Challenges in Creating Evidence in Environmental Health Risk Assessments: The Example of Second-Hand Smoke
Abstract
:1. Introduction
2. Challenges in Creating Evidence in Environmental Health Risk Assessments
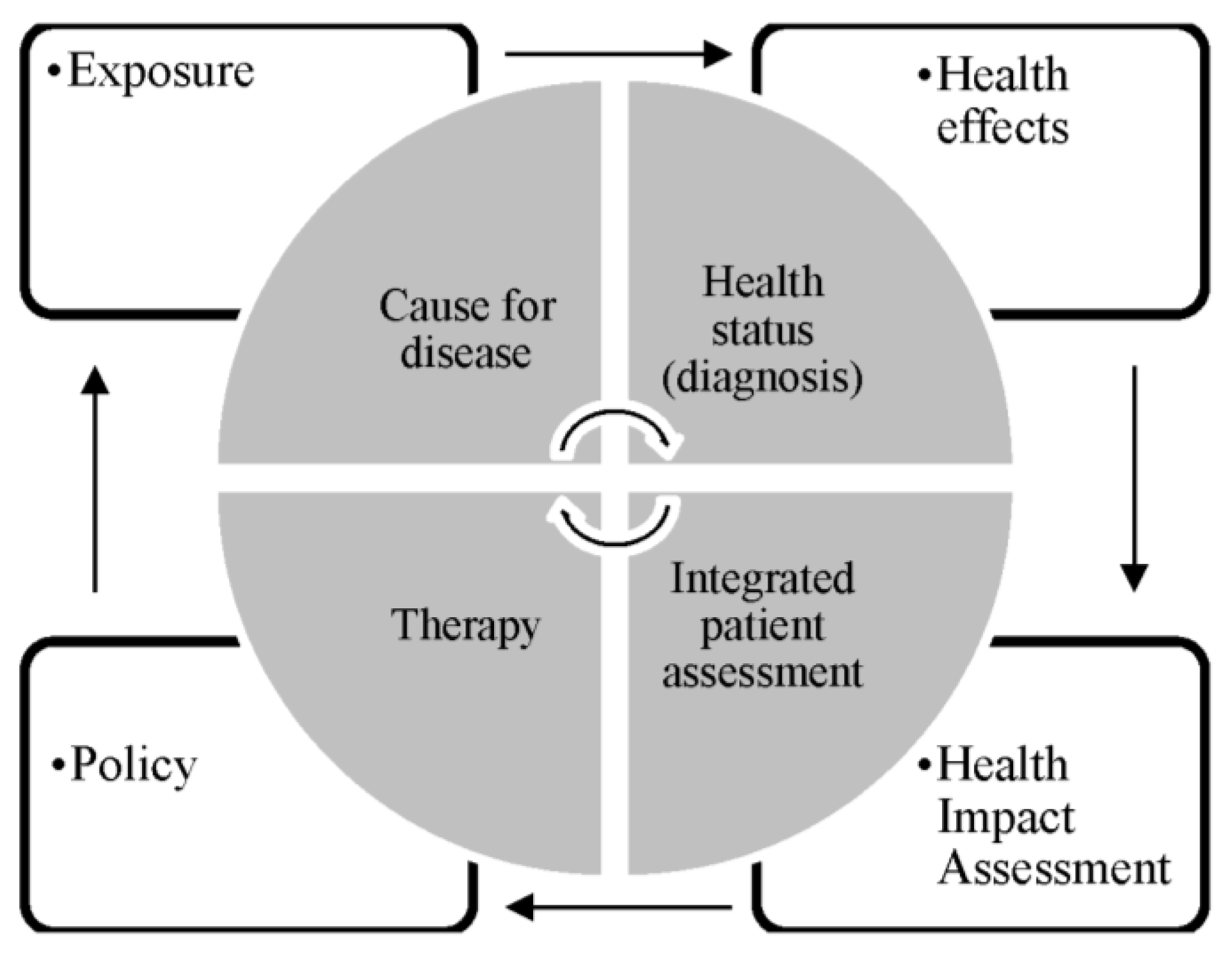
2.1. Selection of Study Design
2.2. Key Sources of Exposure
2.3. Exposure Assessment Method
2.4. Stratification of Results, Bias and Confounding
3. Discussion
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Attena, F. Complexity and indeterminism of evidence-based public health: An analytical framework. Med. Health Care Philos. 2014, 17, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Sackett, D.L.; Rosenberg, W.M.; Gray, J.A.; Haynes, R.B.; Richardson, W.S. Evidence based medicine: What it is and what it isn’t. BMJ 1996, 312, 71–72. [Google Scholar] [CrossRef] [PubMed]
- Victora, C.G.; Habicht, J.P.; Bryce, J. Evidence-based public health: Moving beyond randomized trials. Am. J. Public Health 2004, 94, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Rehfuess, E.A.; Bartram, J. Beyond direct impact: Evidence synthesis towards a better understanding of effectiveness of environmental health interventions. Int. J. Hyg. Environ. Health 2014, 217, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Brownson, R.C.; Fielding, J.E.; Maylahn, C.M. Evidence-based public health: A fundamental concept for public health practice. Annu. Rev. Public Health 2009, 30, 175–201. [Google Scholar] [CrossRef] [PubMed]
- Rychetnik, L.; Frommer, M.; Hawe, P.; Shiell, A. Criteria for evaluating evidence on public health interventions. J. Epidemiol. Commun. Health 2002, 56, 119–127. [Google Scholar] [CrossRef]
- Rychetnik, L.; Hawe, P.; Waters, E.; Barratt, A.; Frommer, M. A glossary for evidence based public health. J. Epidemiol. Commun. Health 2004, 58, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Rushton, L.; Elliott, P. Evaluating evidence on environmental health risks. Br. Med. Bull. 2003, 68, 113–128. [Google Scholar] [CrossRef] [PubMed]
- WHO. Evaluation and use of epidemiological evidence for environmental health risk assessment: Who guideline document. Environ. Health Perspect. 2000, 108, 997–1002. [Google Scholar]
- Künzli, N.; Perez, L. Evidence based public health—The example of air pollution. Swiss Med. Wkly. 2009, 139, 242–250. [Google Scholar] [PubMed]
- Leone, A.; Giannini, D.; Bellotto, C.; Balbarini, A. Passive smoking and coronary heart disease. Curr. Vasc. Pharmacol. 2004, 2, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.E.; Blakely, T.; Kawachi, I.; Woodward, A. Mortality among lifelong nonsmokers exposed to secondhand smoke at home: Cohort data and sensitivity analyses. Am. J. Epidemiol. 2007, 165, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Lightwood, J.M.; Glantz, S.A. Declines in acute myocardial infarction after smoke-free laws and individual risk attributable to secondhand smoke. Circulation 2009, 120, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Tunstall-Pedoe, H.; Brown, C.A.; Woodward, M.; Tavendale, R. Passive smoking by self report and serum cotinine and the prevalence of respiratory and coronary heart disease in the scottish heart health study. J. Epidemiol. Commun. Health 1995, 49, 139–143. [Google Scholar] [CrossRef]
