Insights into Surface Interactions between Metal Organic Frameworks and Gases during Transient Adsorption and Diffusion by In-Situ Small Angle X-ray Scattering
Abstract
:1. Introduction
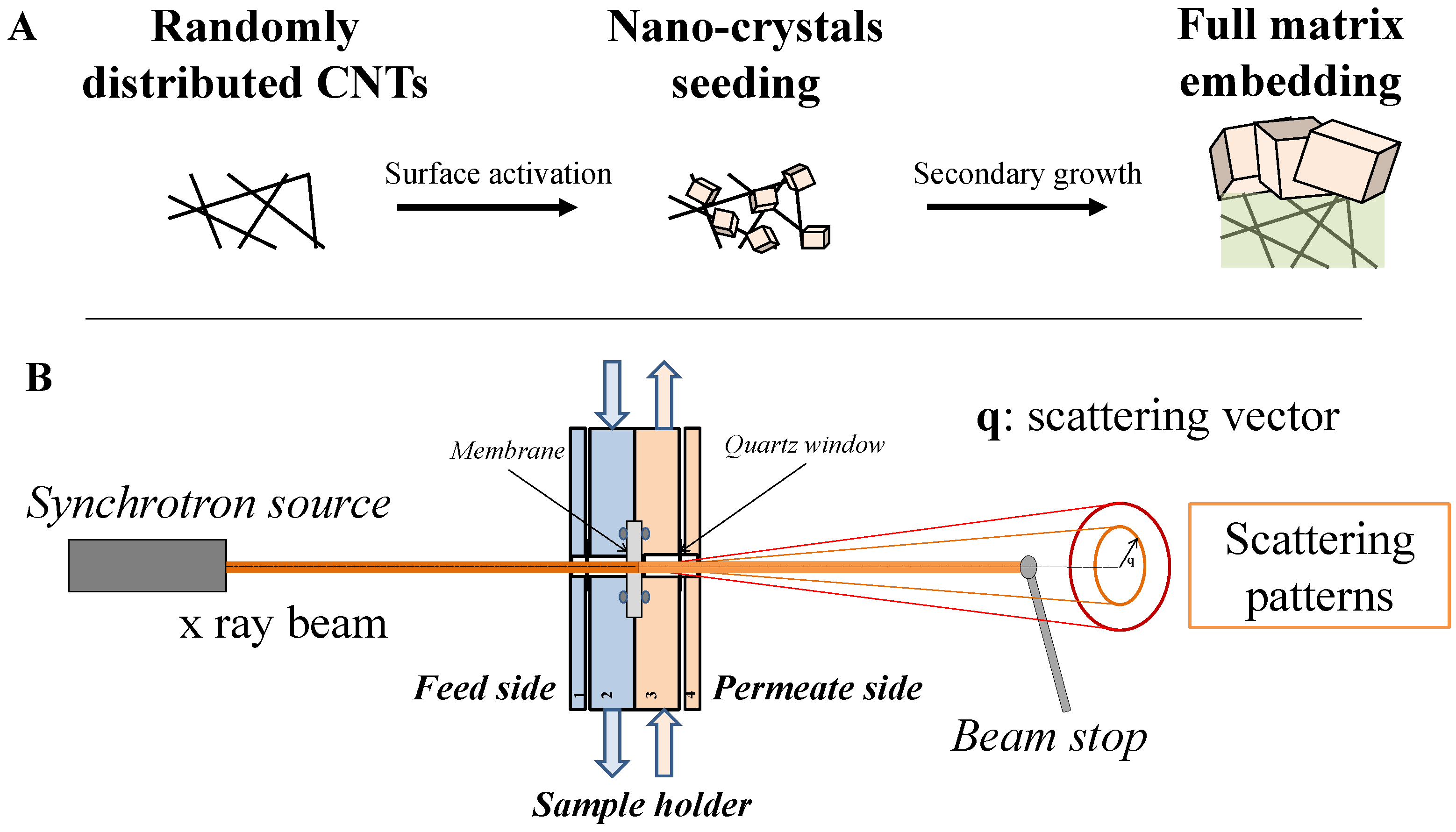
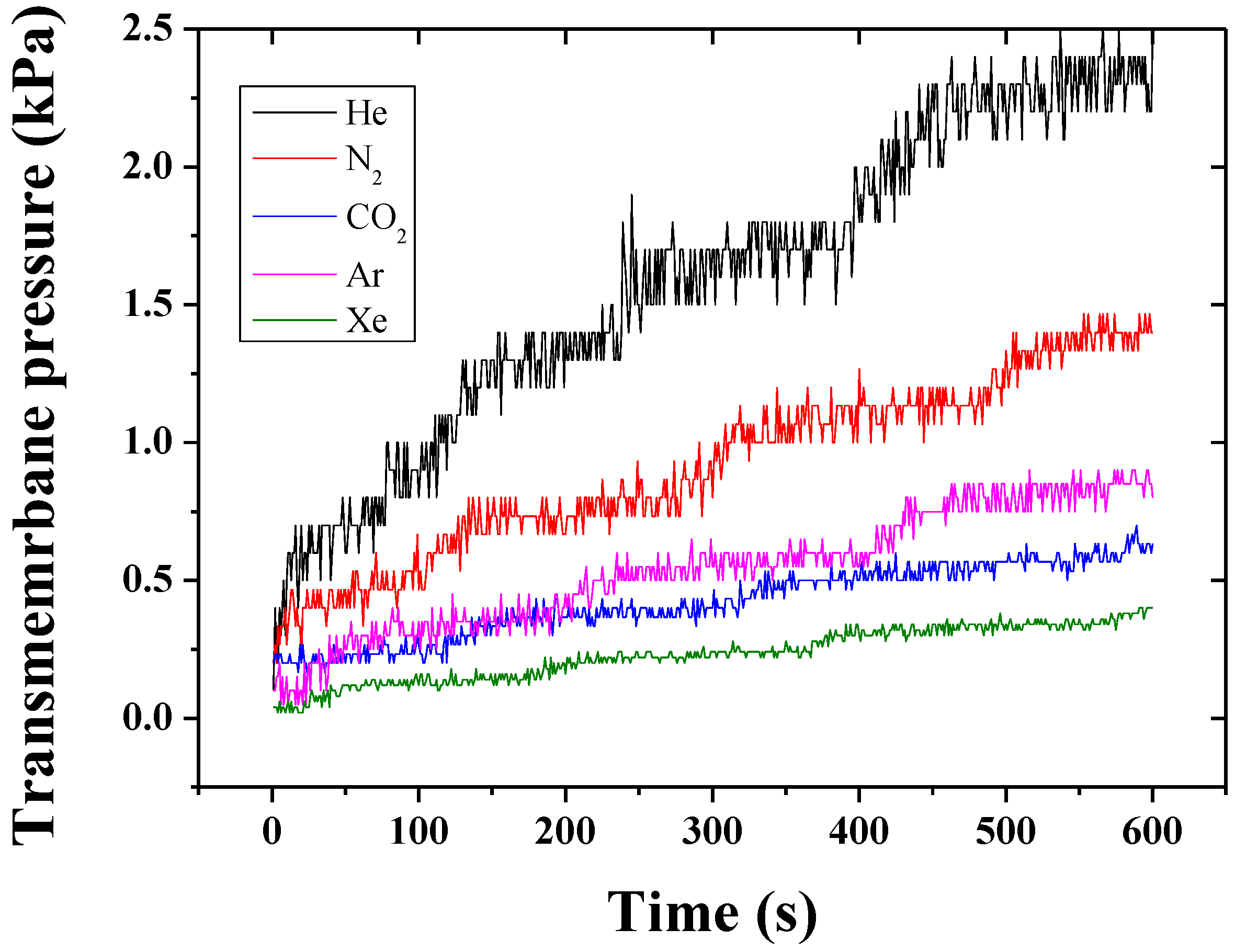
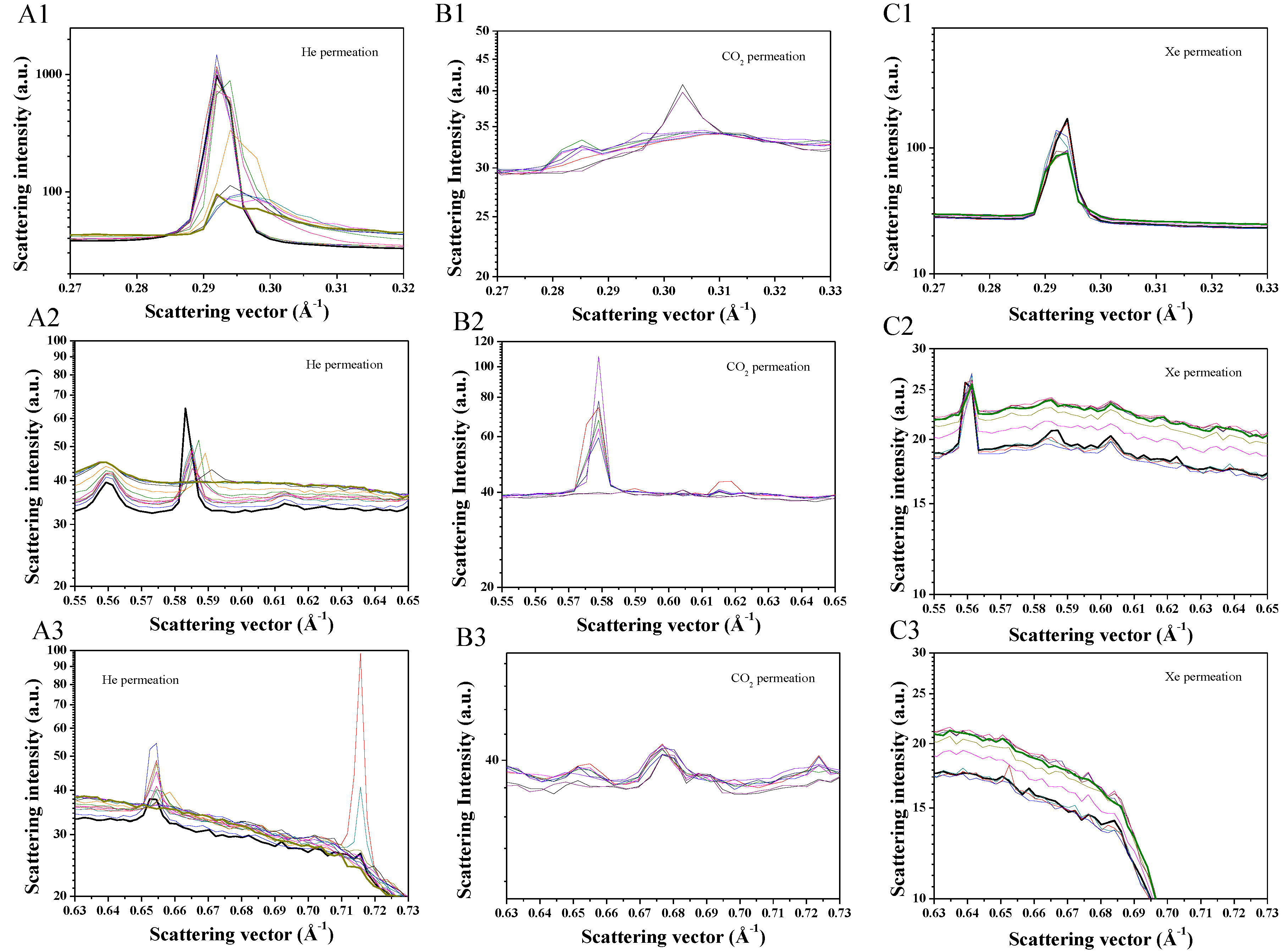
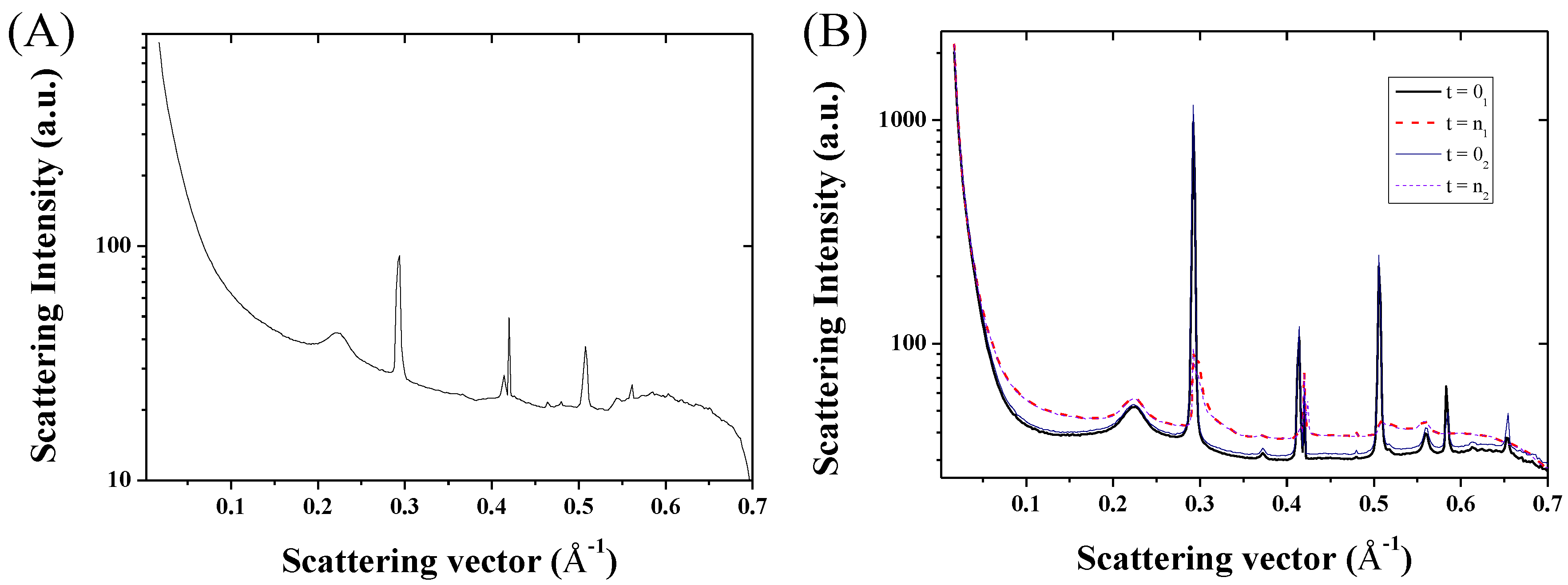
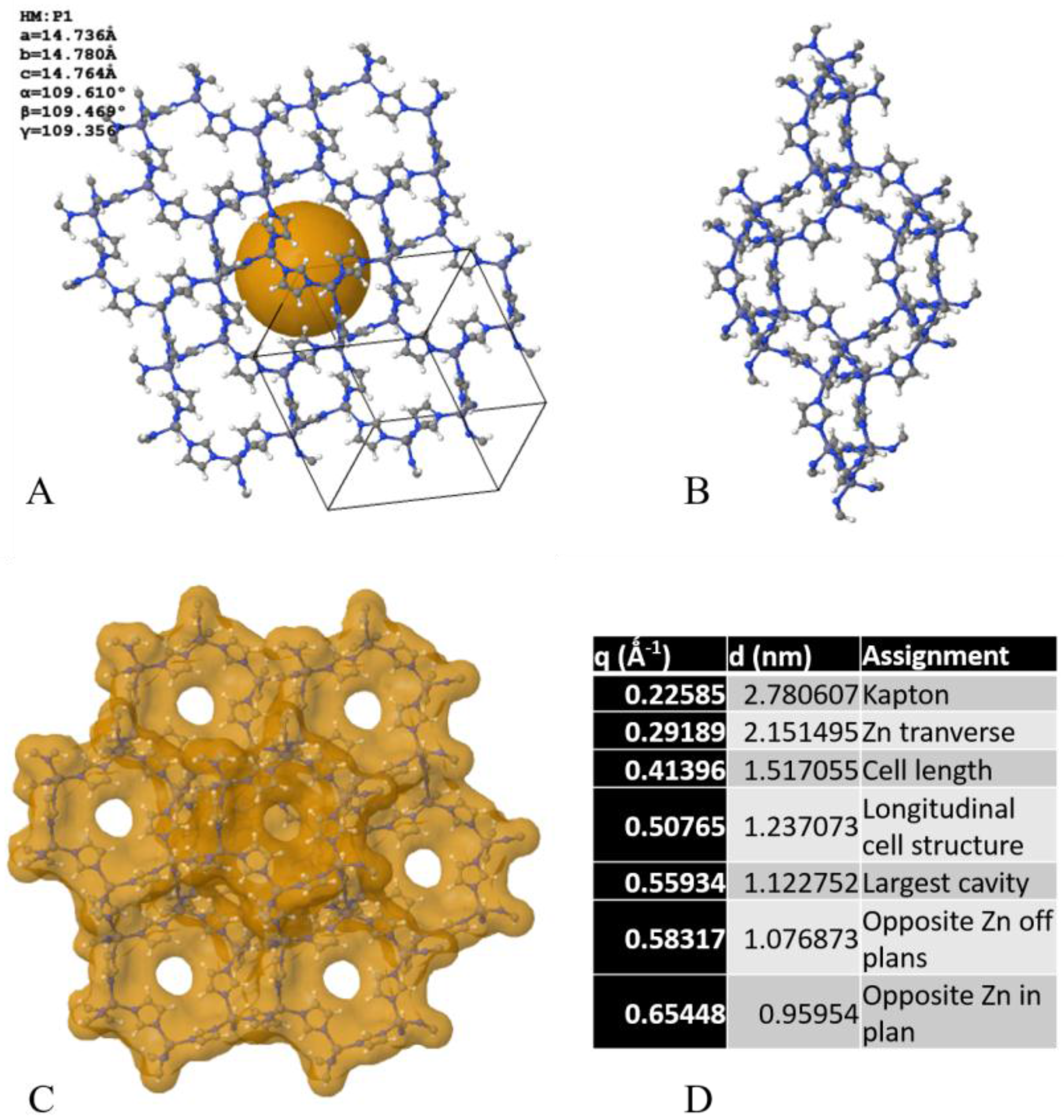
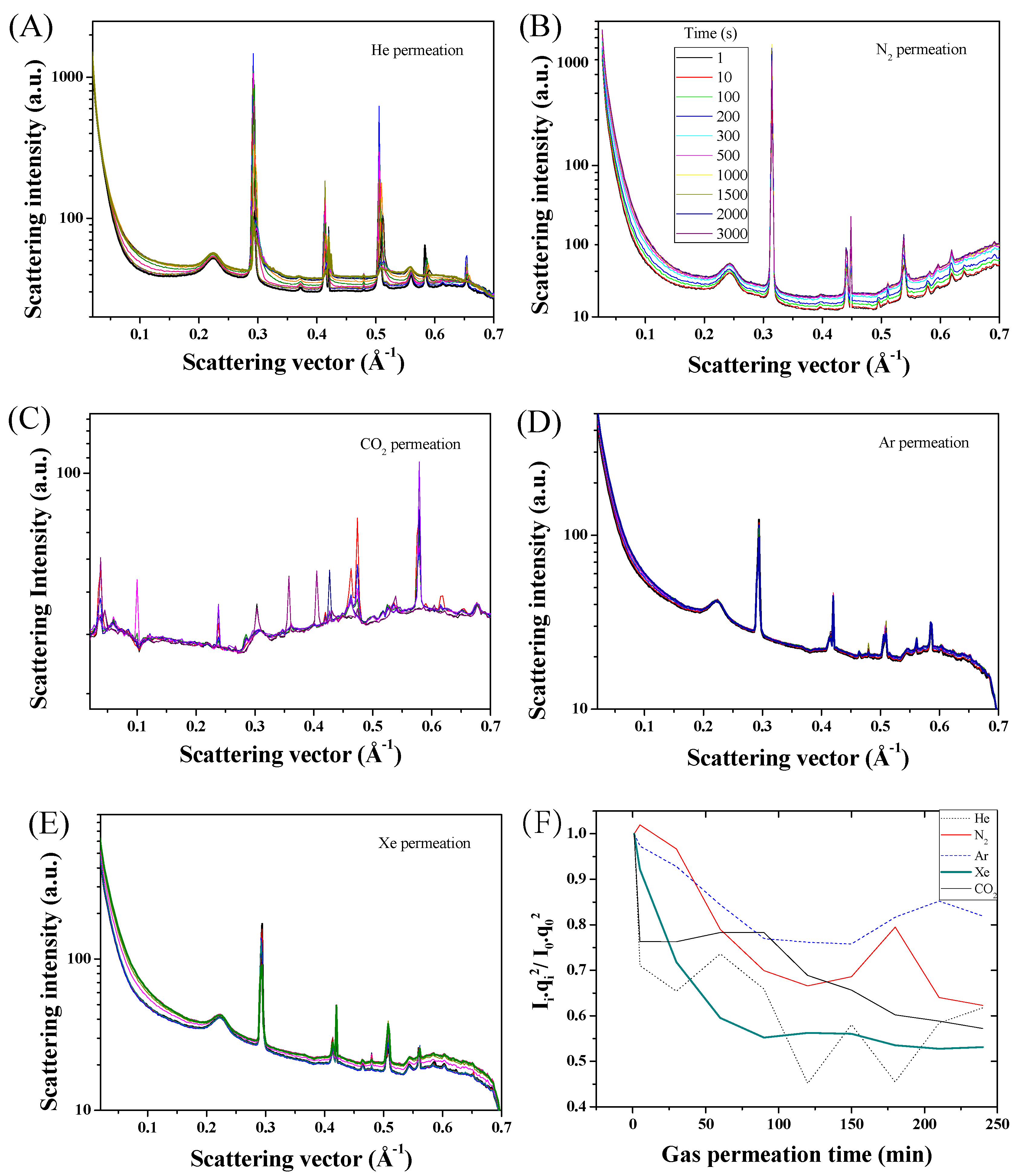
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Membrane Synthesis
3.2. Gas Permeation Tests
3.3. Characterization Techniques
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- Hinds, B.J.; Chopra, N.; Rantell, T.; Andrews, R.; Gavalas, V.; Bachas, L.G. Aligned multiwalled carbon nanotube membranes. Science 2004, 303, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Sears, K.; Dumée, L.; Schütz, J.; She, M.; Huynh, C.; Hawkins, S.; Duke, M.; Gray, S. Recent developments in carbon nanotube membranes for water purification and gas separation. Materials 2010, 3, 127–149. [Google Scholar] [CrossRef] [Green Version]
