Erythronium japonicum Alleviates Inflammatory Pain by Inhibiting MAPK Activation and by Suppressing NF-κB Activation via ERK/Nrf2/HO-1 Signaling Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation and Extraction Procedure
2.3. Cell Culture and Viability Assay
2.4. Nitric Oxide (NO) Assay
2.5. Western Blotting Assay
2.6. Total RNA Isolation and Quantitative Real-Time (qRT-PCR) Assay
2.7. Measurement of Pro-Inflammatory Cytokines
2.8. Cytoplasmic and Nuclear Fractionation
2.9. Animals and Experimental Design
2.10. Von Frey Test to Estimate Mechanical Allodynia
2.11. Extract Fractionation
2.12. Statistical Analysis
3. Results
3.1. EJE Suppresses LPS-Induced Microglial Activation in BV2 Microglia
3.2. EJE Inhibits LPS-Induced Pro-Inflammatory Cytokines in BV2 Microglia
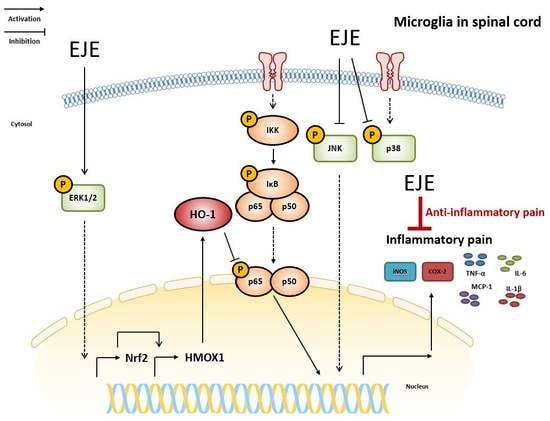
3.3. EJE Inhibits LPS-Induced JNK and p38 Phosphorylation, but Increases ERK1/2 Phosphorylation in BV2 Microglia
3.4. EJE Reduces LPS-Induced NF-ĸB Activation via ERK/Nrf2/HO-1 Signaling in BV2 Microglia
3.5. Oral Administration of EJE Alleviates CFA-Induced Pain Hypersensitivity in Mice and Reduces CFA-Induced Microglial Activation and Neuroinflammation in the Spinal Cord of Mice
3.6. The Butanol Fraction of EJE Mostly Reduces LPS-Induced Microglial Activation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dahlhamer, J.; Lucas, J.; Zelaya, C.; Nahin, R.; Mackey, S.; DeBar, L.; Kerns, R.; Von Korff, M.; Porter, L.; Helmick, C. Prevalence of Chronic Pain and High-Impact Chronic Pain among Adults—United States, 2016. MMWR Morb. Mortal. Wkly Rep. 2018, 67, 1001–1006. [Google Scholar] [CrossRef]
- Thomas, D.A.; Maslin, B.; Legler, A.; Springer, E.; Asgerally, A.; Vadivelu, N. Role of Alternative Therapies for Chronic Pain Syndromes. Curr. Pain Headache Rep. 2016, 20, 29. [Google Scholar] [CrossRef] [PubMed]
- Forouzanfar, F.; Hosseinzadeh, H. Medicinal herbs in the treatment of neuropathic pain: A review. Iran. J. Basic Med. Sci. 2018, 21, 347–358. [Google Scholar] [CrossRef]
- Seo, J.H.; Bang, M.A.; Kim, G.; Cho, S.S.; Park, D.H. Erythronium japonicum attenuates histopathological lung abnormalities in a mouse model of ovalbumin-induced asthma. Int. J. Mol. Med. 2016, 37, 1221–1228. [Google Scholar] [CrossRef] [Green Version]
- Heo, B.G.; Park, Y.S.; Chon, S.U.; Lee, S.Y.; Cho, J.Y.; Gorinstein, S. Antioxidant activity and cytotoxicity of methanol extracts from aerial parts of Korean salad plants. Biofactors 2007, 30, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Bae, C.S.; Yun, C.H.; Ahn, T. Extracts from Erythronium japonicum and Corylopsis coreana Uyeki reduce 1,3-dichloro-2-propanol-mediated oxidative stress in human hepatic cells. Food Sci. Biotechnol. 2019, 28, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Fiebich, B.L.; Batista, C.R.A.; Saliba, S.W.; Yousif, N.M.; de Oliveira, A.C.P. Role of Microglia TLRs in Neurodegeneration. Front. Cell. Neurosci. 2018, 12, 329. [Google Scholar] [CrossRef] [Green Version]
