O4@SiO2 nanoparticles as theranostic agents for simultaneous VEGF siRNA delivery and magnetic resonance cell imaging. Int. J. Nanomed. 2015, 10, 4279–4291. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Estévez, M.C.; Smith, J.E.; Wang, K.; He, X.; Wang, L.; Tan, W. Dye-doped nanoparticles for bioanalysis. Nano Today 2007, 2, 44–50. [Google Scholar] [CrossRef]
- Santra, S.; Tapec, R.; Theodoropoulou, N.; Dobson, J.; Hebard, A.; Tan, W. Synthesis and characterization of silica-coated iron oxide nanoparticles in microemulsion: The effect of nonionic surfactants. Langmuir 2001, 17, 2900–2906. [Google Scholar] [CrossRef]
- Van Blaaderen, A.; Vrij, A. Synthesis and characterization of colloidal dispersions of fluorescent, monodisperse silica spheres. Langmuir 1992, 8, 2921–2931. [Google Scholar] [CrossRef]
- Yamauchi, H.; Ishikawa, T.; Kondo, S. Surface characterization of ultramicro spherical particles of silica prepared by W/O microemulsion method. Colloids Surf. 1989, 37, 71–80. [Google Scholar] [CrossRef]
- Osseo-Asare, K.; Arriagada, F. Preparation of SiO2 nanoparticles in a non-ionic reverse micellar system. Colloids Surf. 1990, 50, 321–339. [Google Scholar] [CrossRef]
- Lindberg, R.; Sjöblom, J.; Sundholm, G. Preparation of silica particles utilizing the sol-gel and the emulsion-gel processes. Colloids Surf. A 1995, 99, 79–88. [Google Scholar] [CrossRef]
- Qhobosheane, M.; Santra, S.; Zhang, P.; Tan, W. Biochemically functionalized silica nanoparticles. Analyst 2001, 126, 1274–1278. [Google Scholar] [CrossRef] [PubMed]
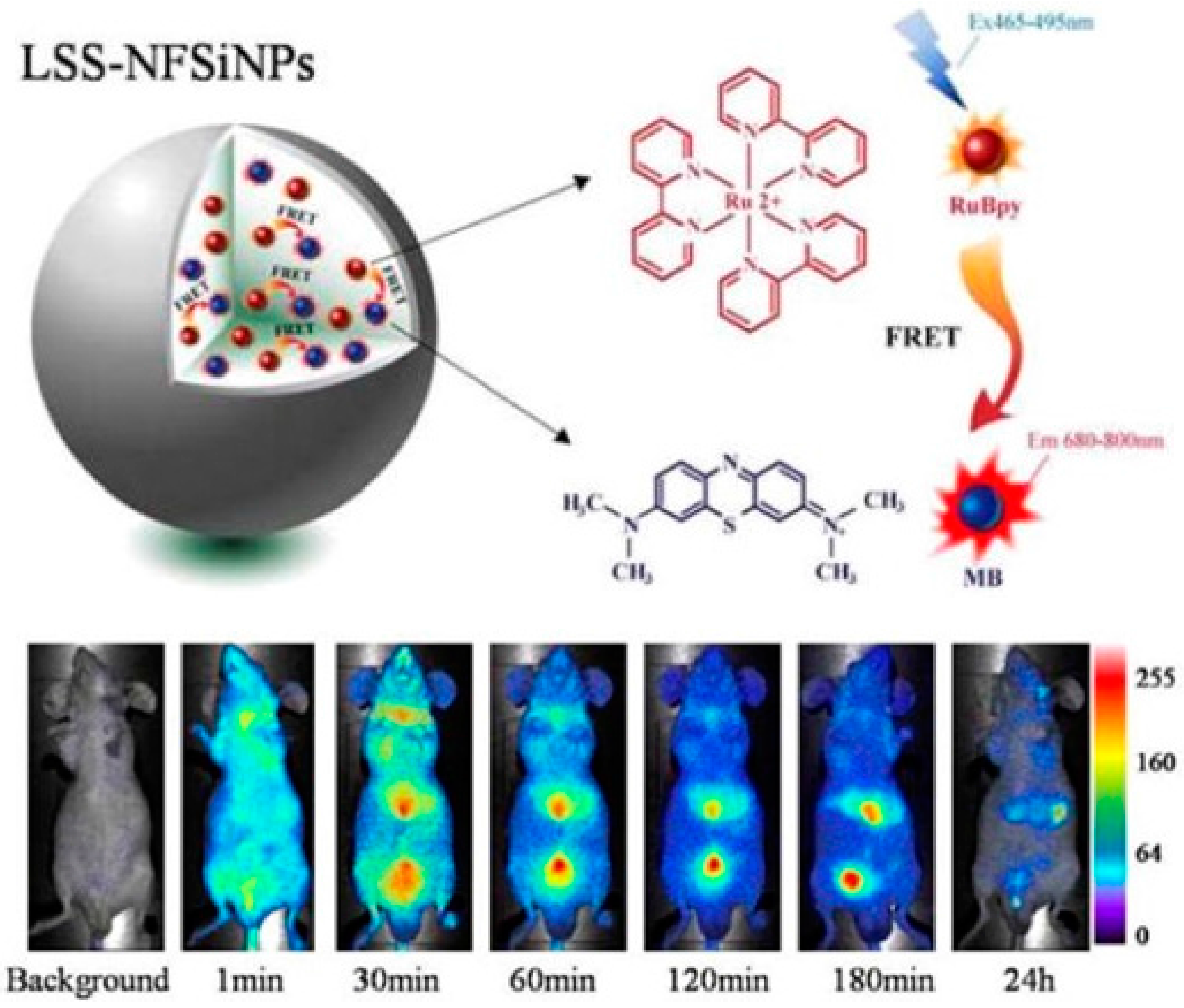