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernandez, J.R.; Ferrari, M.-C.; Gross, R.; Hallett, J.P.; et al. Carbon capture and storage update. Energy Environ. Sci. 2014, 7, 130–189. [Google Scholar] [CrossRef]
- Dumée, L.; Sears, K.; Mudie, S.; Kirby, N.; Skourtis, C.; McDonnell, J.; Lucas, S.; Schütz, J.; Finn, N.; Huynh, C.; et al. Characterization of carbon nanotube webs and yarns with small angle X-ray scattering: Revealing the yarn twist and inter-nanotube interactions and alignment. Carbon 2013, 63, 562–566. [Google Scholar] [CrossRef]
- Drobek, M.; Bechelany, M.; Vallicari, C.; Abou Chaaya, A.; Charmette, C.; Salvador-Levehang, C.; Miele, P.; Julbe, A. An innovative approach for the preparation of confined ZiF-8 membranes by conversion of ZnO ald layers. J. Membr. Sci. 2015, 475, 39–46. [Google Scholar] [CrossRef]
- Drobek, M.; Kim, J.-H.; Bechelany, M.; Vallicari, C.; Julbe, A.; Kim, S.S. Mof-based membrane encapsulated ZnO nanowires for enhanced gas sensor selectivity. ACS Appl. Mater. Interfaces 2016, 8, 8323–8328. [Google Scholar] [CrossRef] [PubMed]
- Shamsaei, E.; Low, Z.X.; Lin, X.; Mayahi, A.; Liu, H.; Zhang, X.; Zhe, L.J.; Wang, H. Rapid synthesis of ultrathin, defect-free ZiF-8 membranes via chemical vapour modification of a polymeric support. Chem. Commun. Camb. Engl. 2015, 51, 11474–11477. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.H.; Nguyen, P.T.; Hill, M.R.; Thornton, A.W.; Konstas, K.; Doherty, C.M.; Mulder, R.J.; Bourgeois, L.; Liu, A.C.Y.; Sprouster, D.J.; et al. Ending aging in super glassy polymer membranes. Angew. Chem. Int. Ed. 2014, 53, 5322–5326. [Google Scholar] [CrossRef] [PubMed]
- Dumée, L.F.; Sears, K.; Schütz, J.A.; Finn, N.; Duke, M.; Mudie, S.; Kirby, N.; Gray, S. Small angle X-ray scattering study of carbon nanotube forests densified into long range patterns by controlled solvent evaporation. J. Colloid