Citrus × Clementina Hort. Juice Enriched with Its By-Products (Peels and Leaves): Chemical Composition, In Vitro Bioactivity, and Impact of Processing
Abstract
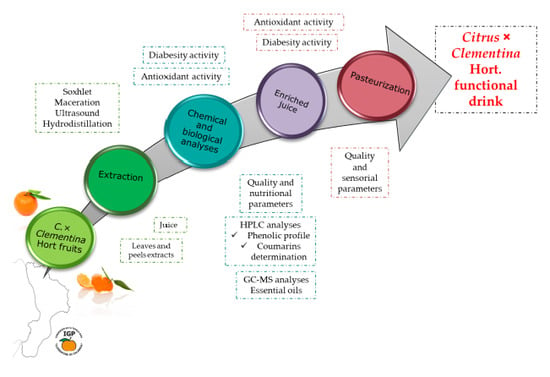
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Materials
2.3. Sample Preparation
2.4. Quality Parameters of C. × Clementina Fruits
2.5. Quality Parameters of C. × Clementina Juice
2.6. Gas Chromatography–Mass Spectrometry (GC–MS) Analyses
2.7. Total Phenol, Flavonoid and Carotenoid Contents of Juice and Extracts
2.8. HPLC–DAD Phenolic Profile
2.9. HPLC Coumarin Determination
2.10. Antioxidant Activity
2.11. Relative Antioxidant Capacity Index (RACI)
2.12. Carbohydrate Hydrolysing-Enzyme Inhibition Study
2.13. Pancreatic Lipase Inhibition Assay
2.14. Sensory Analysis
2.15. Statistical Analysis
3. Results and Discussion
3.1. Quality Parameters
3.2. Extraction Yield and Total Phytochemical Contents
3.3. C. × Clementina Peel Essential Oil Profile
3.4. Phenolic Profile
3.5. Coumarin Determination
3.6. Antioxidant Activity
3.7. Effect on Carbohydrate-Hydrolysing Enzymes and Lipase
3.8. PCA
3.9. Enriched Juice
3.9.1. Quality Characteristics
3.9.2. Antioxidant Activity
3.9.3. Effect of Enriched Juice on α-Amylase and α-Glucosidase and Lipase
3.9.4. Sensory Analysis
3.10. Pasteurization Process
3.10.1. Quality Parameters
3.10.2. Antioxidant Activity
3.10.3. Enzyme Inhibitory Activities
3.10.4. Sensory Analysis
3.10.5. PCA
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Sharma, Y.K.; Mangla, S.K.; Patil, P.P.; Liu, S. When challenges impede the process for circular economy-driven sustainability practices in food supply chain. Manag. Decis. 2019, 57, 995–1017. [Google Scholar] [CrossRef]
- Bayona-Saez, C.; Cruz-Cázares, C.; García-Marco, T.; Sánchez García, M. Open innovation in the food and beverage industry. Manag. Decis. 2017, 55, 526–546. [Google Scholar] [CrossRef] [Green Version]
- Helkar, P.B.; Sahoo, A.K.; Patil, N.J. Food industry by-products used as a functional food ingredient. Int. J. Waste Resour. 2016, 6, 248–254. [Google Scholar]
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food waste: A potential bioresource for extraction of nutraceuticals and bioactive compounds. Biores. Bioproc. 2017, 4, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Rombaut Rombaut, N.; Tixier, S.; Bily, A.; Chemat, F. Green extraction processes of natural products as tools for biorefinery. Biofuel. Bioprod. Bior. 2014, 8, 530–544. [Google Scholar] [CrossRef]
- Arjeh, E.; Akhavan, H.R.; Barzegar, M.; Carbonell-Barrachina, A.A. Bio-active compounds and functional properties of pistachio hull: A review. Trends Food Sci. Technol. 2020, 97, 55–64. [Google Scholar] [CrossRef]
- Caliceti, C.; Capriotti, A.L.; Calabria, D.; Bonvicini, F.; Zenezini Chiozzi, R.; Montone, C.M.; Piovesana, S.; Zangheri, R.; Mirasoli, M.; Simoni, P.; et al. Peptides from cauliflower by-products, obtained by an efficient, ecosustainable, and semi-industrial method, exert protective effects on endothelial function. Oxid. Med. Cell. Longev. 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Lafarga, T.; Hayes, M. Bioactive peptides from meat muscle and by-products: Generation, functionality and application as functional ingredients. Meat Sci. 2014, 98, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Meneguetti, B.T.; dos Santos, M.L.; Oshiro Karen, G.N.; Nogueira Micaella, L.; Carvalho Cristiano, M.E.; Franco Octávio, L. Antimicrobial peptides from fruits and their potential use as biotechnological tools—A Review and Outlook. Front. Microbiol. 2017, 7, 2136. [Google Scholar] [CrossRef] [Green Version]