- He, X.; Wang, Y.; Wang, K.; Chen, M.; Chen, S. Fluorescence resonance energy transfer mediated large stokes shifting near-infrared fluorescent silica nanoparticles for in vivo small-animal imaging. Anal. Chem. 2012, 84, 9056–9064. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; He, X.; Wang, K.; Tan, W.; Wang, Y.; Liu, Y. Noninvasive monitoring of intracellular ph change induced by drug stimulation using silica nanoparticle sensors. Anal. Bioanal. Chem. 2007, 388, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Gerion, D.; Pinaud, F.; Williams, S.C.; Parak, W.J.; Zanchet, D.; Weiss, S.; Alivisatos, A.P. Synthesis and properties of biocompatible water-soluble silica-coated CdSe/ZnS semiconductor quantum dots. J. Phys. Chem. B 2001, 105, 8861–8871. [Google Scholar] [CrossRef]
- Zhao, X.; Tapec-Dytioco, R.; Wang, K.; Tan, W. Collection of trace amounts of DNA/mRNA molecules using genomagnetic nanocapturers. Anal. Chem. 2003, 75, 3476–3483. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Kresge, C.; Leonowicz, M.; Roth, W.; Vartuli, J.; Beck, J. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Shimizu, T.; Kuroda, K.; Kato, C. The preparation of alkyltriinethylaininonium–kaneinite complexes and their conversion to microporous materials. Bull. Chem. Soc. Jpn 1990, 63, 988–992. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomedine 2015, 11, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Zhang, Q.; Li, J.; Shen, X.; Mu, C.; Cai, K. Dendrimerlike mesoporous silica nanoparticles as pH-responsive nanocontainers for targeted drug delivery and bioimaging. ACS Appl. Mater. Interfaces 2015, 7, 7357–7372. [Google Scholar] [CrossRef] [PubMed]
- Nigam, P.; Sarkar, D. Multifunctional silica nanoparticles for pancreatic cancer specific drug delivery and bioimaging. J. Chem. Appl. Biochem. 2015, 2, 110–116. [Google Scholar]
