Redox Status, Dose and Antioxidant Intake in Healthcare Workers Occupationally Exposed to Ionizing Radiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection and Description of Participants
2.2. Dietary Assessment and Dietary Antioxidant Capacity Estimation
2.3. Blood Collection
2.4. Determination of Antioxidant and Oxidant Markers
2.5. Statistics
3. Results
3.1. High Values of Personal Dose Equivalent and Dietary Antioxidant Capacity among Workers from Nuclear Medicine
3.2. Putative Correlation between Impaired Redox Status and Personal Dose Equivalent
3.3. Prominent Imbalance of Redox Status in Workers from Nuclear Medicine
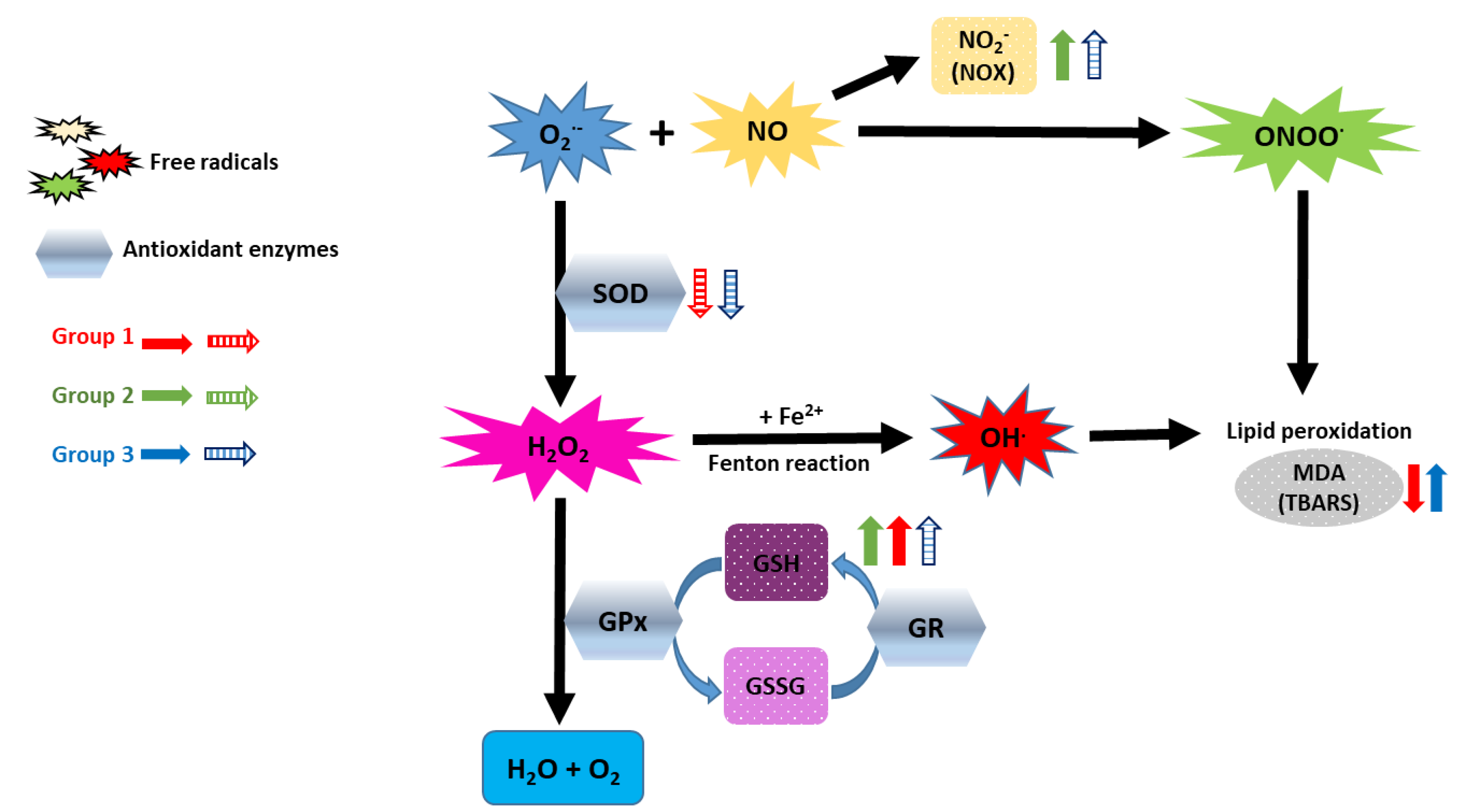
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Doukali, H.; Ben Salah, G.; Hamdaoui, L.; Hajjaji, M.; Tabebi, M.; Ammar-Keskes, L.; Masmoudi, M.E.; Kamoun, H. Oxidative stress and glutathione S-transferase genetic polymorphisms in medical staff professionally exposed to ionizing radiation. Int. J. Radiat. Biol. 2017, 93, 697–704. [Google Scholar] [CrossRef]
- Riley, P.A. Free radicals in biology: Oxidative stress and the effects of ionizing radiation. Int. J. Radiat. Biol. 1994, 65, 27–33. [Google Scholar] [CrossRef]
- Azzam, E.I.; Jay-Gerin, J.P.; Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Kunwar, A.; Barik, A.; Sandur, S.K.; Indira Priyadarsini, K. Differential antioxidant/pro-oxidant activity of dimethoxycurcumin, a synthetic analogue of curcumin. Free Radic. Res. 2011, 45, 959–965. [Google Scholar] [CrossRef]
- Hall, E.J.; Giaccia, A.J. Radiobiology for the Radiologist; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Giardi, M.T.; Touloupakis, E.; Bertolotto, D.; Mascetti, G. Preventive or potential therapeutic value of nutraceuticals against ionizing radiation-induced oxidative stress in exposed subjects and frequent fliers. Int. J. Mol. Sci. 2013, 14, 17168–17192. [Google Scholar] [CrossRef]
- Malekirad, A.A.; Ranjbar, A.; Rahzani, K.; Pilehvarian, A.A.; Rezaie, A.; Zamani, M.J.; Abdollahi, M. Oxidative stress in radiology staff. Environ. Toxicol. Pharm. 2005, 20, 215–218. [Google Scholar] [CrossRef]
- Dainiak, N. Recommendations for assessment of consequences and health risks of low-level exposure to ionizing radiation. Health Phys. 2011, 100, 311–312. [Google Scholar] [CrossRef]
- Baker, J.E.; Moulder, J.E.; Hopewell, J.W. Radiation as a risk factor for cardiovascular disease. Antioxid. Redox Signal. 2011, 15, 1945–1956. [Google Scholar] [CrossRef] [Green Version]