- Steenland, K.; Thun, M.; Lally, C.; Heath, C. Environmental tobacco smoke and coronary heart disease in the American Cancer Society CPS-II cohort. Circulation 1996, 94, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Jousilahti, P.; Patja, K.; Salomaa, V. Environmental tobacco smoke and the risk of cardiovascular disease. Scand. J. Work Environ. Health 2002, 28, 41–51. [Google Scholar] [PubMed]
- Pitsavos, C.; Panagiotakos, D.B.; Chrysohoou, C.; Tzioumis, K.; Papaioannou, I.; Stefanadis, C.; Toutouzas, P. Association between passive cigarette smoking and the risk of developing acute coronary syndromes: The cardio2000 study. Heart Vessels 2002, 16, 127–130. [Google Scholar] [PubMed]
- Steenland, K. Passive smoking and the risk of heart disease. JAMA 1992, 267, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Glantz, S.A.; Parmley, W.W. Passive smoking and heart disease. Epidemiology, physiology, and biochemistry. Circulation 1991, 83, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Enstrom, J.E.; Kabat, G.C. Environmental tobacco smoke and coronary heart disease mortality in the united states—A meta-analysis and critique. Inhal. Toxicol. 2006, 18, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Bonita, R.; Duncan, J.; Truelsen, T.; Jackson, R.T.; Beaglehole, R. Passive smoking as well as active smoking increases the risk of acute stroke. Tob. Control 1999, 8, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Avila-Tang, E.; Elf, J.L.; Cummings, K.M.; Fong, G.T.; Hovell, M.F.; Klein, J.D.; McMillen, R.; Winickoff, J.P.; Samet, J.M. Assessing secondhand smoke exposure with reported measures. Tob. Control 2013, 22, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Bentayeb, M.; Simoni, M.; Norback, D.; Baldacci, S.; Maio, S.; Viegi, G.; Annesi-Maesano, I. Indoor air pollution and respiratory health in the elderly. J. Environ. Sci. Health A 2013, 48, 1783–1789. [Google Scholar] [CrossRef] [PubMed]
- Barnoya, J.; Glantz, S.A. Cardiovascular effects of secondhand smoke: Nearly as large as smoking. Circulation 2005, 111, 2684–2698. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Vupputuri, S.; Allen, K.; Prerost, M.R.; Hughes, J.; Whelton, P.K. Passive smoking and the risk of coronary heart disease—A meta-analysis of epidemiologic studies. N. Engl. J. Med. 1999, 340, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Rostron, B. Mortality risks associated with environmental tobacco smoke exposure in the United States. Nicot. Tob. Res. 2013, 15, 1722–1728. [Google Scholar] [CrossRef] [PubMed]
- Ahijevych, K.; Wewers, M.E. Passive smoking and vascular disease. J. Cardiovasc. Nurs. 2003, 18, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Pron, G.E.; Burch, J.D.; Howe, G.R.; Miller, A.B. The reliability of passive smoking histories reported in a case-control study of lung cancer. Am. J. Epidemiol. 1988, 127, 267–273. [Google Scholar] [PubMed]
