1. Introduction
Tropical soils are often highly weathered and tend to have low fertility [
1]. However, if climate conditions are favorable, dense vegetation of rainforests can develop on those soils. Transformation of forests into farmlands leads to exposure of the vulnerable soil, making it prone to erosion that can lead up to desertification [
2]. Agricultural systems that protect the soil and prevent its degradation are highly required, especially for tropical soils.
Successional agroforestry systems (SAFS) are a promising strategy for more sustainable food production in the tropics [
3]. SAFS seek to mimic the diverse structure of natural forests and at the same time produce marketable products. In comparison with other agroforestry systems, SAFS strive to create food producing systems in advanced stages of succession, managing subclimax or climax plant communities where high niche variability results in high biodiversity and efficient use of resources [
4]. Utilizing native as well as introduced plant species, a forest is created that is structurally analogous to a natural forest of the local area, providing the same ecosystems services, restoring the environment and sustaining local communities. SAFS are designed to follow natural succession, leading to increased complexity and structural diversity in time. Pioneer species, apart from producing marketable goods, create conditions for the successful establishment of climax species. This way, potential productivity of every particular site can be maximally reached.
In agroforestry, carbon (C) is sequestered through incorporation of carbon dioxide (CO
2) into plant biomass by photosynthesis. C is thus stored in pools of aboveground biomass, like timber, branches, and belowground biomass like roots, soil microorganisms, and organic C in soil [
5]. Trees have greater capacity to store C than annual crops and grasses on pastures. Agroforestry systems will therefore present better option for C sequestration [
6,
7].
Apart from the importance of C sequestration for mitigating climate change, soil organic carbon (SOC) is an important indicator of soil health and fertility [
8,
9]. The decrease or increase of SOC can reveal if the land-use system degrades, maintains, or improves the fertility and health of the soil [
10,
11]. SOC, present in the soil as soil organic matter, helps to promote soil fertility directly and indirectly. Its direct effect is connected with the process of mineralization. Through decomposition, organic matter provides a source of nutrients for vegetative and microbial communities. Indirectly, SOC improves physical properties of the soil, leading to improved soil aggregation that in turn positively influences water and air infiltration and root penetration [
10]. Land use can have a profound influence on SOC concentrations [
8,
12]. Removal of vegetation cover during slash and burn agriculture, tillage, or grazing is connected with decreases in SOC concentrations [
13]. Without plant residues, there is no source left on the field that could replenish a decrease in SOC concentration.
Agroforestry, with its tree component, is capable of producing large amounts of biomass. By mimicking forest, SAFS are expected to be especially suitable for SOC replenishment [
14]. However, the possibility of SAFS to replenish SOC depends on the original condition of the soil and environment. Some studies report a gain in SOC under agroforestry, while others do not [
15,
16], and there is no consensus as to whether SAFS can increase SOC.
SAFS are dynamic agricultural systems, and as they mature their features—such as the dominant plant species, structure, and height of the canopy—change dramatically [
4]. Young SAFS stages therefore display attributes more similar to monoculture plantations, while mature systems resemble forests with undisturbed soil covered with litters from trees. Jacobi et al. [
16] proved that SAFS can be more efficient land-use systems than monoculture cultivations in terms of C sequestration, mainly due to C storage in the above ground biomass. However, changes in C sequestration during successional development of SAFS remain poorly investigated.
There are several studies on SAFS from economic and social viewpoints [
16,
17,
18,
19]. However, little is known about their effect on C cycling, soil fertility, and C sequestration, even though farmers are convinced of their resource-use efficiency and ability to conserve soil [
4,
20].
This study therefore aims to clarify changes in C cycling and C sequestration during SAFS development. Filling up these knowledge gaps could help to provide scientific background for the possibility of using SAFS for C sequestration.