- United Nations Scientific Committee on the Effects of Atomic Radiation. In Sources, Effects and Risks of Ionizing Radiation; United Nations Publications: New York, NY, USA, 2018; pp. 1–194.
- Olisekodiaka, M.; Bello, T.; Onuegbu, A.; Olowookere, C.; Om, L.; Lo, O.; Igbeneghu, I.; Olugbenga-bello, A. Evaluation of the serum total antioxidant status level in health workers exposed to low radiation doses in a large Nigerian hospital. Int. J. Res. Rev. Appl. Sci. 2009, 1, 152–156. [Google Scholar]
- Eken, A.; Aydin, A.; Erdem, O.; Akay, C.; Sayal, A.; Somuncu, I. Induced antioxidant activity in hospital staff occupationally exposed to ionizing radiation. Int. J. Radiat. Biol. 2012, 88, 648–653. [Google Scholar] [CrossRef]
- Maffei, F.; Angelini, S.; Forti, G.C.; Violante, F.S.; Lodi, V.; Mattioli, S.; Hrelia, P. Spectrum of chromosomal aberrations in peripheral lymphocytes of hospital workers occupationally exposed to low doses of ionizing radiation. Mutat. Res. 2004, 547, 91–99. [Google Scholar] [CrossRef]
- Montoro, A.; Rodriguez, P.; Almonacid, M.; Villaescusa, J.I.; Verdu, G.; Caballin, M.R.; Barrios, L.; Barquinero, J.F. Biological dosimetry in a group of radiologists by the analysis of dicentrics and translocations. Radiat. Res. 2005, 164, 612–617. [Google Scholar] [CrossRef]
- The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann. ICRP 2007, 37, 1–332. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Agreement between methods of measurement with multiple observations per individual. J. Biopharm. Stat. 2007, 17, 571–582. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J. Agric. Food Chem. 2004, 52, 4026–4037. [Google Scholar] [CrossRef]
- Limberaki, E.; Eleftheriou, P.; Vagdatli, E.; Kostoglou, V.; Petrou, C. Serum antioxidant status among young, middle-aged and elderly people before and after antioxidant rich diet. Hippokratia 2012, 16, 118–123. [Google Scholar]
- Martinez-Fernandez de la Camara, C.; Salom, D.; Sequedo, M.D.; Hervas, D.; Marin-Lambies, C.; Aller, E.; Jaijo, T.; Diaz-Llopis, M.; Millan, J.M.; Rodrigo, R. Altered antioxidant-oxidant status in the aqueous humor and peripheral blood of patients with retinitis pigmentosa. PLoS ONE 2013, 8, e74223. [Google Scholar] [CrossRef] [Green Version]