- Li, J.; Yao, S.Q. “Singapore green”: A new fluorescent dye for microarray and bioimaging applications. Org. Lett. 2008, 11, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Park, S.; Yoon, J.; Shin, I. Recent progress in the development of near-infrared fluorescent probes for bioimaging applications. Chem. Soc. Rev. 2014, 43, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chan, P.S.; Chan, M.S.; Li, K.F.; Lo, P.K.; Mak, N.K.; Cheah, K.W.; Wong, M.S. Two-photon fluorescence probes for imaging of mitochondria and lysosomes. Chem. Commun. 2013, 49, 3428–3430. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.K.; Heo, C.H.; Choi, D.J.; Sen, D.; Joe, E.-H.; Cho, B.R.; Kim, H.M. A ratiometric two-photon fluorescent probe reveals reduction in mitochondrial H2S production in Parkinson’s disease gene knockout astrocytes. J. Am. Chem. Soc. 2013, 135, 9915–9923. [Google Scholar] [CrossRef] [PubMed]
- Santra, S.; Bagwe, R.P.; Dutta, D.; Stanley, J.T.; Walter, G.A.; Tan, W.; Moudgil, B.M.; Mericle, R.A. Synthesis and characterization of fluorescent, radio-opaque, and paramagnetic silica nanoparticles for multimodal bioimaging applications. Adv. Mater. 2005, 17, 2165–2169. [Google Scholar] [CrossRef]
- Licha, K.; Hessenius, C.; Becker, A.; Henklein, P.; Bauer, M.; Wisniewski, S.; Wiedenmann, B.; Semmler, W. Synthesis, characterization, and biological properties of cyanine-labeled somatostatin analogues as receptor-targeted fluorescent probes. Bioconjug. Chem. 2001, 12, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Li, W.P.; Anderson, C.J.; Kao, J.; Nikiforovich, G.V.; Achilefu, S. Synthesis and characterization of a macrocyclic near-infrared optical scaffold. J. Am. Chem. Soc. 2003, 125, 7766–7767. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Duan, J.; Wang, K.; Tan, W.; Lin, X.; He, C. A novel fluorescent label based on organic dye-doped silica nanoparticles for HepG liver cancer cell recognition. J. Nanosci. Nanotechnol. 2004, 4, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Bagwe, R.P.; Yang, C.; Hilliard, L.R.; Tan, W. Optimization of dye-doped silica nanoparticles prepared using a reverse microemulsion method. Langmuir 2004, 20, 8336–8342. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Bagwe, R.P.; Tan, W. Development of organic-dye-doped silica nanoparticles in a reverse microemulsion. Adv. Mater. 2004, 16, 173–176. [Google Scholar] [CrossRef]
- Gunawardana, K.B.; Green, N.S.; Bumm, L.A.; Halterman, R.L. Metal-enhanced fluorescence of dye-doped silica nano particles. J. Fluoresc. 2015, 25, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Aslan, K.; Gryczynski, I.; Malicka, J.; Matveeva, E.; Lakowicz, J.R.; Geddes, C.D. Metal-enhanced fluorescence: An emerging tool in biotechnology. Curr. Opin. Biotechnol. 2005, 16, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Dragan, A.I.; Bishop, E.S.; Casas-Finet, J.R.; Strouse, R.J.; McGivney, J.; Schenerman, M.A.; Geddes, C.D. Distance dependence of metal-enhanced fluorescence. Plasmonics 2012, 7, 739–744. [Google Scholar] [CrossRef]
- Jo, H.; Her, J.; Ban, C. Dual aptamer-functionalized silica nanoparticles for the highly sensitive detection of breast cancer. Biosens. Bioelectron. 2015, 71, 129–136. [Google Scholar] [CrossRef] [PubMed]
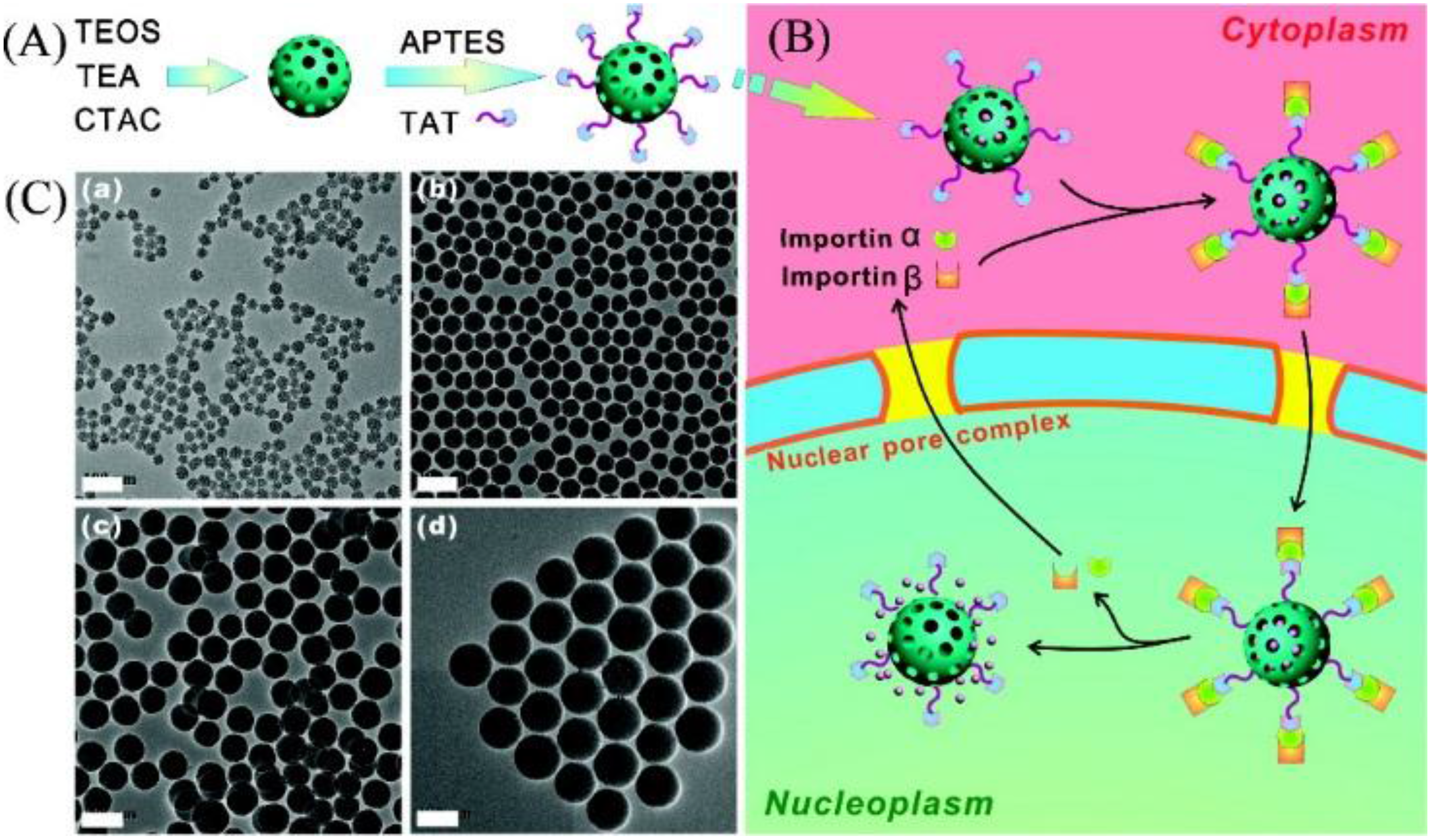