- Montone, C.M.; Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Piovesana, S.; Zenezini Chiozzi, R.; Laganà, A. Characterization of antioxidant and angiotensin-converting enzyme inhibitory peptides derived from cauliflower by-products by multidimensional liquid chromatography and bioinformatics. J. Funct. Foods 2018, 44, 40–47. [Google Scholar] [CrossRef]
- Montone, C.; Capriotti, A.; Cavaliere, C.; la barbera, G.; Piovesana, S.; Zenezini Chiozzi, R.; Laganà, A. Peptidomic strategy for purification and identification of potential ACE-inhibitory and antioxidant peptides in Tetradesmus obliquus microalgae. Anal. Bioanal. Chem. 2018, 410, 3573–3586. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yin, Y.; Zhao, W.; Chen, F.; Liu, J. Application and bioactive properties of proteins and peptides derived from hen eggs: Opportunities and challenges. J. Sci. Food. Agric. 2014, 94, 2839–2845. [Google Scholar] [CrossRef] [PubMed]
- AO (2015) FAOSTAT. Agricultural Statistics Database; World Agricultural Information Center: Rome, Italy, 23009; Available online: http://faostat.fao.org/site/567/default.aspx#ancor (accessed on 4 December 2019).
- Loizzo, M.R.; Leporini, M.; Sicari, V.; Falco, T.; Pellicanò, M.T.; Tundis. R. Investigating the in vitro hypoglycemic and antioxidant properties of Citrus × clementina Hort. Juice. Eur. Food Res. Technol. 2018, 244, 523–534. [Google Scholar] [CrossRef]
- Leporini, M.; Tundis, R.; Sicari, V.; Pellicanò, T.M.; Dugay, A.; Deguin, B.; Loizzo, M.R. Impact of extraction processes on phytochemicals content and biological activity of Citrus × clementina Hort. Ex Tan leaves: New opportunity for under-utilized food by-products. Food Res. Int. 2020, 127, 108742. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 2325/97 of 24 November 1997 Supplementing the Annex to Regulation (EC) No 1107/96 on the Registration of Geographical Indications and Designations of Origin under the Procedure Laid Down in Article 17 of Council Regulation (EEC) No 2081/92; European Commission: Brussels, Belgium, 1997. [Google Scholar]
- Tundis, R.; Bonesi, M.; Sicari, V.; Pellicanò, T.M.; Tenuta, M.C.; Leporini, M.; Menichini, M.; Loizzo, M.R. Poncirus trifoliata (L.) Raf.: Chemical composition, antioxidant properties and hypoglycemic activity via the inhibition of -amylase and -glucosidase enzymes. J. Funct. Foods 2016, 25, 477–485. [Google Scholar] [CrossRef]
- Sdiri, S.; Bermejo, A.; Aleza, P.; Navarro, P.; Salvador, A. Phenolic composition, organic acids, sugars, vitamin C and antioxidant activity in the juice of two new triploid late-season mandarins. Food Res. Int. 2012, 49, 462–468. [Google Scholar] [CrossRef]
- Camarda, L.; Di Stefano, V.; Del Bosco, S.F.; Schillaci, D. Antiproliferative activity of Citrus juices and HPLC evaluation of their flavonoid composition. Fitoterapia 2007, 78, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Boudries, H.; Loupassaki, S.; Ladjal Ettoumi, Y.; Souagui, S.; Bachir Bey, M.; Nabet, N.; Chikhoune, A.; Madani, K.; Chibane, M. Chemical profile, antimicrobial and antioxidant activities of Citrus reticulate and Citrus clementina (L.) essential oils. Food Res. Int. J. 2017, 24, 1782–1792. [Google Scholar]