- Klucinski, P.; Wojcik, A.; Grabowska-Bochenek, R.; Gminski, J.; Mazur, B.; Hrycek, A.; Cieslik, P.; Martirosian, G. Erythrocyte antioxidant parameters in workers occupationally exposed to low levels of ionizing radiation. Ann. Agric. Environ. Med. 2008, 15, 9–12. [Google Scholar]
- Verdon, C.P.; Burton, B.A.; Prior, R.L. Sample pretreatment with nitrate reductase and glucose-6-phosphate dehydrogenase quantitatively reduces nitrate while avoiding interference by NADP+ when the Griess reaction is used to assay for nitrite. Anal. Biochem. 1995, 224, 502–508. [Google Scholar] [CrossRef]
- Peters, R.M.; Shanies, S.A.; Peters, J.C. Fuzzy cluster analysis of positive stress tests, a new method of combining exercise test variables to predict extent of coronary artery disease. Am. J. Cardiol. 1995, 76, 648–651. [Google Scholar] [CrossRef]
- Rousseeuw, L.K.P.J. Finding Groups in Data: An Introduction to Cluster Analysis. Wiley Online Libr. 1990. [Google Scholar] [CrossRef]
- Seong, K.M.; Seo, S.; Lee, D.; Kim, M.J.; Lee, S.S.; Park, S.; Jin, Y.W. Is the Linear No-Threshold Dose-Response Paradigm Still Necessary for the Assessment of Health Effects of Low Dose Radiation? J. Korean Med. Sci. 2016, 31 (Suppl. 1), S10–S23. [Google Scholar] [CrossRef]
- Durovic, B.; Spasic-Jokic, V. Influence of occupational exposure to low-dose ionizing radiation on the plasma activity of superoxide dismutase and glutathione level. Vojn. Pregl. 2008, 65, 613–618. [Google Scholar] [CrossRef]
- Ahmad, I.M.; Temme, J.B.; Abdalla, M.Y.; Zimmerman, M.C. Redox status in workers occupationally exposed to long-term low levels of ionizing radiation: A pilot study. Redox Rep. 2016, 21, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.; Kumari, S.; Salian, S.R.; Uppangala, S.; Kalthur, G.; Challapalli, S.; Chandraguthi, S.G.; Kumar, P.; Adiga, S.K. Genetic Instability in Lymphocytes is Associated With Blood Plasma Antioxidant Levels in Health Care Workers Occupationally Exposed to Ionizing Radiation. Int. J. Toxicol. 2016, 35, 327–335. [Google Scholar] [CrossRef] [Green Version]
- Hagelstrom, A.H.; Gorla, N.B.; Larripa, I.B. Chromosomal damage in workers occupationally exposed to chronic low level ionizing radiation. Toxicol. Lett. 1995, 76, 113–117. [Google Scholar] [CrossRef]
- Koc, U.; Tan, S.; Ertem, A.G.; Gumus, M.; Ozbek, B.; Erel, O. Evaluation of thiol-disulphide homeostasis in radiation workers. Int. J. Radiat. Biol. 2017, 93, 705–710. [Google Scholar] [CrossRef]