- Thun, M.; Henley, J.; Apicella, L. Epidemiologic studies of fatal and nonfatal cardiovascular disease and ETS exposure from spousal smoking. Environ. Health Perspect. 1999, 107 (Suppl. S6), 841–846. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Cohen, A.; Dolor, R.; Coffman, C.J.; Bastian, L.A. The impact of environmental tobacco smoke on women’s risk of dying from heart disease: A meta-analysis. J. Women’s Health 2004, 13, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Oono, I.P.; Mackay, D.F.; Pell, J.P. Meta-analysis of the association between secondhand smoke exposure and stroke. J. Public Health 2011, 33, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Brownson, R.C.; Alavanja, M.C.; Hock, E.T. Reliability of passive smoke exposure histories in a case-control study of lung cancer. Int. J. Epidemiol. 1993, 22, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Eisner, M.D.; Balmes, J.; Katz, P.P.; Trupin, L.; Yelin, E.H.; Blanc, P.D. Lifetime environmental tobacco smoke exposure and the risk of chronic obstructive pulmonary disease. Environ. Health 2005, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emmons, K.M.; Abrams, D.B.; Marshall, R.; Marcus, B.H.; Kane, M.; Novotny, T.E.; Etzel, R.A. An evaluation of the relationship between self-report and biochemical measures of environmental tobacco smoke exposure. Prev. Med. 1994, 23, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Coultas, D.B.; Peake, G.T.; Samet, J.M. Questionnaire assessment of lifetime and recent exposure to environmental tobacco smoke. Am. J. Epidemiol. 1989, 130, 338–347. [Google Scholar] [PubMed]
- Hammond, S.K.; Leaderer, B.P. A diffusion monitor to measure exposure to passive smoking. Environ. Sci. Technol. 1987, 21, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Jefferis, B.J.; Lawlor, D.A.; Ebrahim, S.; Wannamethee, S.G.; Feyerabend, C.; Doig, M.; McMeekin, L.; Cook, D.G.; Whincup, P.H. Cotinine-assessed second-hand smoke exposure and risk of cardiovascular disease in older adults. Heart 2010, 96, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Lightwood, J.M.; Coxson, P.G.; Bibbins-Domingo, K.; Williams, L.W.; Goldman, L. Coronary heart disease attributable to passive smoking: CHD policy model. Am. J. Prev. Med. 2009, 36, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Iribarren, C.; Darbinian, J.; Klatsky, A.L.; Friedman, G.D. Cohort study of exposure to environmental tobacco smoke and risk of first ischemic stroke and transient ischemic attack. Neuroepidemiology 2004, 23, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Whincup, P.H.; Gilg, J.A.; Emberson, J.R.; Jarvis, M.J.; Feyerabend, C.; Bryant, A.; Walker, M.; Cook, D.G. Passive smoking and risk of coronary heart disease and stroke: Prospective study with cotinine measurement. BMJ 2004, 329, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Misailidi, M.; Tzatzarakis, M.N.; Kavvalakis, M.P.; Koutedakis, Y.; Tsatsakis, A.M.; Flouris, A.D. Instruments to assess secondhand smoke exposure in large cohorts of never smokers: The smoke scales. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, M.J.; Tunstall-Pedoe, H.; Feyerabend, C.; Vesey, C.; Saloojee, Y. Comparison of tests used to distinguish smokers from nonsmokers. Am. J. Public Health 1987, 77, 1435–1438. [Google Scholar] [CrossRef] [PubMed]