2. Materials and Methods
2.1. Study Site
The study site is Tomé-Açu in Brazilian state Pará (2°24′53″ S, 48°8′60″ W). The climate is humid tropical with a mean annual temperature of 26.4 °C, and local soils are highly weathered acid Oxisols [
3]. The annual precipitation of 2663 mm is unevenly distributed during two main seasons. The rainy season starts in November and continues to June, while the dry season starts in July and ends in October. During the dry season, monthly precipitation can reach values below 50 mm which can cause water shortages [
21].
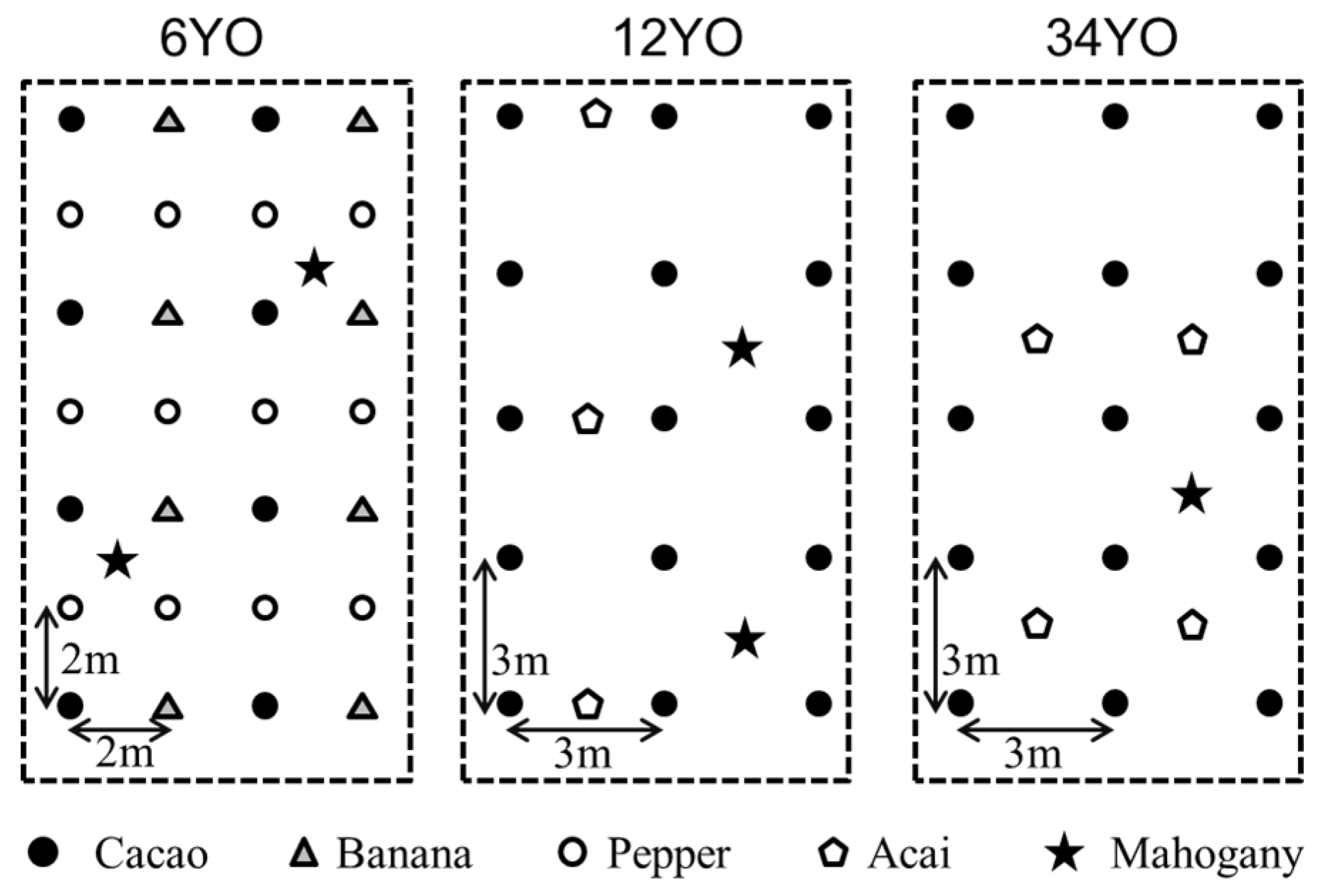
The study was conducted from September 2012 to July 2014 at Konagano farm, a pioneer of SAFS in Brazil. Its vicinity to secondary rainforest and presence of SAFS established in different years makes it an optimal site for comparative research. For our study, three different successional stages of SAFS were selected: SAFS established in 2008 (six-years-old at the time of the study; 6YO), in 2002 (12YO), and in 1980 (34YO). For each SAFS stage, three plots were chosen as replicates. Each SAFS stage covers an area of greater than 4 ha, allocated within 3 km distance. The trees were planted in a square pattern based on a different design for each stage (
Figure 1). Plant species and densities grown on the SAFS stages are listed in
Table 1. As a reference, neighboring secondary forest was also monitored with also three replications. Nitrogen fertilizer was added at a rate of 40, 20, and 0 kg·N·ha
−1·year
−1 to the 6YO, 12YO, and 34YO, respectively. Before the establishment of SAFS, all the plots were used as monoculture pepper plantations.
2.2. Net Biome Productivity
Schulze and Heimann [
22] introduced the term net biome productivity (NBP) to analyze long-term and large-scale changes in C cycle induced by land use. NBP is the amount of C that remains in the system after subtracting respiration and losses like harvest or fires. The C budget of different SAFS stages was analyzed and compared using the following formulas:
where NBP = net biome productivity, NPP = net primary productivity, EF = exported fruit, SR = soil respiration, AGBI = aboveground biomass increase from 2013 to 2014, FR = fruit residue, LF = litterfall.
2.3. Aboveground Biomass Increase