- Einor, D.; Bonisoli-Alquati, A.; Costantini, D.; Mousseau, T.A.; Moller, A.P. Ionizing radiation, antioxidant response and oxidative damage: A meta-analysis. Sci. Total Environ. 2016, 548–549, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Goraca, A.; Skibska, B. Plasma antioxidant status in healthy smoking and non-smoking men. Bratisl. Lek. Listy 2005, 106, 301–306. [Google Scholar] [PubMed]
- Stadtman, E.R. Protein oxidation and aging. Free Radic. Res. 2006, 40, 1250–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, N.J.; Husen, A.Z.; Khoshnaw, N. The Effects of Smoking on IgE, Oxidative Stress and Haemoglobin Concentration. Asian Pac. J. Cancer Prev. 2020, 21, 1069–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fouad, A.A.; Foda, N.T.; Diab, I.H.; Badr El Dine, F.M.M. Evaluation of possible molecular toxicity induced by occupational exposure to lead and concomitant effect of smoking. Environ. Sci. Pollut. Res. Int. 2020, 27, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Isik, B.; Ceylan, A.; Isik, R. Oxidative stress in smokers and non-smokers. Inhal. Toxicol. 2007, 19, 767–769. [Google Scholar] [CrossRef] [PubMed]
- Kander, M.C.; Cui, Y.; Liu, Z. Gender difference in oxidative stress: A new look at the mechanisms for cardiovascular diseases. J. Cell. Mol. Med. 2017, 21, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Pinchuk, I.; Weber, D.; Kochlik, B.; Stuetz, W.; Toussaint, O.; Debacq-Chainiaux, F.; Dolle, M.E.T.; Jansen, E.; Gonos, E.S.; Sikora, E.; et al. Gender- and age-dependencies of oxidative stress, as detected based on the steady state concentrations of different biomarkers in the MARK-AGE study. Redox Biol. 2019, 24, 101204. [Google Scholar] [CrossRef]
- Ahmad, I.M.; Abdalla, M.Y.; Moore, T.A.; Bartenhagen, L.; Case, A.J. Healthcare Workers Occupationally Exposed to Ionizing Radiation Exhibit Altered Levels of Inflammatory Cytokines and Redox Parameters. Antioxidants 2019, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.; Salian, S.R.; Kalthur, G.; Uppangala, S.; Kumari, S.; Challapalli, S.; Chandraguthi, S.G.; Jain, N.; Krishnamurthy, H.; Kumar, P.; et al. Association between sperm DNA integrity and seminal plasma antioxidant levels in health workers occupationally exposed to ionizing radiation. Environ. Res. 2014, 132, 297–304. [Google Scholar] [CrossRef]