- Bonesi, M.; Loizzo, M.R.; Leporini, M.; Tenuta, M.C.; Passalacqua, N.G.; Tundis, R. Comparative evaluation of petitgrain oils from six Citrus species alone and in combination as potential functional anti-radicals and antoxidant agents. Plant Biosyst. 2017, 152, 986–993. [Google Scholar] [CrossRef]
- Russo, D.; Bonomo, M.G.; Salzano, G.; Martelli, G.B.G.; Milella, L. Nutraceutical properties of Citrus clementina juices. Pharmacologyonline 2012, 1, 84–93. [Google Scholar]
- Mahato, N.; Sharma, K.; Sinha, M.; Choa, M.H. Citrus waste derived nutra-pharmaceuticals for health benefits: Current trends and future perspectives. J. Funct. Foods 2018, 40, 307–316. [Google Scholar] [CrossRef]
- Kaur, G.; Mukundan, S.; Wani, V.; Kumar, M.S. Nutraceuticals in the management and prevention of metabolic syndrome. Austin. J. Pharmacol. Ther. 2015, 3, 1063. [Google Scholar]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Zaid, H.; Saad, B.; Mahdi, A.A.; Tamrakar, A.K.; Haddad, P.S.; Afifi, F.U. Medicinal plants and natural active compounds for diabetes and/or obesity treatment, evidence-based complementary and alternative medicine. Evid. Based Compl. Alt. Med. 2015, 1, 1–2. [Google Scholar]
- Graf, B.L.; Raskin, I.; Cefalu, W.T.; Ribnicky, D.M. Plant-derived therapeutics for the treatment of metabolic syndrome. Curr. Opin. Investig. Drugs 2010, 11, 1107–1115. [Google Scholar]
- Simmons, R.K.; Alberti, K.G.; Gale, E.A.; Colagiuri, S.; Tuomilehto, J.; Qiao, Q.; Ramachandran, A.; Tajima, N.; Brajkovich Mirchov, I.; Ben-Nakhi, A.; et al. The metabolic syndrome: Useful concept or clinical tool? Report of a WHO expert consultation. Diabetologia 2010, 53, 600–605. [Google Scholar] [CrossRef]
- Rabie, M.A.; Soliman, A.Z.; Diaconeasa, Z.S.; Constantin, B. Effect of pasteurization and shelf life on the physicochemical properties of Physalis (Physalis peruviana L.) juice. J. Food Process. Preserv. 2014, 39, 1051–1060. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.J. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioprocess Tech. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Gao, X.; Ohlander, M.; Jeppsson, N.; Björk, L.; Trajkovski, V. Changes in antioxidant effects and their relationship to phytonutrients in fruits of Sea buckthorn (Hippophaer hamnoides L.) during maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Pugliese, A.; Bonesi, M.; Tenuta, M.C.; Menichini, F.; Xiao, J.; Tundis, R. Chemical composition and bioactivity of dried fruits and honey of Ficus carica cultivars Dottato, San Francesco and Citrullara. J. Agric. Food Chem. 2016, 64, 2467–2474. [Google Scholar] [CrossRef]
- Sun, T.; Tanumihardjo, S.A. An integrated approach to evaluate food antioxidant capacity. J. Food Sci. 2007, 72, 159–165. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Rao, J.M. Diabetes mellitus and multiple therapeutic approaches of phytochemicals: Present status and future prospects. Current Sci. 2002, 83, 30–38. [Google Scholar]
- El-shiekh, R.A.; Al-Mahdy, D.A.; Hifnawy, M.S.; Abdel-Sattar, E.A. In-vitro screening of selected traditional medicinal plants for their anti-obesity and anti-oxidant activities. S. Afr. J Bot. 2019, 123, 43–50. [Google Scholar] [CrossRef]