- Pan, L.; He, Q.; Liu, J.; Chen, Y.; Ma, M.; Zhang, L.; Shi, J. Nuclear-targeted drug delivery of TAT peptide-conjugated monodisperse mesoporous silica nanoparticles. J. Am. Chem. Soc. 2012, 134, 5722–5725. [Google Scholar] [CrossRef] [PubMed]
- Nakielny, S.; Dreyfuss, G. Transport of proteins and rnas in and out of the nucleus. Cell 1999, 99, 677–690. [Google Scholar] [CrossRef]
- Patel, S.S.; Belmont, B.J.; Sante, J.M.; Rexach, M.F. Natively unfolded nucleoporins gate protein diffusion across the nuclear pore complex. Cell 2007, 129, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Mackey, M.A.; El-Sayed, M.A. Nuclear targeting of gold nanoparticles in cancer cells induces DNA damage, causing cytokinesis arrest and apoptosis. J. Am. Chem. Soc. 2010, 132, 1517–1519. [Google Scholar] [CrossRef] [PubMed]
- Kubitscheck, U.; Grünwald, D.; Hoekstra, A.; Rohleder, D.; Kues, T.; Siebrasse, J.P.; Peters, R. Nuclear transport of single molecules dwell times at the nuclear pore complex. J. Cell Biol. 2005, 168, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Jo, C.; Lee, H.J.; Oh, M. One-pot synthesis of silica@coordination polymer core–shell microspheres with controlled shell thickness. Adv. Mater. 2011, 23, 1716–1719. [Google Scholar] [CrossRef] [PubMed]
- Kreno, L.E.; Hupp, J.T.; van Duyne, R.P. Metal−organic framework thin film for enhanced localized surface plasmon resonance gas sensing. Anal. Chem. 2010, 82, 8042–8046. [Google Scholar] [CrossRef] [PubMed]
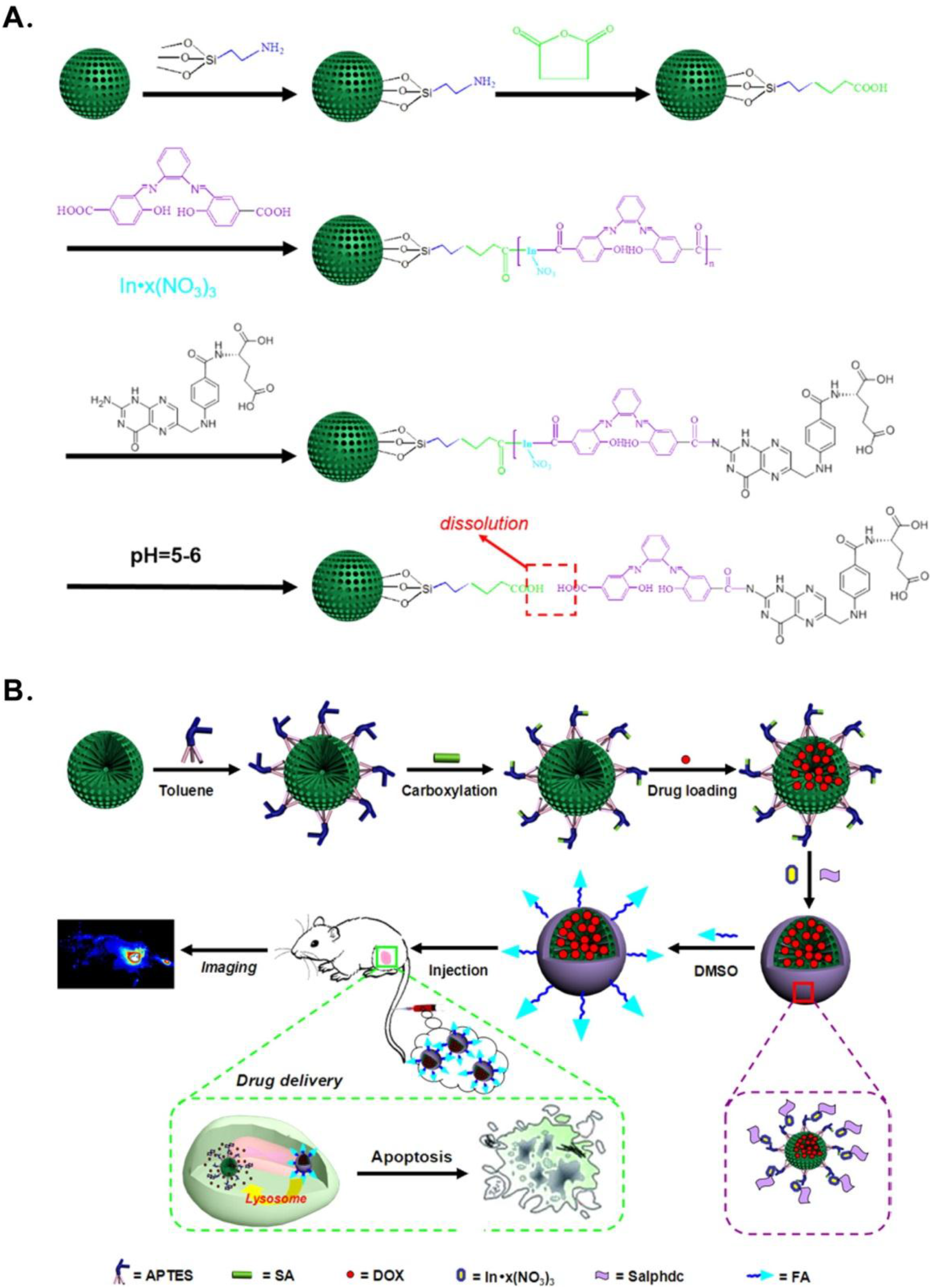
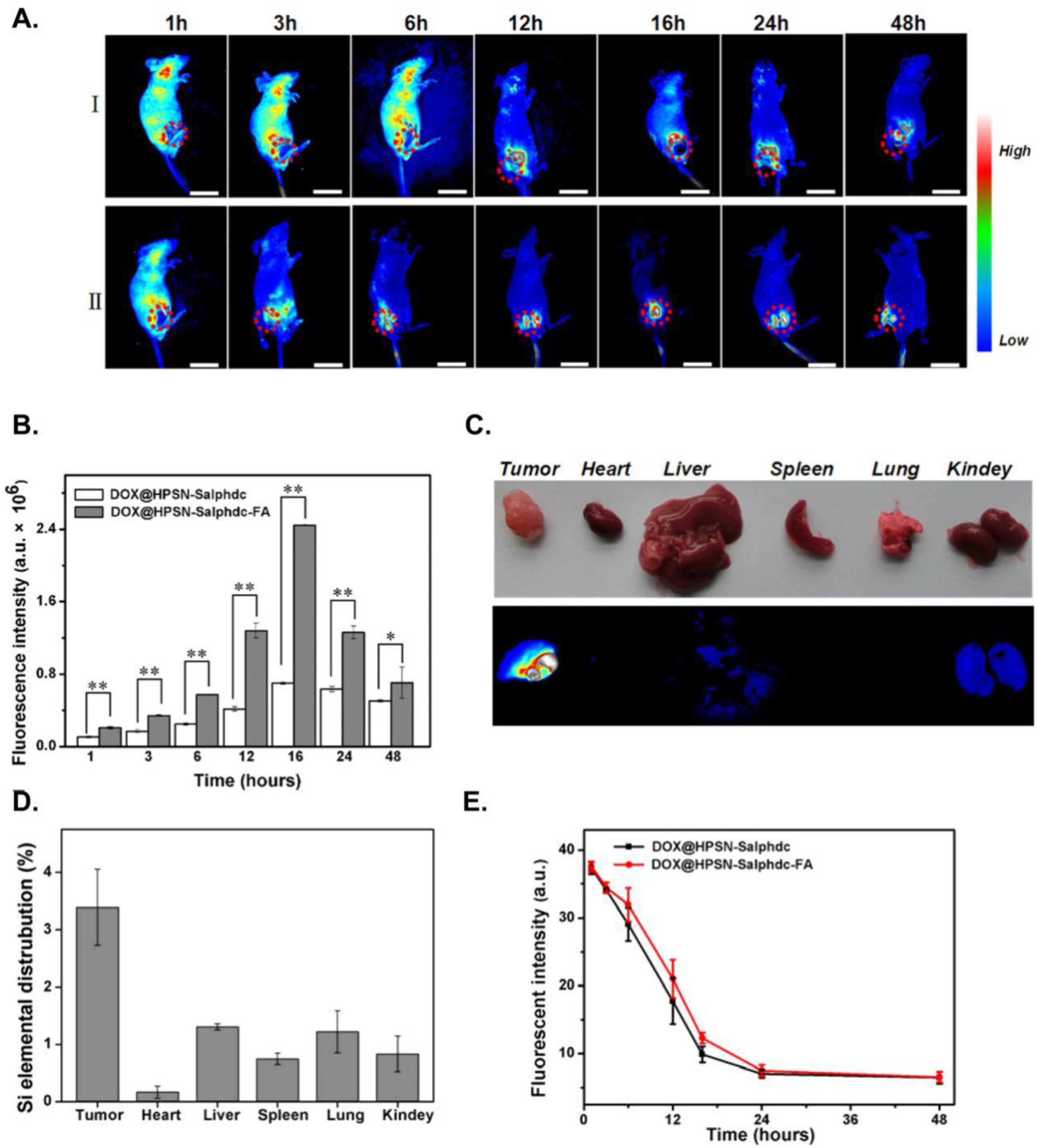
- Sudimack, J.; Lee, R.J. Targeted drug delivery via the folate receptor. Adv. Drug Del. Rev. 2000, 41, 147–162. [Google Scholar] [CrossRef]