- Zamulaieva, I.A.; Orlova, N.V.; Smirnova, S.G.; Proskuriakov, S.; Saenko, A.S. The correlation between intracellular level of nitric oxide and frequency of gene somatic mutations after low dose radiation exposure. Radiats. Biol. Radioecol. 2007, 47, 86–92. [Google Scholar]
- Kamodyová, N.; Tóthová, L.; Celec, P. Salivary markers of oxidative stress and antioxidant status: Influence of external factors. Dis. Markers 2013, 34, 313–321. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, M.; Lee, S.G.; Davis, C.G.; Kenny, A.; Koo, S.I.; Chun, O.K. Plasma total antioxidant capacity is associated with dietary intake and plasma level of antioxidants in postmenopausal women. J. Nutr. Biochem. 2012, 23, 1725–1731. [Google Scholar] [CrossRef]
- Gawron-Skarbek, A.; Guligowska, A.; Prymont-Przymińska, A.; Godala, M.; Kolmaga, A.; Nowak, D.; Szatko, F.; Kostka, T. Dietary Vitamin C, E and β-Carotene Intake Does Not Significantly Affect Plasma or Salivary Antioxidant Indices and Salivary C-Reactive Protein in Older Subjects. Nutrients 2017, 9, 729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Yang, M.; Lee, S.G.; Davis, C.G.; Koo, S.I.; Chun, O.K. Dietary total antioxidant capacity is associated with diet and plasma antioxidant status in healthy young adults. J. Acad. Nutr. Diet. 2012, 112, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Kolarzyk, E.; Pietrzycka, A.; Zając, J.; Morawiecka-Baranek, J. Relationship between dietary antioxidant index (DAI) and antioxidants level in plasma of Kraków inhabitants. Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2017, 26, 393–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corsetto, P.A.; Montorfano, G.; Klersy, C.; Massimino, L.; Infantino, V. Fatty Acid Profile and Antioxidant Status Fingerprint in Sarcopenic Elderly Patients: Role of Diet and Exercise. Nutrients 2019, 11, 2569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Luca, C.; Deeva, I.; Mariani, S.; Maiani, G.; Stancato, A.; Korkina, L. Monitoring antioxidant defenses and free radical production in space-flight, aviation and railway engine operators, for the prevention and treatment of oxidative stress, immunological impairment, and pre-mature cell aging. Toxicol. Ind. Health 2009, 25, 259–267. [Google Scholar] [CrossRef]
- Murcia, M.A.; Jimenez, A.M.; Martinez-Tome, M. Evaluation of the antioxidant properties of Mediterranean and tropical fruits compared with common food additives. J. Food Prot. 2001, 64, 2037–2046. [Google Scholar] [CrossRef]