- Casacchia, T.; Occhiuzzi, M.A.; Grande, F.; Rizzuti, B.; Granieri, M.C.; Rocca, C.; Gattuso, A.; Garofalo, A.; Angelone, T.; Statti, G. A pilot study on the nutraceutical properties of the Citrus hybrid Tacle® as a dietary source of polyphenols for supplementation in metabolic disorders. J. Funct Foods 2019, 52, 370–381. [Google Scholar] [CrossRef]
- Boudries, H.; Souagui, S.; Nabet, N.; Ydjedd, S.; Kefalas, P.; Madani, K.; Chibane, M. Valorisation of Clementine peels for the recovery of minerals and antioxidants: Evaluation and characterization by LC-DAD-MS of solvent extracts. Food Res. Int. J. 2015, 22, 1218–1226. [Google Scholar]
- Levaj, B.; Dragović-Uzelac, V.; Bursać Kovačević, D.; Krasnići, N. Determination of flavonoids in pulp and peels of mandarin fruits. Agric. Conspec. Sci. 2009, 74, 221–226. [Google Scholar]
- Buettner, A.; Mestres, M.; Guasch, A.F.J.; Schieberle, P. Chemical composition of essential oil from the peel of Satsuma mandarin. Eur. Food Res. Technol. 2003, 216, 11–14. [Google Scholar] [CrossRef]
- Pitarokili, D.; Loukis, O.; Tzakou, A.; Harvala, C. Metabolites from Salvia fruticosa as antifungal agents in soilborne pathogens. J. Agric. Food Chem. 2003, 51, 3294–3301. [Google Scholar] [CrossRef]
- Ruberto, G.; Rapisarda, P. Essential oils of new pigmented Citrus hybrids: Citrus sinensis L. osbeck x C. clementina Hort. ex Tanaka. J. Food Sci. 2012, 67, 2778–2780. [Google Scholar] [CrossRef]
- Bermejo, A.; Llosa’, M.J.; Cano, A. Analysis of bioactive compounds in seven Citrus cultivars. Food Sci. Tech. Int. 2011, 17, 55–62. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Tran, T.T.; Hua, T.M.; Diep, T.T.; Nguyen Chau, D.K.; Duus, F.; Le, T.N. Investigation of peels and leaf essential oils of Citrus clementina Hort. ex Tan. growing in the south of Vietnam. J. Essent. Oil Res. 2016, 28, 96–103. [Google Scholar] [CrossRef]
- Lota, M.L.; De Rocca Serra, D.; Tomi, F.; Casanova, J. Chemical variability of peels and leaf essential oils of 15 species of mandarins. Biochem. Syst. Ecol. 2001, 29, 77–104. [Google Scholar] [CrossRef]
- El-hawary, S.S.; Taha, F.K.; Abdel-Monem, R.A.; Kirillos, N.F.; Mohamed, A.A. Chemical composition and biological activities of peels and leaves essential oils of four cultivars of Citrus deliciosa var. tangarina. Am. J. Essent. Oil. 2013, 1, 1–6. [Google Scholar]
- Rapisarda, P.; Pannuzzo, P.; Romano, G.; Russo, G. Juice components of a new pigmented Citrus hybrid Citrus sinensis (L.) Osbeck × Citrus clementina Hort. ex Tan. J. Agric. Food Chem. 2003, 51, 1611–1616. [Google Scholar] [CrossRef]
- Milella, L.; Caruso, M.; Galgano, F.; Favati, F.; Padula, M.C.; Martelli, G. Role of the cultivar in choosing clementine fruits with a high level of health-promoting compounds. J. Agric. Food Chem. 2011, 59, 5293–5298. [Google Scholar] [CrossRef]
- Xu, G.; Liu, D.; Chen, J.; Ye, X.; Ma, Y.; Shi, J. Juice components and antioxidant capacity of Citrus varieties cultivated in China. Food Chem. 2008, 106, 545–551. [Google Scholar] [CrossRef]
- Nogata, Y.; Sakamoto, K.; Shiratsuchi, H.; Ishii, T.; Yano, M.; Ohta, H. Flavonoid composition of fruit tissues of Citrus species. Biosci. Biotechnol. Biochem. 2006, 7, 178–192. [Google Scholar] [CrossRef] [Green Version]
- Tumbas, V.T.; Ćetkovic, G.S.; Djilas, S.M.; Canadanovic-Brunet, J.M.; Vulic, J.J.; Knez, Z. Antioxidant activity of mandarin (Citrus reticulata) peel. Acta Period. Technol. 2010, 40, 195–203. [Google Scholar] [CrossRef]