Interface Sci. 2013, 407, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Favvas, E.; Stefanopoulos, K.; Vairis, A.; Nolan, J.; Joensen, K.; Mitropoulos, A. In situ SAXS investigation of dibromomethane adsorption in ordered mesoporous silica. Adsorption 2013, 19, 331–338. [Google Scholar] [CrossRef]
- Dumée, L.; Lee, J.; Sears, K.; Tardy, B.; Duke, M.; Gray, S. Fabrication of thin film composite poly(amide)-carbon-nanotube supported membranes for enhanced performance in osmotically driven desalination systems. J. Membr. Sci. 2013, 427, 422–430. [Google Scholar] [CrossRef] [Green Version]
- Dumee, L.; He, L.; Hill, M.; Zhu, B.; Duke, M.; Schutz, J.; She, F.; Wang, H.; Gray, S.; Hodgson, P.; et al. Seeded growth of ZiF-8 on the surface of carbon nanotubes towards self-supporting gas separation membranes. J. Mater. Chem. A 2013, 1, 9208–9214. [Google Scholar] [CrossRef]
- First, E.L.; Floudas, C.A. Mofomics: Computational pore characterization of metal–organic frameworks. Microporous Mesoporous Mater. 2013, 165, 32–39. [Google Scholar] [CrossRef]
- Fairen-Jimenez, D.; Moggach, S.A.; Wharmby, M.T.; Wright, P.A.; Parsons, S.; Duren, T. Opening the gate: Framework flexibility in ZiF-8 explored by experiments and simulations. J. Am. Chem. Soc. 2011, 133, 8900–8902. [Google Scholar] [CrossRef] [PubMed]
- McEwen, J.; Hayman, J.-D.; Ozgur Yazaydin, A. A comparative study of CO2, CH4 and N2 adsorption in ZiF-8, Zeolite-13X and BPL activated carbon. Chem. Phys. 2013, 412, 72–76. [Google Scholar] [CrossRef]
- Dumee, L.; Hill, M.R.; Duke, M.; Velleman, L.; Sears, K.; Schutz, J.; Finn, N.; Gray, S. Activation of gold decorated carbon nanotube hybrids for targeted gas adsorption and enhanced catalytic oxidation. J. Mater. Chem. 2012, 22, 9374–9378. [Google Scholar] [CrossRef]
- Novaković, S.B.; Bogdanović, G.A.; Heering, C.; Makhloufi, G.; Francuski, D.; Janiak, C. Charge-density distribution and electrostatic flexibility of ZiF-8 based on high-resolution X-ray diffraction data and periodic calculations. Inorg. Chem. 2015, 54, 2660–2670. [Google Scholar] [CrossRef] [PubMed]