- Kielian, T. Toll-like receptors in central nervous system glial inflammation and homeostasis. J. Neurosci. Res. 2006, 83, 711–730. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Kuzmich, N.N.; Sivak, K.V.; Chubarev, V.N.; Porozov, Y.B.; Savateeva-Lyubimova, T.N.; Peri, F. TLR4 Signaling Pathway Modulators as Potential Therapeutics in Inflammation and Sepsis. Vaccines 2017, 5, 34. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.R.; Xu, H.; Tao, M.; Xu, L.H.; Fu, X.C. Ligustilide Relieves Complete Freund’s Adjuvant-Induced Mechanical Hyperalgesia through Inhibiting the Activation of Spinal c-Jun N-terminal Kinase/c-Jun Pathway in Rats. Pharmacogn. Mag. 2017, 13, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Nasseri, B.; Zaringhalam, J.; Daniali, S.; Manaheji, H.; Abbasnejad, Z.; Nazemian, V. Thymulin treatment attenuates inflammatory pain by modulating spinal cellular and molecular signaling pathways. Int. Immunopharmacol. 2019, 70, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Paine, A.; Eiz-Vesper, B.; Blasczyk, R.; Immenschuh, S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem. Pharmacol. 2010, 80, 1895–1903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Yang, F.; Liu, F.; Li, D.; Yang, T. NRF2/HO-1 activation via ERK pathway involved in the anti-neuroinflammatory effect of Astragaloside IV in LPS induced microglial cells. Neurosci. Lett. 2018, 666, 104–110. [Google Scholar] [CrossRef]
- Li, C.; Chen, T.; Zhou, H.; Zhang, C.; Feng, Y.; Tang, F.; Hoi, M.P.; He, C.; Zheng, Y.; Lee, S.M. Schisantherin A Attenuates Neuroinflammation in Activated Microglia: Role of Nrf2 Activation through ERK Phosphorylation. Cell Physiol. Biochem. 2018, 47, 1769–1784. [Google Scholar] [CrossRef]
- Sun, G.Y.; Chen, Z.; Jasmer, K.J.; Chuang, D.Y.; Gu, Z.; Hannink, M.; Simonyi, A. Quercetin Attenuates Inflammatory Responses in BV-2 Microglial Cells: Role of MAPKs on the Nrf2 Pathway and Induction of Heme Oxygenase-1. PLoS ONE 2015, 10, e0141509. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhang, Z.; Cheng, Z.; Zhang, J.; Xu, S.; Liu, H.; Jia, H.; Jin, Y. Spinal Heme Oxygenase-1 (HO-1) Exerts Antinociceptive Effects against Neuropathic Pain in a Mouse Model of L5 Spinal Nerve Ligation. Pain Med. 2016, 17, 220–229. [Google Scholar] [CrossRef] [Green Version]
- Redondo, A.; Chamorro, P.A.F.; Riego, G.; Leanez, S.; Pol, O. Treatment with Sulforaphane Produces Antinociception and Improves Morphine Effects during Inflammatory Pain in Mice. J. Pharmacol. Exp. Ther. 2017, 363, 293–302. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Gu, M.Y.; Xu, J.L.; Zhang, L.J.; Ryu, S.Y.; Yang, H.O. Anti-Neuroinflammatory Effects of 12-Dehydrogingerdione in LPS-Activated Microglia through Inhibiting Akt/IKK/NF-κB Pathway and Activating Nrf-2/HO-1 Pathway. Biomol. Ther. 2019, 27, 92–100. [Google Scholar] [CrossRef]
- Sun, X.; Zeng, H.; Wang, Q.; Yu, Q.; Wu, J.; Feng, Y.; Deng, P.; Zhang, H. Glycyrrhizin ameliorates inflammatory pain by inhibiting microglial activation-mediated inflammatory response via blockage of the HMGB1-TLR4-NF-kB pathway. Exp. Cell Res. 2018, 369, 112–119. [Google Scholar] [CrossRef]