- Jang, H.; Lee, C.; Nam, G.-E.; Quan, B.; Choi, H.J.; Yoo, J.S.; Piao, Y. In vivo magnetic resonance and fluorescence dual imaging of tumor sites by using dye-doped silica-coated iron oxide nanoparticles. J. Nanopart. Res. 2016, 18, 1–11. [Google Scholar] [CrossRef]
- Thorek, D.L.; Ulmert, D.; Diop, N.-F.M.; Lupu, M.E.; Doran, M.G.; Huang, R.; Abou, D.S.; Larson, S.M.; Grimm, J. Non-invasive mapping of deep-tissue lymph nodes in live animals using a multimodal PET/MRI nanoparticle. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wu, X.; Wang, K.; Shi, B.; Hai, L. Methylene blue-encapsulated phosphonate-terminated silica nanoparticles for simultaneous in vivo imaging and photodynamic therapy. Biomaterials 2009, 30, 5601–5609. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Shi, H.; Li, Z.; Shen, H.; Ma, K.; Li, B.; Shen, S.; Jin, Y. A multifunctional mesoporous silica nanocomposite for targeted delivery, controlled release of doxorubicin and bioimaging. Colloids Surf. B 2013, 110, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Wallrabe, H.; Periasamy, A. Imaging protein molecules using FRET and FLIM microscopy. Curr. Opin. Biotechnol. 2005, 16, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Förster, T. Zwischenmolekulare energiewanderung und fluoreszenz. Ann. Phys. 1948, 437, 55–75. [Google Scholar] [CrossRef]
- Jang, E.S.; Lee, S.Y.; Cha, E.-J.; Sun, I.-C.; Kwon, I.C.; Kim, D.; Kim, Y.I.; Kim, K.; Ahn, C.-H. Fluorescent dye labeled iron oxide/silica core/shell nanoparticle as a multimodal imaging probe. Pharm. Res. 2014, 31, 3371–3378. [Google Scholar] [CrossRef] [PubMed]