- Polivkova, Z.; Smerak, P.; Demova, H.; Houska, M. Antimutagenic effects of lycopene and tomato puree. J. Med. Food 2010, 13, 1443–1450. [Google Scholar] [CrossRef]
- Martinez-Tome, M.; Jimenez, A.M.; Ruggieri, S.; Frega, N.; Strabbioli, R.; Murcia, M.A. Antioxidant properties of Mediterranean spices compared with common food additives. J. Food Prot. 2001, 64, 1412–1419. [Google Scholar] [CrossRef]
- Hammerstone, J.F.; Lazarus, S.A.; Schmitz, H.H. Procyanidin content and variation in some commonly consumed foods. J. Nutr. 2000, 130, 2086S–2092S. [Google Scholar] [CrossRef]
- Mak, J.C. Potential role of green tea catechins in various disease therapies: Progress and promise. Clin. Exp. Pharm. Physiol. 2012, 39, 265–273. [Google Scholar] [CrossRef]
- Darvesh, A.S.; Carroll, R.T.; Bishayee, A.; Novotny, N.A.; Geldenhuys, W.J.; Van der Schyf, C.J. Curcumin and neurodegenerative diseases: A perspective. Expert Opin. Investig. Drugs 2012, 21, 1123–1140. [Google Scholar] [CrossRef] [PubMed]


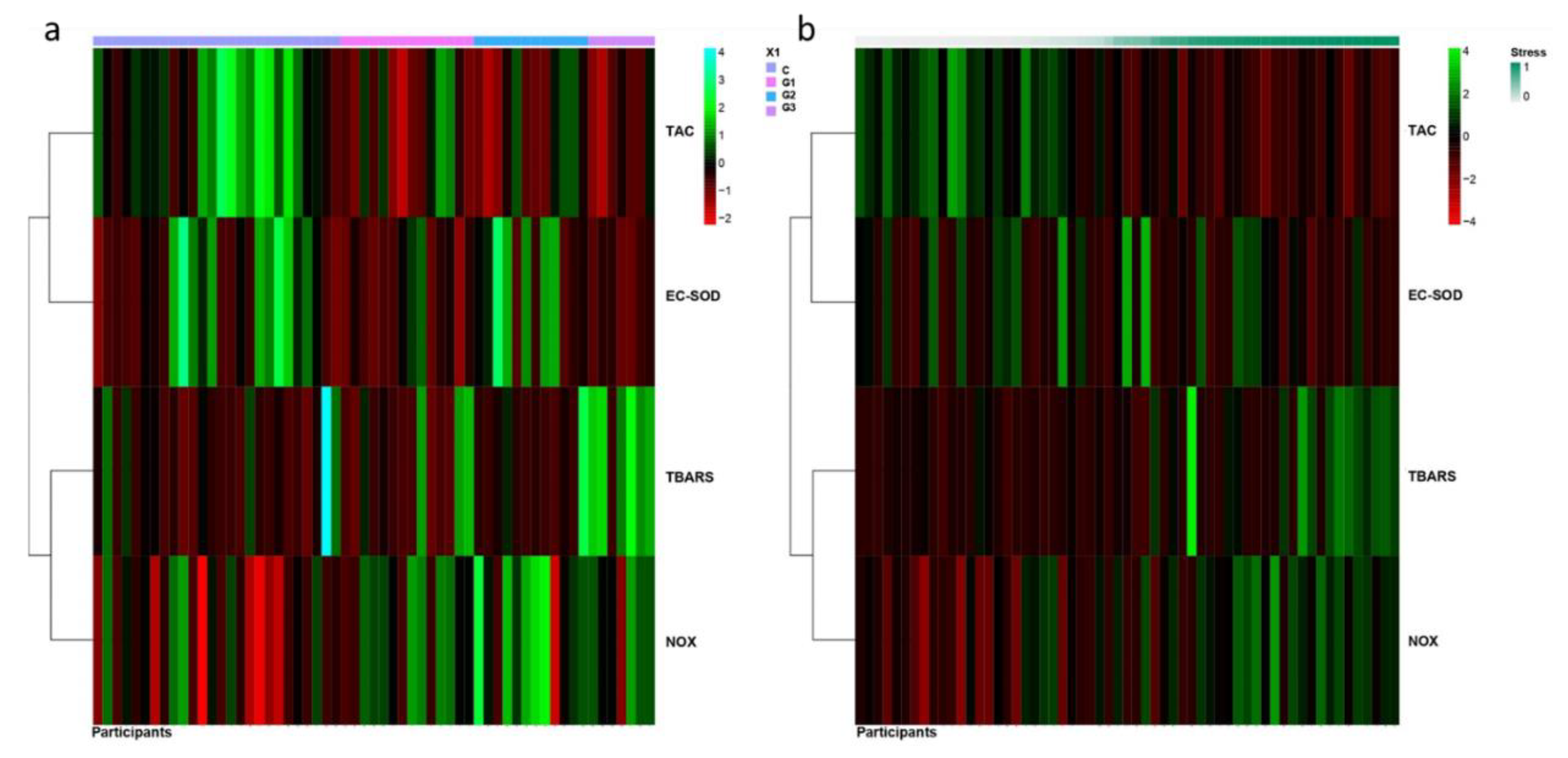
| Characteristics | Control | Group 1 | Group 2 | Group 3 |
|---|---|---|---|---|
| N of subjects | 28 | 14 9 nurses, 4 physicians, 1 radiographer | 18 6 nurses, 6 physicians,3 physicists, 3 radiographers | 10 5 nurses 1 physician 1 pharmacist 3 radiographers |
| Males | 10 | 3 | 8 | 1 |
| Females | 18 | 11 | 10 | 9 |
| Age (yr) Mean (SEM) | 43 (2) | 48 (1) | 50 (3) | 48 (1) |
| Smokers (n smokers/group) | 3/28 | 4/14 | 4/18 | 5/10 |
| High Cholesterol (n subjects with >220 mg/dL/group) | 6/28 | 1/14 | 0/18 | 1/10 |
| Hypertension (n of subject with ≥140/90 mm Hg/group) | 1/28 | 2/14 | 0/18 | 0/10 |
| Vitamins/minerals supplements (n of subject/group) | 1/28 | 1/14 | 0/18 | 0/10 |
| ORAC (μmol TE/100 g) Mean (SEM) | 12599 (1449) | 17796 (2615) | 17272 (3039) | 23368 (3716) * |
| 19074 (1822) (Groups 1–3) | ||||
| Hp(10)12 (mSv) Mean (SEM) [Range] | ---- | 0.22 (0.15) [0.0–1.9] | 0.05 (0.03) [0.0–0.6] | 0.45 (0.17) a,b [0.0–1.4] |
| 0.2 (0.07) [0.0–1.9] (Groups 1–3) | ||||
| Hp(0.07)12 (mSv) Mean (SEM) [Range] | ---- | 11.05 (5.66) [1.8–38.7] | 0.12 (0.08) c [0.0–0.5] | 3.14 (1.46) [0.0–11.1] |
| 4.7 (2.0) [0.0–38.7] (Groups 1–3) | ||||
| Cumulative Hp(10) (mSv) Mean (SEM) [Range] | ---- | 5.16 (1.43) [0.0–13.7] | 5.56 (2.67) [0.0–49.8] | 9.8 (6.4) [0.0–65.3] |
| 6.44 (1.95) [0.1–65.3] (Groups 1–3) | ||||