- Inoue, K.; Tsuda, M. Microglia in neuropathic pain: Cellular and molecular mechanisms and therapeutic potential. Nat. Rev. Neurosci. 2018, 19, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, Y.Q.; Qadri, Y.J.; Serhan, C.N.; Ji, R.R. Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.D.; Zhao, L.X.; Wang, X.T.; Gao, Y.J.; Zhang, Z.J. Ligustilide inhibits microglia-mediated proinflammatory cytokines production and inflammatory pain. Brain Res. Bull. 2014, 109, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Y.; Tan, M.S.; Yu, J.T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Jin, C.Y.; Kim, C.H.; Yoo, Y.H.; Choi, S.H.; Kim, G.Y.; Yoon, H.M.; Park, H.T.; Choi, Y.H. Isorhamnetin alleviates lipopolysaccharide-induced inflammatory responses in BV2 microglia by inactivating NF-κB, blocking the TLR4 pathway and reducing ROS generation. Int. J. Mol. Med. 2019, 43, 682–692. [Google Scholar] [CrossRef] [Green Version]
- Waetzig, V.; Czeloth, K.; Hidding, U.; Mielke, K.; Kanzow, M.; Brecht, S.; Goetz, M.; Lucius, R.; Herdegen, T.; Hanisch, U.K. c-Jun N-terminal kinases (JNKs) mediate pro-inflammatory actions of microglia. Glia 2005, 50, 235–246. [Google Scholar] [CrossRef]
- Ji, R.R.; Suter, M.R. p38 MAPK, microglial signaling, and neuropathic pain. Mol. Pain 2007, 3, 33. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Zhang, Y.; Lu, W.; Gao, X.; Xu, C.; Bao, H. Caffeic acid phenethyl ester attenuates neuropathic pain by suppressing the p38/NF-κB signal pathway in microglia. J. Pain Res. 2018, 11, 2709–2719. [Google Scholar] [CrossRef] [Green Version]
- Taves, S.; Berta, T.; Liu, D.L.; Gan, S.; Chen, G.; Kim, Y.H.; Van de Ven, T.; Laufer, S.; Ji, R.R. Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: Sex-dependent microglial signaling in the spinal cord. Brain Behav. Immun. 2016, 55, 70–81. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.F.; Shen, X.Y.; Lio, C.K.; Dai, Y.; Cheng, C.S.; Liu, J.X.; Yao, Y.D.; Yu, Y.; Xie, Y.; Luo, P.; et al. Activation of Nrf2/HO-1 Pathway by Nardochinoid C Inhibits Inflammation and Oxidative Stress in Lipopolysaccharide-Stimulated Macrophages. Front. Pharmacol. 2018, 9, 911. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Yang, H.; Son, G.W.; Park, H.R.; Park, C.S.; Jin, Y.H.; Park, Y.S. Eriodictyol Protects Endothelial Cells against Oxidative Stress-Induced Cell Death through Modulating ERK/Nrf2/ARE-Dependent Heme Oxygenase-1 Expression. Int. J. Mol. Sci. 2015, 16, 14526–14539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.Y.; Kang, K.A.; Kim, K.C.; Cha, J.W.; Kim, E.H.; Hyun, J.W. Morin Induces Heme Oxygenase-1 via ERK-Nrf2 Signaling Pathway. J. Cancer Prev. 2013, 18, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Sandberg, M.; Patil, J.; D’Angelo, B.; Weber, S.G.; Mallard, C. NRF2-regulation in brain health and disease: Implication of cerebral inflammation. Neuropharmacology 2014, 79, 298–306. [Google Scholar] [CrossRef] [Green Version]