- Sathe, T.R.; Agrawal, A.; Nie, S. Mesoporous silica beads embedded with semiconductor quantum dots and iron oxide nanocrystals: Dual-function microcarriers for optical encoding and magnetic separation. Anal. Chem. 2006, 78, 5627–5632. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, B.; Dong, S. Bifunctional nanostructure of magnetic core luminescent shell and its application as solid-state electrochemiluminescence sensor material. J. Phys. Chem. B 2007, 111, 10448–10452. [Google Scholar] [CrossRef] [PubMed]
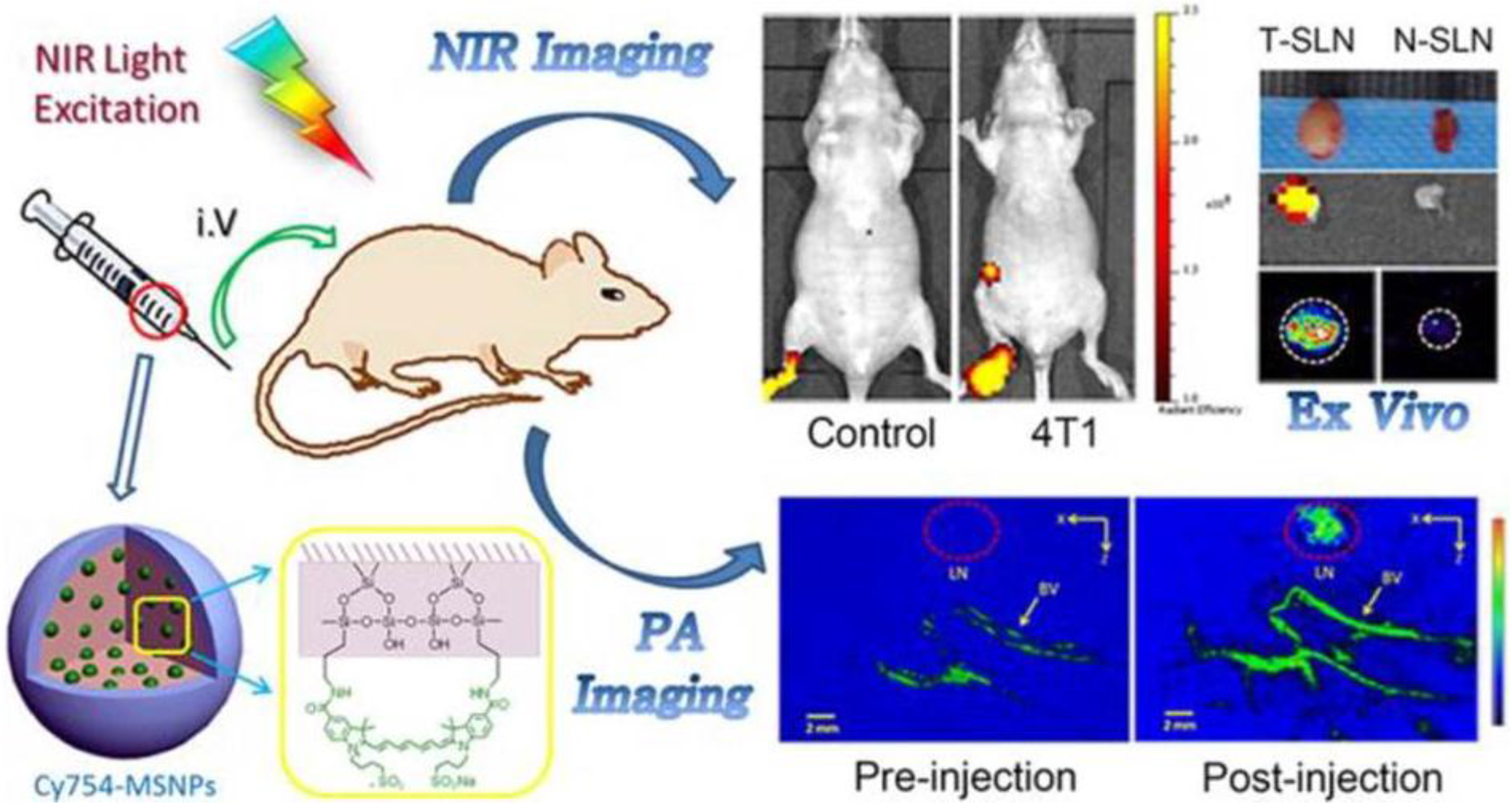
- Liu, Z.; Rong, P.; Yu, L.; Zhang, X.; Yang, C.; Guo, F.; Zhao, Y.; Zhou, K.; Wang, W.; Zeng, W. Dual-modality noninvasive mapping of sentinel lymph node by photoacoustic and near-infrared fluorescent imaging using dye-loaded mesoporous silica nanoparticles. Mol. Pharm. 2015, 12, 3119–3128. [Google Scholar] [CrossRef] [PubMed]
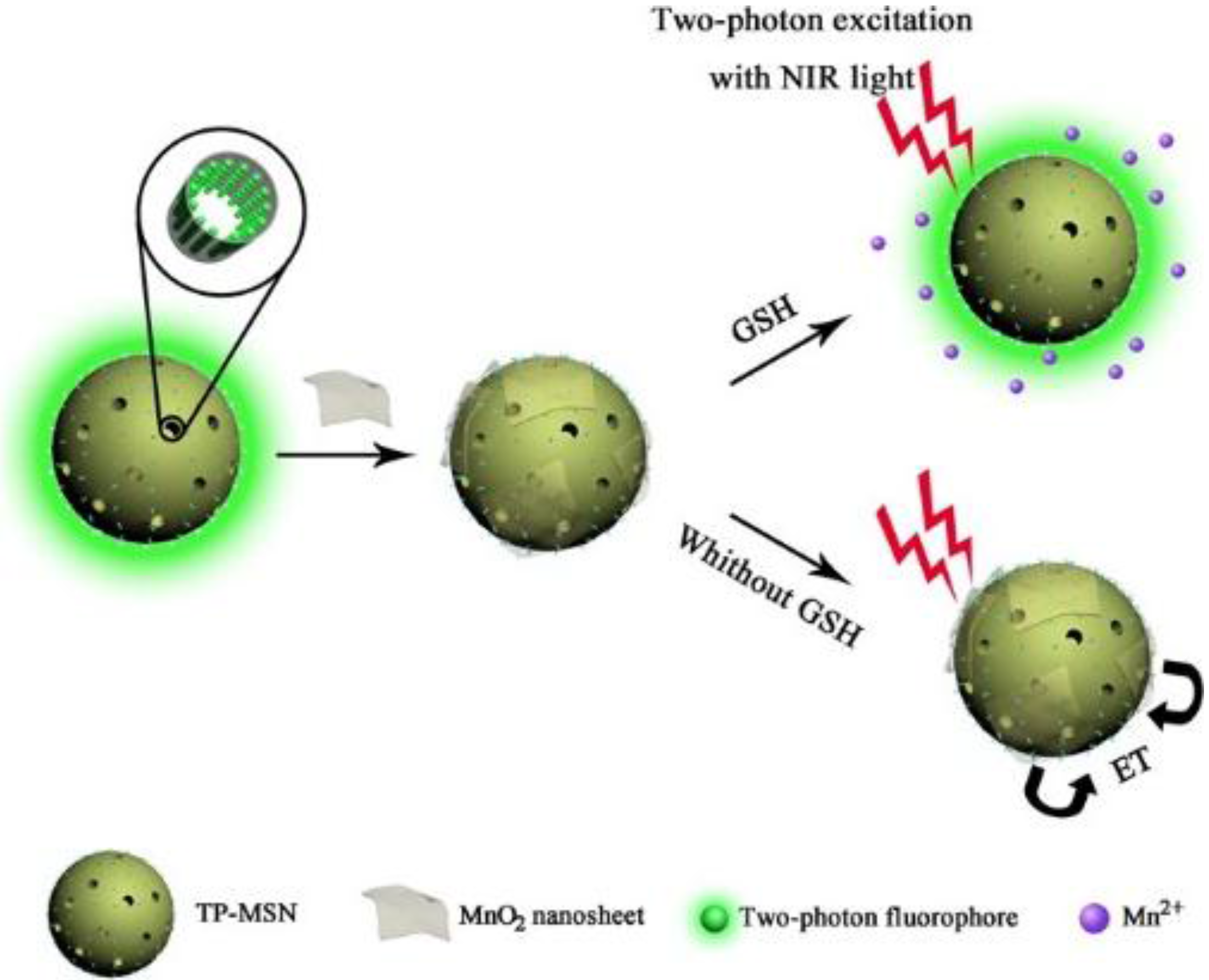
- Meng, H.-M.; Jin, Z.; Lv, Y.; Yang, C.; Zhang, X.-B.; Tan, W.; Yu, R.-Q. Activatable two-photon fluorescence nanoprobe for bioimaging of glutathione in living cells and tissues. Anal. Chem. 2014, 86, 12321–12326. [Google Scholar] [CrossRef] [PubMed]
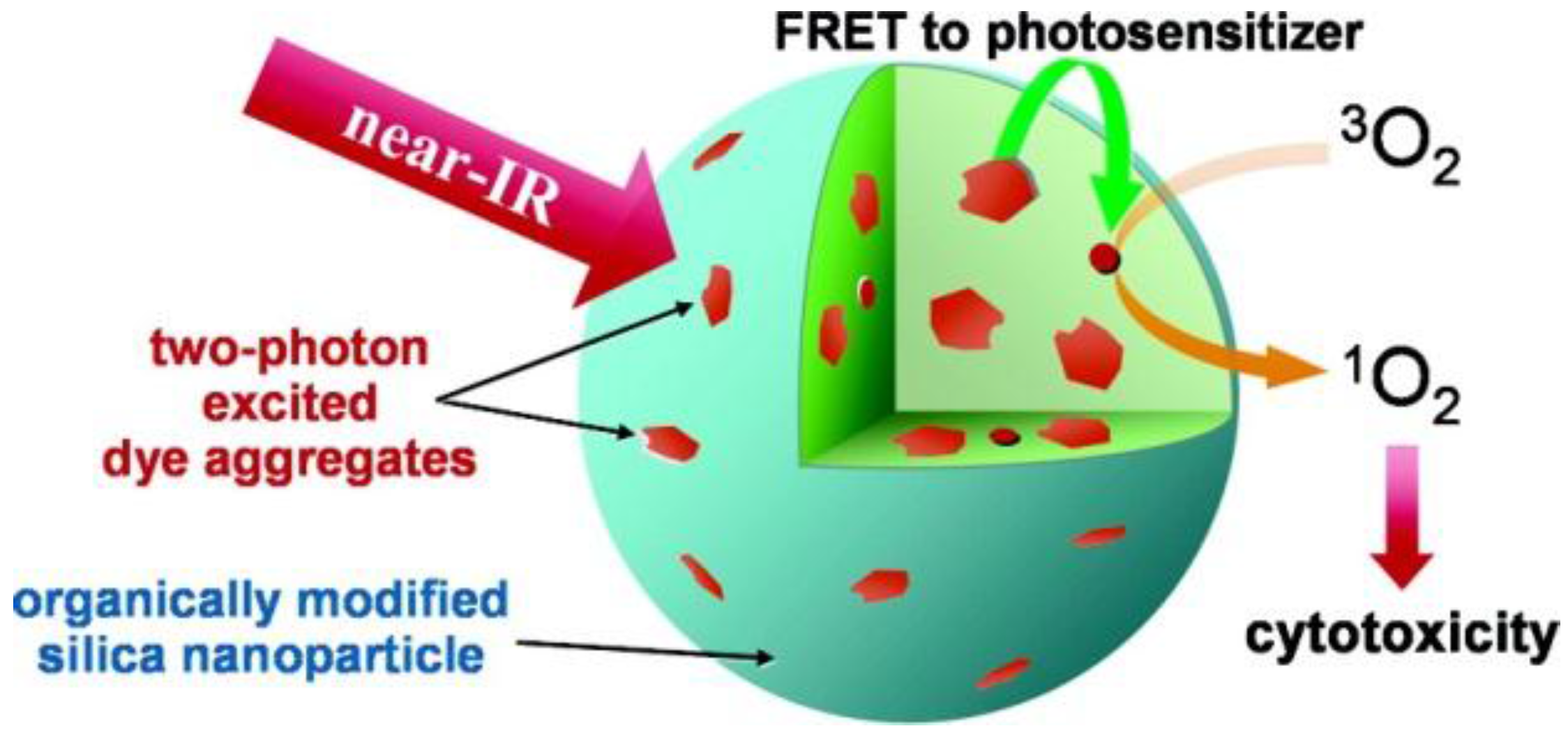
- Kim, S.; Ohulchanskyy, T.Y.; Pudavar, H.E.; Pandey, R.K.; Prasad, P.N. Organically modified silica nanoparticles co-encapsulating photosensitizing drug and aggregation-enhanced two-photon absorbing fluorescent dye aggregates for two-photon photodynamic therapy. J. Am. Chem. Soc. 2007, 129, 2669–2675. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.W.; Wang, Y.H.; Lai, C.H.; Yang, M.J.; Chen, C.Y.; Chou, P.T.; Chan, C.S.; Chi, Y.; Chen, Y.C.; Hsiao, J.K. Iridium-complex-functionalized Fe3O4/SiO2 core/shell nanoparticles: A facile three-in-one system in magnetic resonance imaging, luminescence imaging, and photodynamic therapy. Small 2008, 4, 218–224. [Google Scholar] [CrossRef] [PubMed]