- Bernert, J.T., Jr.; McGuffey, J.E.; Morrison, M.A.; Pirkle, J.L. Comparison of serum and salivary cotinine measurements by a sensitive high-performance liquid chromatography-tandem mass spectrometry method as an indicator of exposure to tobacco smoke among smokers and nonsmokers. J. Anal. Toxicol. 2000, 24, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L.; Kuyt, F.; Jacob, P., III; Jones, R.T.; Osman, A.L. Cotinine disposition and effects. Clin. Pharmacol. Ther. 1983, 34, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Nondahl, D.M.; Cruickshanks, K.J.; Schubert, C.R. A questionnaire for assessing environmental tobacco smoke exposure. Environ. Res. 2005, 97, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Hawamdeh, A.; Kasasbeh, F.A.; Ahmad, M.A. Effects of passive smoking on children’s health: A review. East. Mediterr. Health J. 2003, 9, 441–447. [Google Scholar] [PubMed]
- Jarvis, M.; Tunstall-Pedoe, H.; Feyerabend, C.; Vesey, C.; Salloojee, Y. Biochemical markers of smoke absorption and self reported exposure to passive smoking. J. Epidemiol. Commun. Health 1984, 38, 335–339. [Google Scholar] [CrossRef]
- Jarvis, M.J.; Russell, M.A.; Benowitz, N.L.; Feyerabend, C. Elimination of cotinine from body fluids: Implications for noninvasive measurement of tobacco smoke exposure. Am. J. Public Health 1988, 78, 696–698. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Tavendale, R.; Tunstall-Pedoe, H. Environmental tobacco smoke and prevalent coronary heart disease among never smokers in the scottish monica surveys. Occup. Environ. Med. 2004, 61, 790–792. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.A.; la Vecchia, C.; Levi, F. The antiestrogenic effect of cigarette smoking in women. Am. J. Obstet. Gynecol. 1990, 162, 502–514. [Google Scholar] [CrossRef]
- Geisler, J.; Omsjo, I.H.; Helle, S.I.; Ekse, D.; Silsand, T.; Lonning, P.E. Plasma oestrogen fractions in postmenopausal women receiving hormone replacement therapy: Influence of route of administration and cigarette smoking. J. Endocrinol. 1999, 162, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.C.; Signorello, L.B.; Lipworth, L.; Lagiou, P.; Mantzoros, C.S.; Trichopoulos, D. Predictors of sex hormone levels among the elderly: A study in greece. J. Clin. Epidemiol. 1998, 51, 837–841. [Google Scholar] [CrossRef]
- Bolego, C.; Poli, A.; Paoletti, R. Smoking and gender. Cardiovasc. Res. 2002, 53, 568–576. [Google Scholar] [CrossRef]