| Variables | Estimate | S. Error | p | Estimate | S. Error | p | Estimate | S. Error | p | Estimate | S. Error | p | Estimate | S. Error | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TAC | EC-SOD | GSG/GSSG | TBARS | NOX | |||||||||||
| Age | 0.043 | 0.028 | 0.126 | 0.026 | 0.029 | 0.371 | −0.032 | 0.041 | 0.426 | −0.009 | 0.029 | 0.763 | −0.004 | 0.029 | 0.888 |
| Gender | 0.749 | 0.626 | 0.232 | −0.202 | 0.672 | 0.764 | −2.745 | 1.293 | 0.034 | −0.302 | 0.707 | 0.669 | −1.385 | 0.658 | 0.035 |
| Tobacco | −3.455 | 0.929 | <0.001 | 0.51 | 0.837 | 0.542 | −0.637 | 1.673 | 0.703 | 0.874 | 0.898 | 0.33 | 0.905 | 0.781 | 0.247 |
| High Cholesterol | 1.455 | 0.979 | 0.137 | −0.043 | 0.956 | 0.964 | 2.138 | 1.27 | 0.092 | −0.978 | 0.927 | 0.291 | 1.136 | 0.932 | 0.222 |
| ORAC | −0.102 | 0.037 | 0.006 | 0.022 | 0.035 | 0.525 | 0.016 | 0.079 | 0.844 | 0.011 | 0.038 | 0.772 | 0.028 | 0.036 | 0.443 |
| C vs. Group 1 | −2.364 | 1.058 | 0.025 | −1.276 | 0.919 | 0.165 | 4.983 | 2.186 | 0.023 | −2.34 | 0.959 | 0.015 | 1.276 | 0.927 | 0.169 |
| C vs. Group 2 | −2.009 | 0.841 | 0.017 | 0.87 | 0.818 | 0.288 | 4.031 | 1.868 | 0.031 | 0.081 | 0.743 | 0.913 | 2.701 | 0.893 | 0.002 |
| C vs. Group 3 | −3.176 | 1.032 | 0.002 | −1.696 | 1.031 | 0.1 | 2.769 | 1.604 | 0.084 | 4.428 | 1.682 | 0.008 | 1.845 | 0.959 | 0.054 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sebastià, N.; Olivares-González, L.; Montoro, A.; Barquinero, J.-F.; Canyada-Martinez, A.J.; Hervás, D.; Gras, P.; Villaescusa, J.I.; Martí-Bonmatí, L.; Muresan, B.T.; et al. Redox Status, Dose and Antioxidant Intake in Healthcare Workers Occupationally Exposed to Ionizing Radiation. Antioxidants 2020, 9, 778. https://doi.org/10.3390/antiox9090778
Sebastià N, Olivares-González L, Montoro A, Barquinero J-F, Canyada-Martinez AJ, Hervás D, Gras P, Villaescusa JI, Martí-Bonmatí L, Muresan BT, et al. Redox Status, Dose and Antioxidant Intake in Healthcare Workers Occupationally Exposed to Ionizing Radiation. Antioxidants. 2020; 9(9):778. https://doi.org/10.3390/antiox9090778
Chicago/Turabian StyleSebastià, Natividad, Lorena Olivares-González, Alegría Montoro, Joan-Francesc Barquinero, Antonio José Canyada-Martinez, David Hervás, Pilar Gras, Juan Ignacio Villaescusa, Luis Martí-Bonmatí, Bianca Tabita Muresan, and et al. 2020. "Redox Status, Dose and Antioxidant Intake in Healthcare Workers Occupationally Exposed to Ionizing Radiation" Antioxidants 9, no. 9: 778. https://doi.org/10.3390/antiox9090778