Aboveground biomass was calculated twice, in 2013 and 2014, according to a suitable formula for each tree species on a separate basis (
Table 2). In the case of banana, the plants were cut down in 2013 and 2014 and dried and weighted for their biomass directly in an area of 5 m by 5 m from each plot. Since not all plant species existed in all SAFS stages (
Table 1), the presence of the species in each of the SAFS stages, 20 cacao, 10 mahogany, 20 acai, and 3 banana trees were randomly selected for analysis from each plot. Pepper plants were neglected from the balance because of their small contribution to overall biomass. For the other calculations, we measured tree diameter at breast height and tree height using a clinometer. To count annual aboveground biomass increase, we subtracted aboveground biomass measured in 2013 from that measured in 2014. Carbon contents were determined by analysis of representative samples obtained from each plant by CHN-analyzer (Yanako MT-6).
2.4. Exported Fruit and Fruit Residue
In order to analyze the biomass of exported fruit and fruit residue, 20 cacao, 10 pepper, and 20 acai trees were randomly selected for analysis from each plot and analyzed for C content as previously described. Subsamples from plant organs were dried in an oven at 75 °C for two days to determine dry weight. Cacao was harvested in June/July and in February, acai continuously from June to November, and pepper from July to September. Cacao fruits were separately weighted for husks (fruit residue) and beans (exported fruit). Husks were left on the ground as mulch while beans were removed from the field. Acai fruit-bunches were separated into strings (fruit residue) and fruits (exported fruit). Strings were left on the ground as mulch while fruits were removed from the field. Pepper was removed from the field without leaving residue. Bananas were not harvested. Banana trees were cut down after a few years and left on the field as mulch.
2.5. Litterfall
Litterfall from trees was measured continuously from November 2012 to July 2014 in all stages of SAFS, as well as in the secondary forest, and analyzed for C content, as previously described. In each plot, we used three 1000 mm by 1000 mm square nets for debris catchment. Every month, the contents of the nets were weighted and a selected sample was dried in an oven at 75 °C for two days to determine dry weight.