- Syapin, P.J. Regulation of haeme oxygenase-1 for treatment of neuroinflammation and brain disorders. Br. J. Pharmacol. 2008, 155, 623–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jazwa, A.; Cuadrado, A. Targeting heme oxygenase-1 for neuroprotection and neuroinflammation in neurodegenerative diseases. Curr. Drug Targets 2010, 11, 1517–1531. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M. Microglia in the spinal cord and neuropathic pain. J. Diabetes Investig. 2016, 7, 17–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, R.R.; Chamessian, A.; Zhang, Y.Q. Pain regulation by non-neuronal cells and inflammation. Science 2016, 354, 572–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.J.; Peng, J.; Xu, Y.N.; Zeng, W.J.; Zhang, J.; Wei, X.; Mai, C.L.; Lin, Z.J.; Liu, Y.; Murugan, M.; et al. Microglia Are Indispensable for Synaptic Plasticity in the Spinal Dorsal Horn and Chronic Pain. Cell Rep. 2019, 27, 3844–3859. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.Q.; Liu, Z.; Liu, Z.H.; Chen, S.P.; Li, M.; Shahveranov, A.; Ye, D.W.; Tian, Y.K. Interleukin-6: An emerging regulator of pathological pain. J. Neuroinflamm. 2016, 13, 141. [Google Scholar] [CrossRef] [Green Version]
- Kwon, M.S.; Shim, E.J.; Seo, Y.J.; Choi, S.S.; Lee, J.Y.; Lee, H.K.; Suh, H.W. Differential modulatory effects of cholera toxin and pertussis toxin on pain behavior induced by TNF-α, interleukin-1β and interferon-gamma injected intrathecally. Arch. Pharm. Res. 2005, 28, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Sommer, C.; Schmidt, C.; George, A. Hyperalgesia in experimental neuropathy is dependent on the TNF receptor 1. Exp. Neurol. 1998, 151, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.J.; Hao, J.X.; Andell-Jonsson, S.; Poli, V.; Bartfai, T.; Wiesenfeld-Hallin, Z. Nociceptive responses in interleukin-6-deficient mice to peripheral inflammation and peripheral nerve section. Cytokine 1997, 9, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Gui, W.S.; Wei, X.; Mai, C.L.; Murugan, M.; Wu, L.J.; Xin, W.J.; Zhou, L.J.; Liu, X.G. Interleukin-1β overproduction is a common cause for neuropathic pain, memory deficit, and depression following peripheral nerve injury in rodents. Mol. Pain 2016, 12. [Google Scholar] [CrossRef] [Green Version]
- White, F.A.; Feldman, P.; Miller, R.J. Chemokine signaling and the management of neuropathic pain. Mol. Interv. 2009, 9, 188–195. [Google Scholar] [CrossRef] [Green Version]
- You, M.K.; Kim, M.S.; Rhyu, J.; Bang, M.A.; Kim, H.A. Inhibitory effect of Erythronium japonicum on the human breast cancer cell metastasis. Nutr. Res. Pract. 2015, 9, 17–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, W.; Qi, R.; Zhang, J.; Wang, Z.; Wang, H.; Hu, C.; Zhao, Y.; Bie, M.; Wang, Y.; Fu, Y.; et al. Chlorogenic acid inhibits LPS-induced microglial activation and improves survival of dopaminergic neurons. Brain Res. Bull. 2012, 88, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, H.S.; Notsu, Y.; Ban, H.S.; Kim, Y.P.; Ishihara, K.; Hirasawa, N.; Jung, S.H.; Lee, Y.S.; Lim, S.S.; et al. Effects of hyperin, isoquercitrin and quercetin on lipopolysaccharide-induced nitrite production in rat peritoneal macrophages. Phytother. Res. 2008, 22, 1552–1556. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Sapkota, K.; Kim, S.; Kim, H.; Kim, S.J. Kaempferol acts through mitogen-activated protein kinases and protein kinase B/AKT to elicit protection in a model of neuroinflammation in BV2 microglial cells. Br. J. Pharmacol. 2011, 164, 1008–1025. [Google Scholar] [CrossRef] [Green Version]
- Heitman, E.; Ingram, D.K. Cognitive and neuroprotective effects of chlorogenic acid. Nutr. Neurosci. 2017, 20, 32–39. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, T.; Pei, Q.; Liu, S.; Yuan, H. Pharmacokinetics and tissue distribution study of chlorogenic Acid from lonicerae japonicae flos following oral administrations in rats. Evid.-Based Complement. Altern. Med. 2014, 2014, 979414. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Paliwal, P.; Mukherjee, S.; Patnaik, N.; Krishnamurthy, S.; Patnaik, R. Pharmacokinetics and brain penetration study of chlorogenic acid in rats. Xenobiotica 2019, 49, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Grabska-Kobylecka, I.; Kaczmarek-Bak, J.; Figlus, M.; Prymont-Przyminska, A.; Zwolinska, A.; Sarniak, A.; Wlodarczyk, A.; Glabinski, A.; Nowak, D. The Presence of Caffeic Acid in Cerebrospinal Fluid: Evidence that Dietary Polyphenols Can Cross the Blood-Brain Barrier in Humans. Nutrients 2020, 12, 1531. [Google Scholar] [CrossRef] [PubMed]






| Species | Gene | Primer Sequence (5′-3′) | |
|---|---|---|---|
| Forward | Reverse | ||
| Mouse | TNF-α | CCTCTCTCTAATCAGCCCTCTG | GAGGACCTGGGAGTAGATGAG |
| IL-1β | ATGATGGCTTATTACAGTGGCAA | GTCGGAGATTCGTAGCTGGA | |
| IL-6 | ACTCACCTCTTCAGAACGAATTG | CCATCTTTGGAAGGTTCAGGTTG | |
| MCP-1 | CAGCCAGATGCAATCAATGCC | TGGAATCCTGAACCCACTTCT | |
| HMOX1 | AAGCCGAGAATGCTGAGTTCA | GCCGTGTAGATATGGTACAAGGA | |
| G6PD | CACAGTGGACGACATCCGAAA | AGCTACATAGGAATTACGGGCAA | |
| NQO1 | AGGATGGGAGGTACTCGAATC | AGGCGTCCTTCCTTATATGCTA | |
| FTL1 | CCATCTGACCAACCTCCGC | CGCTCAAAGAGATACTCGCC | |
| GAPDH | GGAGCGAGATCCCTCCAAAAT | GGCTGTTGTCATACTTCTCATGG | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Kim, Y.T. Erythronium japonicum Alleviates Inflammatory Pain by Inhibiting MAPK Activation and by Suppressing NF-κB Activation via ERK/Nrf2/HO-1 Signaling Pathway. Antioxidants 2020, 9, 626. https://doi.org/10.3390/antiox9070626
Park J, Kim YT. Erythronium japonicum Alleviates Inflammatory Pain by Inhibiting MAPK Activation and by Suppressing NF-κB Activation via ERK/Nrf2/HO-1 Signaling Pathway. Antioxidants. 2020; 9(7):626. https://doi.org/10.3390/antiox9070626
Chicago/Turabian StylePark, Joon, and Yun Tai Kim. 2020. "Erythronium japonicum Alleviates Inflammatory Pain by Inhibiting MAPK Activation and by Suppressing NF-κB Activation via ERK/Nrf2/HO-1 Signaling Pathway" Antioxidants 9, no. 7: 626. https://doi.org/10.3390/antiox9070626