Continuous-Flow Processes in Heterogeneously Catalyzed Transformations of Biomass Derivatives into Fuels and Chemicals
Abstract
:1. Introduction

- (1) Continuous flow processing allows a better of control of reaction conditions. This is advantageous when dealing with highly reactive feedstocks such as those derived from biomass. Furthermore, as will be shown in some of the examples detailed bellow, continuous approaches offer more flexibility to modify conditions through the course of a reaction thereby allowing an optimum control of intermediates in consecutive A → B → C type of reactions. Double-bed reactors, operating at different temperature conditions, could in principle be designed to achieve the desired and optimum control of reactivity in consecutive reactions involving several intermediates.
- (2) Flow processing also facilitates scaling up which is an important point taking into consideration that many of the biomass processes are still in the lab scale. The development of flow technologies will thus contribute to the commercialization of biomass technologies in the near future.
- (3) Since the chemical composition of biomass feedstocks is normally very different from that of the final products, multiple processing steps are typically required in such transformations, negatively affecting the economy of the process. The utilization of flow processing technologies allows intensification of the chemical processes, thereby significantly contributing to simplify technologies, as detailed in some of the examples of the present review.
- (4) Unlike batch processing, fixed-bed flow technologies do not require catalyst separation after reaction and regeneration, if required, is readily performed over the same catalytic bed. In the case of biomass processing, easy regeneration is crucial since the high reactivity of biomass derivatives typically leads to overreactions generating carbonaceous deposits and tars that poison and deposit on the catalysts surface.