2.6. Soil Respiration
Soil respiration was measured by using the closed chamber method. Carbon dioxide (CO2) concentration was measured continually from April 2013 to July 2014 in 6-min intervals by a CO2 sensor (Testo 535). For every plot, three replications were conducted for soil with litter and three replications for soil without litter. The CO2 flux from soil without litter was referred to as soil respiration, while the difference between the CO2 flux with litter and that without litter was defined as litter respiration.
Soil respiration is composed of heterotrophic respiration (soil organisms) and autotrophic respiration (roots). Autotrophic respiration can represent 20% to 80% of soil respiration [
26]. However, to estimate NBP, only heterotrophic respiration is used. The values of soil respiration were therefore adjusted accordingly to omit root respiration. This provided a range of possible values for heterotrophic respiration.
2.7. Soil Organic Carbon
Soil sampling was performed by digging a 30-cm deep profile in 6YO, 12YO, 34YO, and secondary forest plots, as well as in the road in the secondary forest. The road was attached to the 34YO plot and was created at the same time as that the pot. It was added to the analysis of SOC as a reference value. At each plot, four soil core sample replications (100 cm3) were taken for analysis of soil bulk density and C content as previously described.
Different land use systems lead to different soil bulk densities and thus comparing C contents to the same soil depth will lead to errors because soil layers of the same depth can contain different masses of soil. Therefore, SOC contents were compared using equivalent soil masses. Using this method, soil layers are not defined by depth in cm, but rather by specific mass in Mg·ha
−1. This method allows for the comparison of soil masses even if their soil bulk densities are different. As recommended by Wendt and Hauser [
27], an excel spreadsheet [
28] was used to calculate SOC concentration in equivalent soil masses, using data of bulk density, depth, and SOC concentration collected by soil sampling. In this study, soil mass of 5000 Mg·ha
−1 was used.
2.8. Statistics
Statistical analysis was made in IBM SPSS Software version 22 (Armonk, NY, USA). To explore differences in soil respiration, Welch’s ANOVA was performed followed by Games-Howell post-hoc test at level of significance p < 0.05.
Analysis of litterfall was conducted using one-way ANOVA (F(3,80) = 6.539, p = 0.001) followed by Tukey’s post-hoc test. Litterfall data were sqrt-transformed in order to comply with Shapiro-Wilk test of normal distribution.
Differences in soil organic C content were analyzed by one-way ANOVA (F(4,15) = 122.907, p = 0.000) followed by Tukey’s post-hoc test.
3. Results
Exported fruit and residue from Cacao was significantly highest in 12YO with 3.7 ± 0.8 Mg·C·ha
−1·year
−1, followed by 34YO and 6YO (
Table 3). Exported fruit and residue from Acai was almost the same for 12YO and 34YO, ranging from 1.5 to 1.6 Mg·C·ha
−1·year
−1. Pepper was harvested only in 6YO, resulting in 1.0 ± 0.2 Mg·C·ha
−1·year
−1. The sum of all the exported fruit and fruit residue was highest for 12YO with 5.2 ± 1.0 Mg·C·ha
−1·year
−1, followed by 34YO with 4.5 ± 0.9 Mg·C·ha
−1·year
−1. The lowest value was measured in 6YO with 2.7 ± 0.5 Mg·C·ha
−1·year
−1. Similarly, the sum of all the exported fruit without fruit residue was highest in 12YO with 4.1 ± 0.8 Mg·C·ha
−1·year
−1, followed by 34YO with 3.5 ± 0.7 Mg·C·ha
−1·year
−1. The lowest value was measured in 6YO with 2.3 ± 0.4 Mg·C·ha
−1·year
−1.