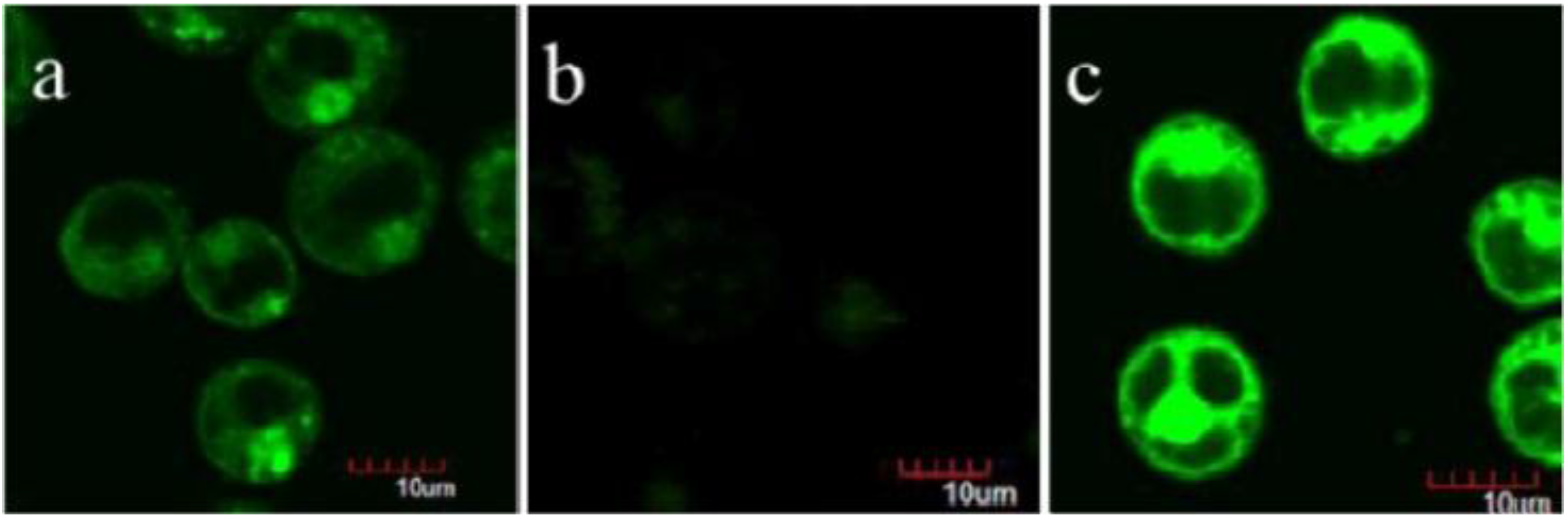
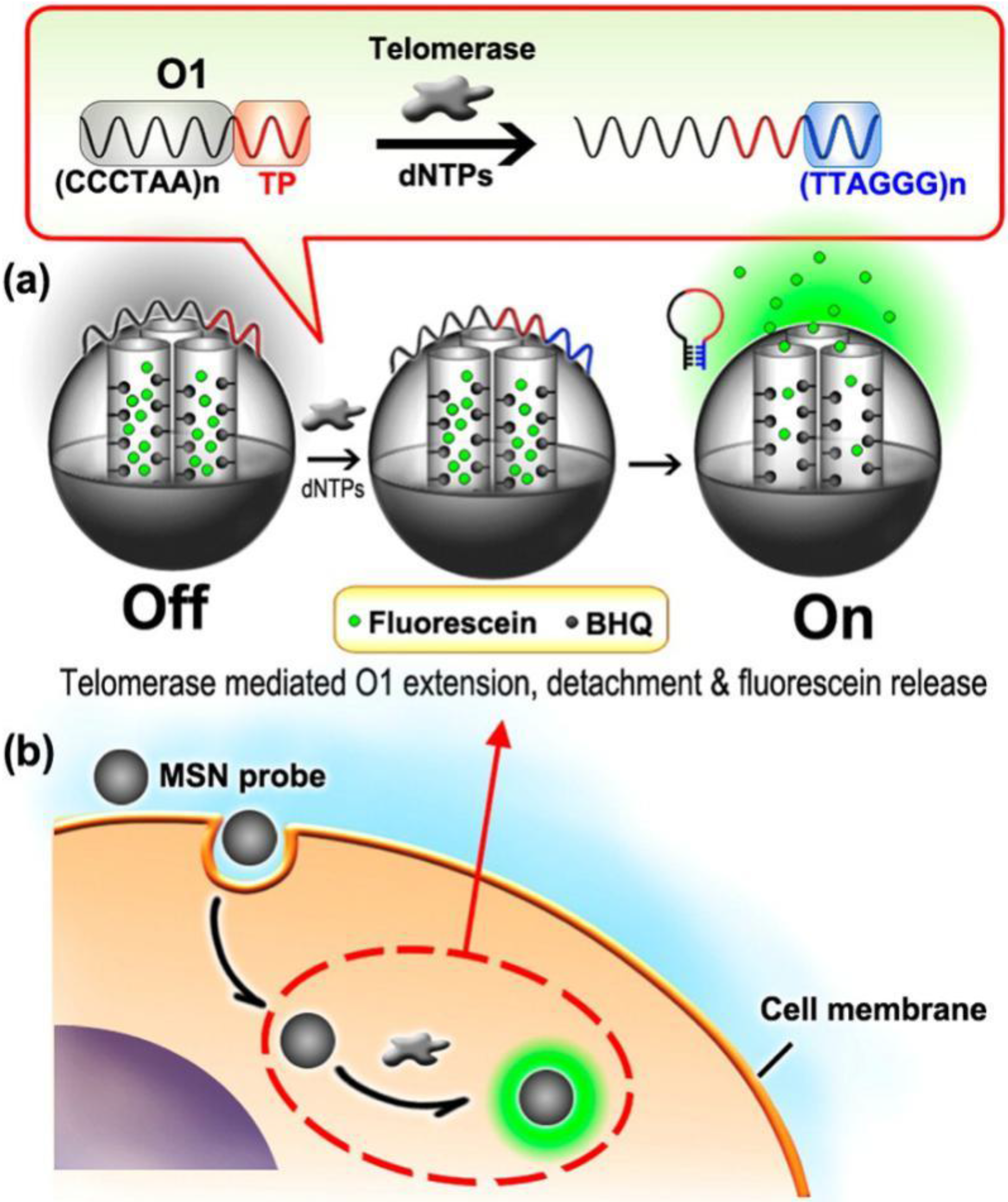
- Qian, R.; Ding, L.; Ju, H. Switchable fluorescent imaging of intracellular telomerase activity using telomerase-responsive mesoporous silica nanoparticle. J. Am. Chem. Soc. 2013, 135, 13282–13285. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.; Sebastian, R.; Rodrigues, A.; Fernandes, F.; Baleizão, C.; Farinha, J. New visible and nir highly photostable fluorescent silica nanoparticles for laser scanning imaging applications. Microsc. Microanal. 2013, 19, 105–106. [Google Scholar] [CrossRef]
- Wang, L.; Tan, W. Multicolor fret silica nanoparticles by single wavelength excitation. Nano Lett. 2006, 6, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Biffi, S.; Petrizza, L.; Rampazzo, E.; Voltan, R.; Sgarzi, M.; Garrovo, C.; Prodi, L.; Andolfi, L.; Agnoletto, C.; Zauli, G. Multiple dye-doped NIR-emitting silica nanoparticles for both flow cytometry and in vivo imaging. RSC Adv. 2014, 4, 18278–18285. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.-H.; Hu, X.-X.; Zhang, X.-B. Dye-Doped Fluorescent Silica Nanoparticles for Live Cell and In Vivo Bioimaging. Nanomaterials 2016, 6, 81. https://doi.org/10.3390/nano6050081
Zhang W-H, Hu X-X, Zhang X-B. Dye-Doped Fluorescent Silica Nanoparticles for Live Cell and In Vivo Bioimaging. Nanomaterials. 2016; 6(5):81. https://doi.org/10.3390/nano6050081
Chicago/Turabian StyleZhang, Wen-Han, Xiao-Xiao Hu, and Xiao-Bing Zhang. 2016. "Dye-Doped Fluorescent Silica Nanoparticles for Live Cell and In Vivo Bioimaging" Nanomaterials 6, no. 5: 81. https://doi.org/10.3390/nano6050081