- (5) Many of the biomass processes will require oxygen removal steps to produce the final product. Oxygen is generally removed from biomass molecules in the form of H2O or COx (e.g., CO and CO2). When operating under batch conditions, these gases build up in the reactor leading to increasing pressure and, potentially, new and uncontrolled processes. Flow operation allows continuous removal of these gases which may not interfere in the main catalytic process.
- (6) The microwave-to-flow paradigm, recently highlighted by Kappe’s group [4] is a smart approach to translating batch microwave chemistries to more scalable flow conditions upon mimicking the relatively high pressures and temperatures obtained in a microwave experiment in a continuous flow reactor equipped with a back pressure regulator [4]. The proposed methodology is envisaged to be particularly useful for biomass valorisation practises which could be in principle screened in a quick and efficient manner under microwave batch conditions and then translated to flow chemistry protocols after optimisation of reaction parameters, catalysts and conditions.
2. Continuous-Flow Transformations of Biomass Derivatives into Fuels and Chemicals
2.1. Ethanol

2.2. Furans
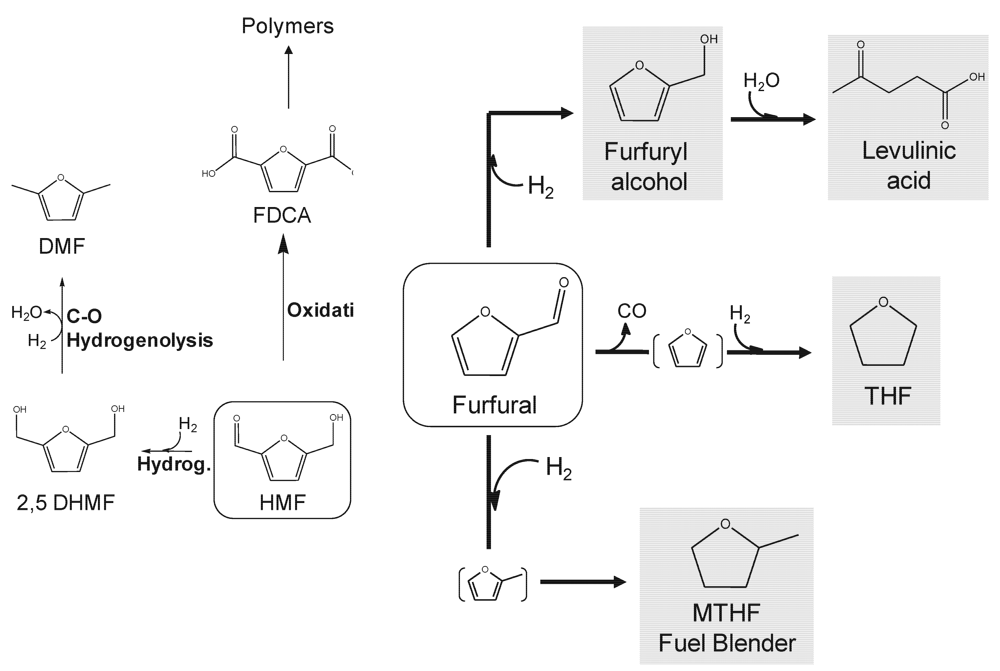
2.3. Organic Acids



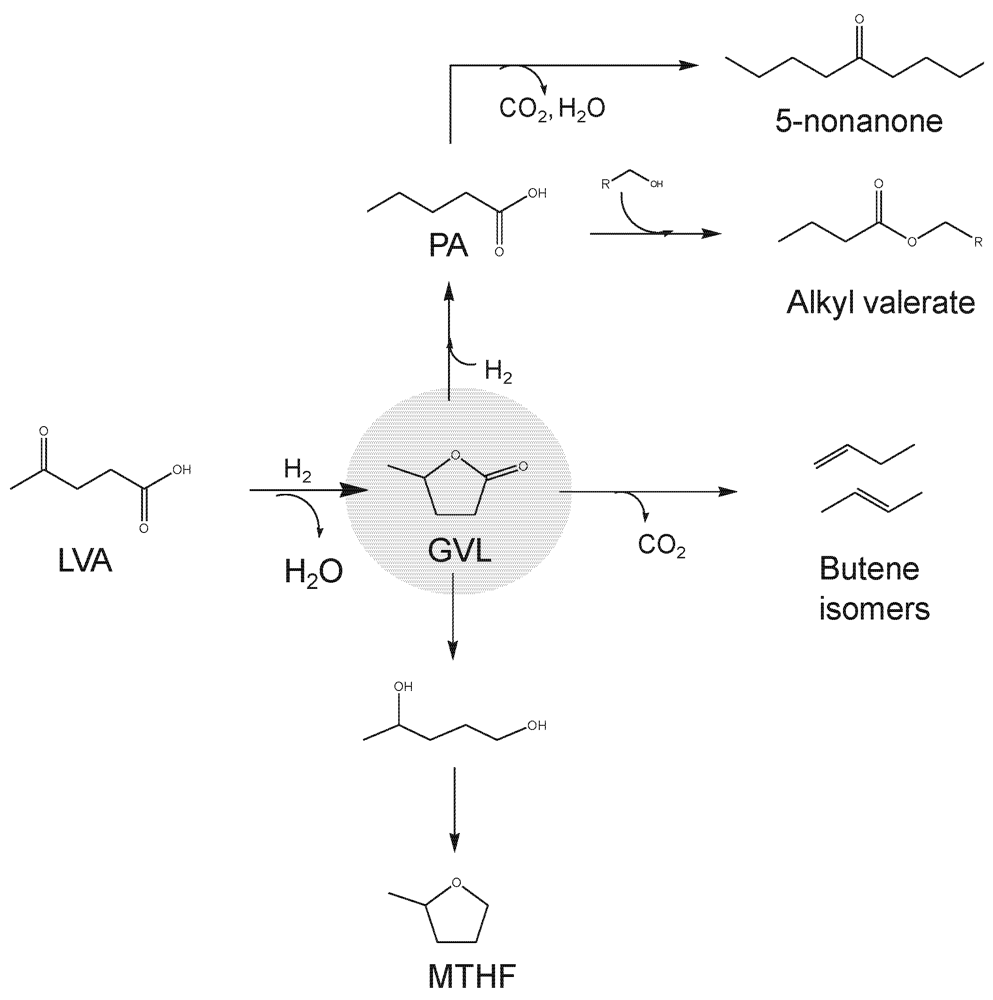
2.4. Polyols and Sugars

2.5. Synthesis Gas
3. Conclusions and Future Prospects
- - Design of novel flow processes for an efficient and effective biomass conversion
- - Design of water-tolerant and stable catalysts able to perform aqueous chemistries in high yields to products controlling the selectivity and reactivity of biomass-derived intermediates
- - Development of low environmental impact technologies based on multi-step reactors, cheap and readily available transition metal (bifunctional) catalysts, mild reaction conditions, etc.