The biomass increase of cacao tree was comparable in 6YO and 34YO reaching 0.9 Mg·C·ha
−1·year
−1 (
Table 4). 12YO showed a lower increase with 0.7 ± 0.3 Mg·C·ha
−1·year
−1. Mahogany growth was the most intensive in 6YO with 0.9 ± 0.2 Mg·C·ha
−1·year
−1, followed by 12YO and 34YO with 0.8 ± 0.4 and 0.2 ± 0.1 Mg·C·ha
−1·year
−1, respectively. Acai palm growth was higher in 12YO than in 34YO with 0.8 ± 0.3 and 0.5 ± 0.2 Mg·C·ha
−1·year
−1, respectively. Banana was grown only in 6YO with a yearly biomass increase of 0.5 ± 0.1 Mg·C·ha
−1·year
−1. When comparing total biomass increases, then 6YO and 12YO, both with 2.3 Mg·C·ha
−1·year
−1, were more productive than 34YO with 1.6 Mg·C·ha
−1·year
−1.
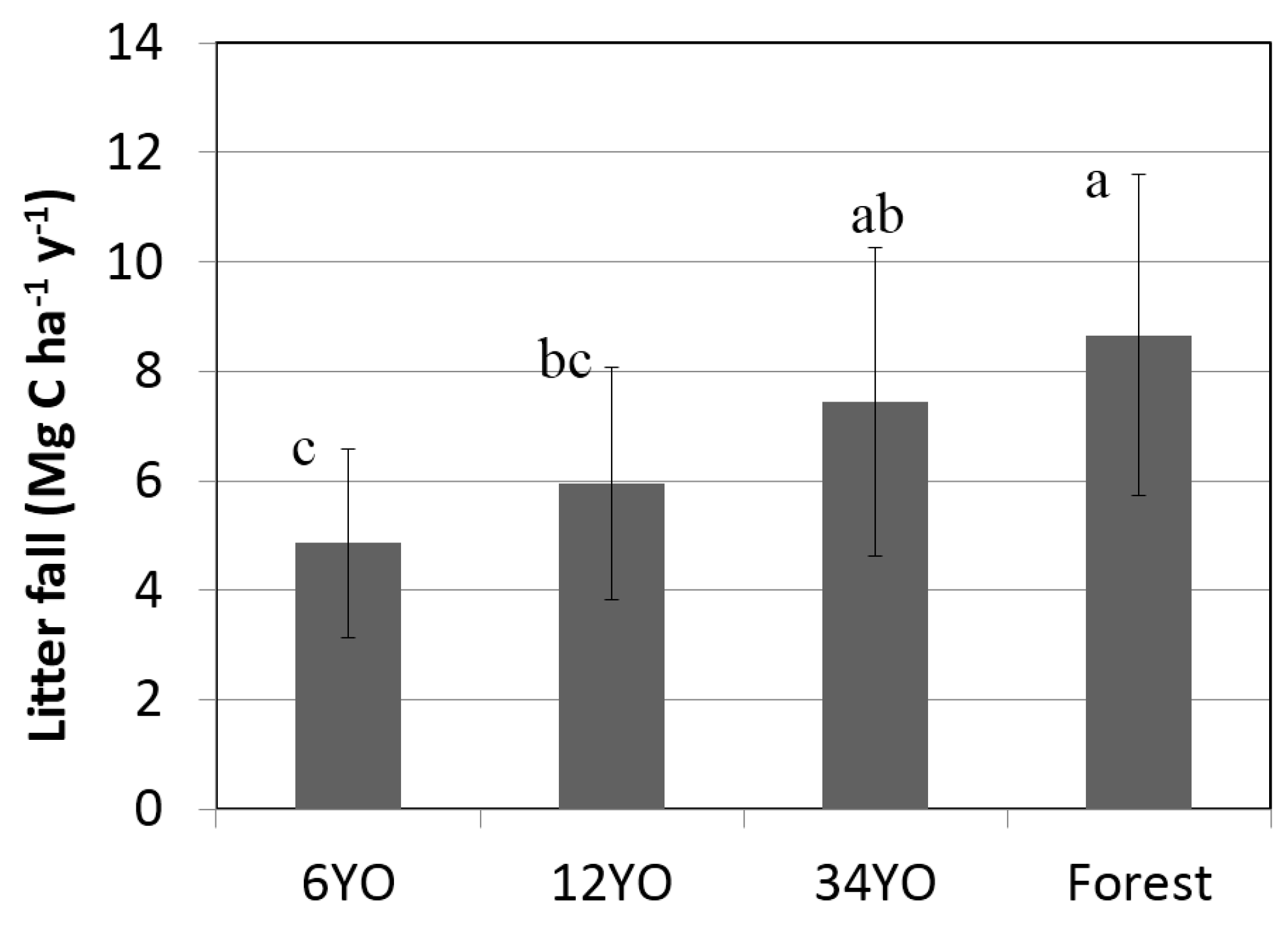
Annual litterfall increased with age of SAFS (
Figure 2). Secondary forest and 34YO reached the highest values with 8.7 ± 2.9 and 7.4 ± 2.8 Mg·C·ha
−1·year
−1, respectively. A lower value was found in 12YO with 5.9 ± 2.1 Mg·C·ha
−1·year
−1. Litterfall was lowest in 6YO with 4.9 ± 1.7 Mg·C·ha
−1·year
−1.