- McElduff, P.; Dobson, A.J.; Jackson, R.; Beaglehole, R.; Heller, R.F.; Lay-Yee, R. Coronary events and exposure to environmental tobacco smoke: A case-control study from australia and new zealand. Tob. Control 1998, 7, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, A.; Gotsis, W.; Frishman, W. Second-hand tobacco smoke and cardiovascular disease risk: An epidemiological review. Cardiol. Rev. 2013, 21, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Fung, W.H.; Zhang, Q.; Yip, G.W.K.; Chan, C.K.; Yu, C.M. Effect of household passive smoking exposure on the risk of ischaemic heart disease in never-smoke female patients in hong kong. Tob. Control 2009, 18, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Iribarren, C.; Friedman, G.D.; Klatsky, A.L.; Eisner, M.D. Exposure to environmental tobacco smoke: Association with personal characteristics and self reported health conditions. J. Epidemiol. Commun. Health 2001, 55, 721–728. [Google Scholar] [CrossRef]
- Johannessen, A.; Bakke, P.S.; Hardie, J.A.; Eagan, T.M. Association of exposure to environmental tobacco smoke in childhood with chronic obstructive pulmonary disease and respiratory symptoms in adults. Respirology 2012, 17, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Coultas, D.B. Passive smoking and risk of adult asthma and copd: An update. Thorax 1998, 53, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Enstrom, J.E.; Kabat, G.C. Environmental tobacco smoke and tobacco related mortality in a prospective study of californians, 1960–98. BMJ 2003, 326, 1057. [Google Scholar] [CrossRef] [PubMed]
- Reardon, J.Z. Environmental tobacco smoke: Respiratory and other health effects. Clin. Chest Med. 2007, 28, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Pekkanen, J.; Pearce, N. Environmental epidemiology: Challenges and opportunities. Environ. Health Perspect. 2001, 109, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Neutra, R.R.; Trichopoulos, D. The place of epidemiology in environmental decisions: Needed support for the development of risk assessment policy. Environ. Health Perspect. 1993, 101 (Suppl. S4), 67–69. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J. Methodological frontiers in environmental epidemiology. Environ. Health Perspect. 1993, 101, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Rychetnik, L.; Bauman, A.; Laws, R.; King, L.; Risse, D.; Nutbeam, D. Translating research for evidence-based public health: Key concepts and future directions. J. Epidemiol. Commun. Health 2012, 66, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, F. Challenges in Creating Evidence in Environmental Health Risk Assessments: The Example of Second-Hand Smoke. Challenges 2016, 7, 2. https://doi.org/10.3390/challe7010002
Fischer F. Challenges in Creating Evidence in Environmental Health Risk Assessments: The Example of Second-Hand Smoke. Challenges. 2016; 7(1):2. https://doi.org/10.3390/challe7010002
Chicago/Turabian StyleFischer, Florian. 2016. "Challenges in Creating Evidence in Environmental Health Risk Assessments: The Example of Second-Hand Smoke" Challenges 7, no. 1: 2. https://doi.org/10.3390/challe7010002