Acknowledgments
References
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar]
- Serrano-Ruiz, J.C.; Luque, R.; Sepúlveda-Escribano, A. Transformations of biomass-derived platform molecules: From high added-value chemicals to fuels via aqueous-phase processing. Chem. Soc. Rev. 2011, 40, 5266–5281. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar]
- Glasnov, T.N.; Kappe, C.O. The microwave-to-flow paradigm: Translating high-temperature batch microwave chemistry to scalable continuous-flow processes. Chem. Eur. J. 2011, 17, 11956–11968. [Google Scholar] [CrossRef]
- Ethanol producer magazine, International Ethanol Report 2010. Available online: http://www.ethanolproducer.com/articles/6696/international-ethanol-report-2010 (accessed on 10 May 2012).
- Haryanto, A.; Fernando, S.; Murali, N.; Adhikari, S. Current status of hydrogen production techniques by steam reforming of ethanol: A review. Energy Fuels 2005, 19, 2098–2106. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H. A review on reforming bio-ethanol for hydrogen production. Int. J. Hydrog. Energy 2007, 32, 3238–3247. [Google Scholar]
- Batista, M.S.; Assaf, E.M.; Assaf, J.M.; Ticianelli, E.A. Double bed reactor for the simultaneous steam reforming of ethanol and water gas shift reactions. Int. J. Hydrog. Energy 2006, 31, 1204–1209. [Google Scholar]
- Bedia, J.; Barrionuevo, R.; Rodríguez-Mirasol, J.; Cordero, T. Ethanol dehydration to ethylene on acid carbon catalysts. Appl. Catal. B 2001, 103, 302–310. [Google Scholar]
- Ethanol producer magazine, Braskem starts up ethanol-to-ethylene plant. Available online: http://www.ethanolproducer.com/articles/7022/braskem-starts-up-ethanol-to-ethylene-plant (accessed on 10 May 2012).
- Takai, T.; Mochizuki, D.; Umeno, M. Method of producing propylene containing biomass-origin carbon. European Patent Application EP1953129 (A1), 2008. Mitsui Chemicals Inc.. [Google Scholar]
- Tu, Y.; Chen, Y. Effects of alkaline-earth oxide additives on silica-supported copper catalysts in ethanol dehydrogenation. Ind. Eng. Chem. Res. 1998, 37, 2618–2622. [Google Scholar]
- Roman-Leshkov, Y.; Barrett, C.J.; Liu, Z.Y.; Dumesic, J.A. Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates. Nature 2007, 447, 982–986. [Google Scholar]
- Lilga, M.A.; Richard, T.H.; Gray, M. Production of Oxidized Derivatives of 5-Hydroxymethylfurfural (HMF). Top. Catal. 2010, 53, 1264–1269. [Google Scholar]
- Surapas, S.; Pham, T.; Prasomsri, T.; Sooknoi, T.; Mallinson, R.G.; Resasco, D.E. Conversion of furfural and 2-methylpentanal on Pd/SiO2 and Pd–Cu/SiO2 catalysts. J. Catal. 2011, 280, 17–27. [Google Scholar]
- Sitthisa, S.; Resasco, D.E. Hydrodeoxygenation of furfural over supported metal catalysts: A comparative study of Cu, Pd and Ni. Catal. Lett. 2011, 141, 784–791. [Google Scholar] [CrossRef]
- García-Suárez, E.J.; Balu, A.M.; Tristany, M.; García, A.B.; Philippot, K.; Luque, R. Versatile dual hydrogenation–oxidation nanocatalysts for the aqueous transformation of biomass-derived platform molecules. Green Chem. 2012, 14, 1434–1439. [Google Scholar] [CrossRef]
- X-Cube™—Catalysis Made Simple. ThalesNano Nanotechnology Inc: Graphisoft Park, Hungary, 2009. Available online: http://www.thalesnano.com/products/x-cube (accessed on 10 May 2012).