Net primary productivity increased with age of SAFS. Highest values were recorded for Forest, 13.7–18.7 and lowest for 6YO, 9.8 ± 1.8 Mg·C·ha
−1·year
−1 (
Table 5).
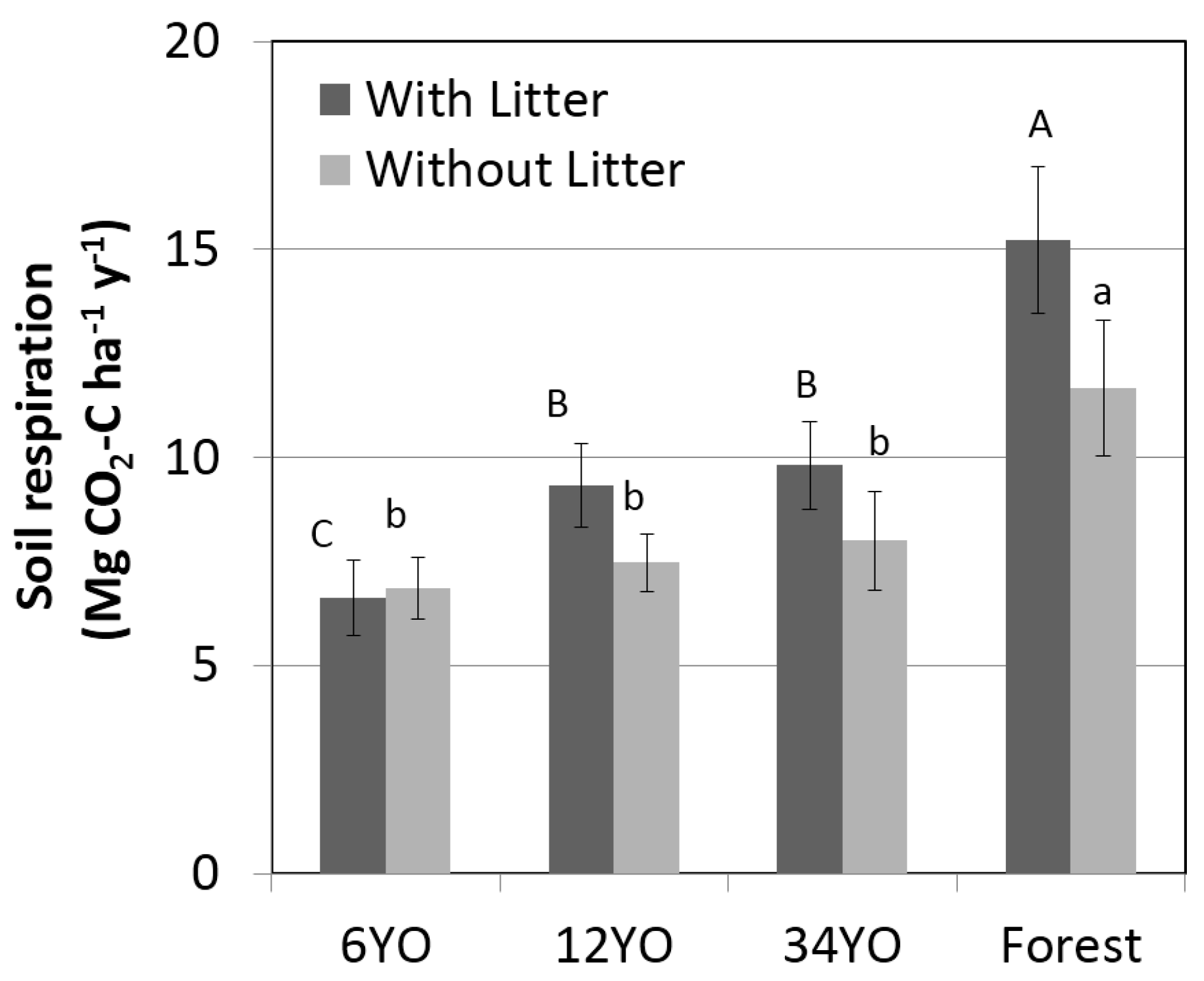
Soil respiration with litter increased with age of SAFS and was highest in the secondary forest with 15.2 ± 1.8 Mg·C·ha
−1·year
−1, followed by 34YO with 9.8 ± 1.0 Mg·C·ha
−1·year
−1 (
Figure 3). 12YO and 6YO had lower values of 9.3 ± 1.0 and 6.6 ± 0.9 Mg·C·ha
−1·year
−1, respectively. Soil respiration without litter showed the same tendency and increased with age of SAFS, ranging from 6.9 ± 0.7 Mg·C·ha
−1·year
−1 in 6YO to 11.7 ± 1.6 Mg·C·ha
−1·year
−1 in the secondary forest. The difference between soil respiration with and without litter corresponds to soil litter respiration. While this was negligible in 6YO, it was similar in 12YO and 34YO, reaching 1.9 and 1.8 Mg·C·ha
−1·year