- Greeves, N. ZiF-8 Metal Organic Framework. Available online: http://www.chemtube3d.com/solidstate/MOF-ZIF8.htm (accessed on 22 October 2015).
- Huynh, C.P.; Hawkins, S.C. Understanding the synthesis of directly spinnable carbon nanotube forests. Carbon 2010, 48, 1105–1115. [Google Scholar] [CrossRef]
- Merenda, A.; des Ligneris, E.; Sears, K.; Chaffraix, T.; Magniez, K.; Cornu, D.; Schütz, J.A.; Dumée, L.F. Assessing the temporal stability of surface functional groups introduced by plasma treatments on the outer shells of carbon nanotubes. Sci. Rep. 2016. [Google Scholar] [CrossRef] [PubMed]

| Gas | Mw (g·mol−1) | αTH over N2 | Kinetic Diameter (Å) | Permeance (KPa·s−1) | αXP |
|---|---|---|---|---|---|
| He | 2 | 0.26 | 2.6 | 0.0029 | 0.86 |
| N2 | 28 | 1 | 3.6 | 0.0025 | 1 |
| CO2 | 44 | 1.25 | 3.3 | 0.0018 | 1.38 |
| Ar | 39.9 | 1.19 | 3.4 | 0.0022 | 1.13 |
| Xe | 131 | 2.16 | 3.96 | 0.0012 | 2.08 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumée, L.F.; He, L.; Hodgson, P.; Kong, L. Insights into Surface Interactions between Metal Organic Frameworks and Gases during Transient Adsorption and Diffusion by In-Situ Small Angle X-ray Scattering. Membranes 2016, 6, 41. https://doi.org/10.3390/membranes6030041
Dumée LF, He L, Hodgson P, Kong L. Insights into Surface Interactions between Metal Organic Frameworks and Gases during Transient Adsorption and Diffusion by In-Situ Small Angle X-ray Scattering. Membranes. 2016; 6(3):41. https://doi.org/10.3390/membranes6030041
Chicago/Turabian StyleDumée, Ludovic F., Li He, Peter Hodgson, and Lingxue Kong. 2016. "Insights into Surface Interactions between Metal Organic Frameworks and Gases during Transient Adsorption and Diffusion by In-Situ Small Angle X-ray Scattering" Membranes 6, no. 3: 41. https://doi.org/10.3390/membranes6030041