- Lange, J.P.; van der Heide, E.; van Buijtenen, J.; Price, R. Furfural-A promising platform for lignocellulosic biofuels. ChemSusChem 2012, 5, 150–166. [Google Scholar]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass. Volume 1—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; U.S. Department of Energy: Washington, DC, USA, 2004; pp. 1–67. [Google Scholar]
- Fan, Y.; Zhou, C.; Zhu, X. Selective catalysis of lactic acid to produce commodity chemicals. Catal. Rev. 2009, 51, 293–324. [Google Scholar] [CrossRef]
- Holm, M.S.; Saravanamurugan, S.; Taarning, E. Conversion of sugars to lactic acid derivatives using heterogeneous zeotype catalysts. Science 2010, 328, 602–605. [Google Scholar]
- Mok, W.S.; Antal, M.J.; Jones, M. Formation of acrylic acid from lactic acid in supercritical water. J. Org. Chem. 1989, 54, 4596–4602. [Google Scholar]
- Gunter, G.C.; Langford, R.H.; Jackson, J.E.; Miller, D.J. Catalysts and supports for conversion of lactic acid to acrylic acid and 2, 3-pentanedione. Ind. Eng. Chem. Res. 1995, 34, 974–980. [Google Scholar]
- Sawicki, R.A. Catalyst for Dehydration of Lactic Acid to Acrylic Acid. U.S. Patent 4729978, 1998. [Google Scholar]
- Serrano-Ruiz, J.C.; Dumesic, J.A. Catalytic upgrading of lactic acid to fuels and chemicals by dehydration/hydrogenation and C–C coupling reactions. Green Chem. 2009, 11, 1101–1104. [Google Scholar]
- Samuel, U.R.; Kohler, W.; Gamer, A.O.; Keuser, U. Propionic acid and derivatives. In Ullmann’s Encyclopedia of Industrial Chemistry; VCH Publishers Inc: Weinheim, Germany, 1993. [Google Scholar]
- Gunter, G.C.; Miller, D.J.; Jackson, J.E. Formation of 2,3-pentanedione from lactic acid over supported phosphate catalysts. J. Catal. 1994, 148, 252–260. [Google Scholar]
- Serrano-Ruiz, J.C.; Dumesic, J.A. Catalytic processing of lactic acid over Pt/Nb2O5. ChemSusChem 2009, 2, 581–586. [Google Scholar] [CrossRef]
- Renz, M. Ketonization of carboxylic acids by decarboxylation: Mechanism and scope. Eur. J. Org. Chem. 2005, 2005(6), 979–988. [Google Scholar] [CrossRef]
- Fitzpatrick, S.W. Production of levulinic acid from carbohydrate-containing materials. U.S. Patent 5608105, 1997. [Google Scholar]
- Yan, Z.; Lin, L.; Liu, S. Synthesis of γ-Valerolactone by Hydrogenation of Biomass-derived Levulinic Acid over Ru/C Catalyst. Energy Fuels 2009, 23, 3853–3858. [Google Scholar]
- Upare, P.P.; Lee, J.M.; Hwang, D.W.; Halligudi, S.B.; Hwang, Y.K.; Chang, J.S. Selective hydrogenation of levulinic acid to γ-valerolactone over carbon-supported noble metal catalysts. J. Ind. Eng. Chem. 2011, 17, 287–292. [Google Scholar] [CrossRef]
- Deng, L.; Li, J.; Lai, D.M.; Fu, Y.; Guo, Q.X. Catalytic conversion of biomass-derived carbohydrates into γ-valerolactone without using an external H2 supply. Angew. Chem. Int. Ed. 2009, 48, 6529–6532. [Google Scholar]
- Bozell, J.J.; Moens, L.; Elliott, D.C.; Wang, Y.; Neuenscwander, G.G.; Fritzpatrick, S.W.; Bilski, R.J.; Jarnefeld, J.L. Production of levulinic acid and use as a platform chemical for derived products. Res. Conserv. Recycl. 2000, 28, 227–239. [Google Scholar] [CrossRef]
- Lange, J.P.; Price, R.; Ayoub, P.M.; Louis, J.; Petrus, L.; Clarke, L.; Gosselink, H. Valeric biofuels: A platform of cellulosic transportation fuels. Angew. Chem. Int. Ed. 2010, 49, 4479–4483. [Google Scholar]
- Serrano-Ruiz, J.C.; Wang, D.; Dumesic, J.A. Catalytic upgrading of levulinic acid to 5-nonanone. Green Chem. 2010, 12, 574–577. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; Braden, D.J.; West, R.M.; Dumesic, J.A. Conversion of cellulose to hydrocarbon fuels by progressive removal of oxygen. Appl. Catal. B 2010, 100, 184–189. [Google Scholar]