−1, respectively. The highest litter respiration was measured in the secondary forest, with 3.6 Mg·C·ha
−1·year
−1.
Net biome productivity based on Equation (1), using the measured values above, was highest in the secondary forest and ranged from 0.8 up to 12.8 Mg·C·ha
−1·year
−1 (
Table 5). For 34YO, the value ranged from 1.8 to 8.2 Mg·C·ha
−1·year
−1. The lowest values were measured in 12YO and 6YO, ranging from 1.6 to 6.1 and 2.2 to 6.3 Mg·C·ha
−1·year
−1, respectively.
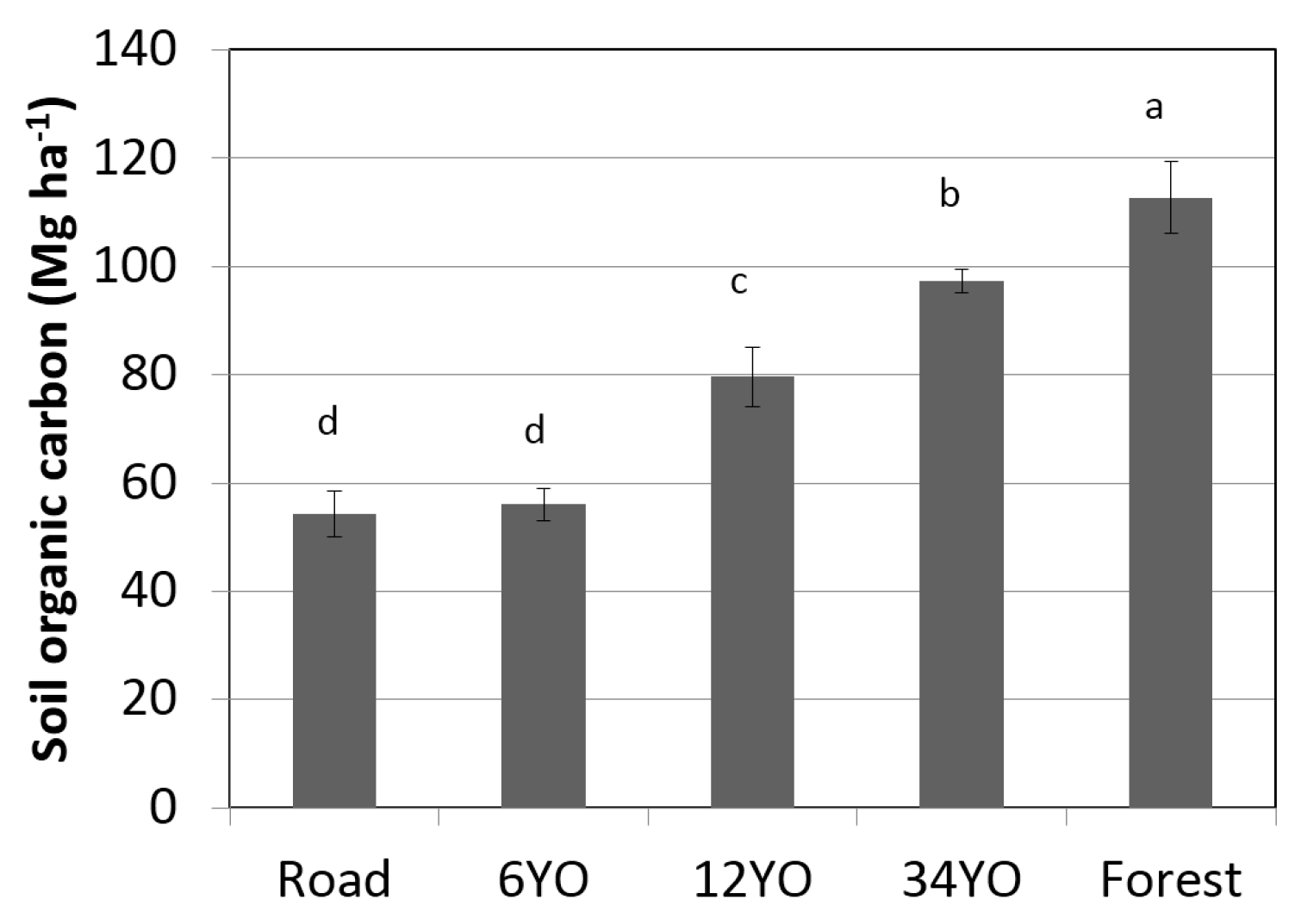
Soil organic carbon at equivalent soil mass 0–5000 Mg ha
−1 significantly increased with age of SAFS and was highest in secondary forest with 112.7 ± 6.6 Mg·C·ha
−1·year
−1, followed by 34YO with 97.4 ± 2.2 Mg·C·ha
−1·year
−1 (
Figure 4). 12YO reached 79.6 ± 5.5 Mg·C·ha
−1·year
−1. The lowest values were measured in 6YO and in the road with 56.0 ± 3.1 and 54.3 ± 4.2 Mg·C·ha
−1·year
−1, respectively.
5. Conclusions
This study compared different successional stages of SAFS, mapped their C flows, and assessed their ability to improve soil fertility and sequester C. Results indicated that with increasing age of SAFS, their ability to sequester, accumulate, and recycle C and nutrients increases. Raised pools of nutrients coupled with their more dynamic turnover lead to reduced reliance on external fertilizer inputs. In time, SAFS are able to produce more biomass with less fertilizer, implying high resource use efficiency.
Soils under mature SAFS had significantly higher concentrations of SOC, resulting in higher quality soils. SAFS therefore were shown to be an innovative agricultural system, capable of improving vulnerable tropical soils, and at the same time sequestering C. In terms of preventing soil degradation in tropical regions, SAFS are a promising land use system.