- Martin-Alonso, D.; Bond, J.Q.; Dumesic, J.A. Catalytic conversion of biomass to biofuels. Green Chem. 2010, 12, 1493–1513. [Google Scholar]
- Bond, J.Q.; Martin-Alonso, D.; Wang, D.; West, R.M.; Dumesic, J.A. Integrated catalytic conversion of γ-valerolactone to liquid alkenes for transportation fuels. Science 2010, 327, 1110–1114. [Google Scholar]
- Pagliaro, M.; Rossi, M. Future of Glycerol, New Usages for a Versatile Raw Material; RSC publishing: Cambridge, UK, 2008. [Google Scholar]
- Soares, R.R.; Simonetti, D.A.; Dumesic, J.A. Glycerol as a source for fuels and chemicals by low-temperature catalytic processing. Angew. Chem. Int. Ed. 2006, 45, 3982–3985. [Google Scholar]
- Simonetti, D.A.; Rass-Hansen, J.; Kunkes, E.L.; Soares, R.R.; Dumesic, J.A. Coupling of glycerol processing with Fischer-Tropsch synthesis for production of liquid fuels. Green Chem. 2007, 9, 1073–1083. [Google Scholar]
- Kunkes, E.L.; Soares, R.R.; Simonetti, D.A.; Dumesic, J.A. An integrated catalytic approach for the production of hydrogen by glycerol reforming coupled with water-gas shift. Appl. Catal. Environ. 2009, 90, 693–698. [Google Scholar]
- Zhou, C.H.C.; Beltramini, J.N.; Fan, Y.X.; Lu, G.Q.M. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem. Soc. Rev. 2007, 37, 527–549. [Google Scholar]
- Kunkes, E.L.; Simonetti, D.A.; West, R.M.; Serrano-Ruiz, J.C.; Gartner, C.A.; Dumesic, J.A. Catalytic conversion of biomass to monofunctional hydrocarbons and targeted liquid-fuel classes. Science 2008, 322, 417–421. [Google Scholar]
- Serrano-Ruiz, J.C.; Dumesic, J.A. Catalytic routes for the conversion of biomass into liquid hydrocarbon transportation fuels. Energy Environ. Sci. 2011, 4, 83–99. [Google Scholar]
- Dry, M.E. The Fischer-Tropsch process: 1950–2000. Catal. Today 2002, 71, 227–241. [Google Scholar]
- Biomass to Fuel Conversion; Primus Green Energy Ltd.: Hillsborough, NJ, USA, 2012. Available online: http://www.primusge.com/how-it-works/biomass-to-fuel-conversion/ (accessed on 15 June 2012).
- Sundrop Fuels, Inc. Available online: http://www.sundropfuels.com/ (accessed on 15 June 2012).
- Lange, J.P. Methanol synthesis: A short review of technology improvements. Catal. Today 2001, 64, 3–8. [Google Scholar]
- Exxon Mobil, Research and Engineering, Methanol to gasoline (MTG). Available online: http://www.exxonmobil.com/Apps/RefiningTechnologies/files/sellsheet_09_mtg_brochure.pdf (accessed on 15 June 2012).
- Haldor Topsøe. Available online: http://www.topsoe.com/business_areas/gasification_based/Processes/Gasoline_TIGAS.aspx (accessed on 15 June 2012).
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Serrano-Ruiz, J.C.; Luque, R.; Campelo, J.M.; Romero, A.A. Continuous-Flow Processes in Heterogeneously Catalyzed Transformations of Biomass Derivatives into Fuels and Chemicals. Challenges 2012, 3, 114-132. https://doi.org/10.3390/challe3020114
Serrano-Ruiz JC, Luque R, Campelo JM, Romero AA. Continuous-Flow Processes in Heterogeneously Catalyzed Transformations of Biomass Derivatives into Fuels and Chemicals. Challenges. 2012; 3(2):114-132. https://doi.org/10.3390/challe3020114
Chicago/Turabian StyleSerrano-Ruiz, Juan Carlos, Rafael Luque, Juan Manual Campelo, and Antonio A. Romero. 2012. "Continuous-Flow Processes in Heterogeneously Catalyzed Transformations of Biomass Derivatives into Fuels and Chemicals" Challenges 3, no. 2: 114-132. https://doi.org/10.3390/challe3020114