- Dugrand, A.; Olry, A.; Duval, T.; Hehn, A.; Froelicher, Y.; Bourgaud, F. Coumarin and furanocoumarin quantitation in Citrus peels via ultraperformance liquid chromatography coupled with mass spectrometry (UPLC-MS). J. Agric. Food Chem. 2013, 61, 10677–10684. [Google Scholar] [CrossRef]
- Lin, H.; Kenaan, C.; Hollenberg, P.F. Identification of the residue in human CYP3A4 that is covalently modified by bergamottin and the reactive intermediate that contributes to the grapefruit juice effect. Drug Metab. Dispos. 2012, 40, 998–1006. [Google Scholar] [CrossRef] [Green Version]
- Bailey, D.G.; Dresser, G.; Arnold, J.M. Grapefruit-medication interactions: Forbidden fruit or avoidable consequences? Can. Med. Assoc. J. 2013, 185, 309–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, R.; Albergamo, A.; Arrigo, S.; Gentile, F.; Dugo, G. Solid-phase microextraction-gas chromatography and ultra-high performance liquid chromatography applied to the characterization of lemon wax, a waste product from Citrus industry. J. Chromatogr. A 2019, 1603, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Dugrand-Judek, A.; Olry, A.; Hehn, A.; Costantino, G.; Ollitrault, P.; Froelicher, Y.; Bourgaud, F. The distribution of coumarins and furanocoumarins in Citrus species closely matches Citrus phylogeny and reflects the organization of biosynthetic pathways. PLoS ONE 2015, 10, e0142757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez-Pelayo, J.; Martínez-Quiñones, C.; Gil, J.; Durango, D. Coumarins from the peels of Citrus grown in Colombia: Composition, elicitation and antifungal activity. Heliyon 2019, 5, e01937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourgaud, F.; Hehn, A.; Larbat, R.; Doerper, S.; Gontier, E.; Kellner, S.; Matern, U. Biosynthesis of coumarins in plants: a major pathway still to be unravelled for cytochrome P450 enzymes. Phytochem. Rev. 2006, 5, 293–308. [Google Scholar] [CrossRef]
- Melough, M.M.; Lee, S.G.; Cho, E.; Kim, K.; Provatas, A.A.; Perkins, C.; Park, M.K.; Qureshi, A.; Chun, O.K. Identification and quantitation of furocoumarins in popularly consumed foods in the U.S. using QuEChERS extraction coupled with UPLC-MS/MS analysis. J. Agric. Food Chem. 2017, 65, 5049–5055. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, A.; Cannazza, G.; Capriotti, A.L.; Citti, C.; La Barbera, G.; Laganà, A.; Montone, C.M.; Piovesana, S.; Cavaliere, C. A new software-assisted analytical workflow based on high-resolution mass spectrometry for the systematic study of phenolic compounds in complex matrices. Talanta 2020, 209, 120573. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Sicari, V.; Tundis, R.; Leporini, M.; Falco, T.; Calabrò, V. The influence of ultrafiltration of Citrus limon L. Burm. cv Femminello Comune juice on its chemical composition and antioxidant and hypoglycemic properties. Antioxidants 2019, 8, 23. [Google Scholar] [CrossRef] [Green Version]
- Ghasemi, K.; Ghasemi, Y.; Ebrahimzadeh, M.A. Antioxidant activity, phenol and flavonoid contents of 13 Citrus species peels and tissues. Pak. J. Pharm. Sci. 2009, 22, 277–281. [Google Scholar]
- Kang, H.J.; Chawla, S.P.; Jo, C.; Kwon, J.H.; Byun, M.W. Studies on the development of functional powder from Citrus peel. Bioresour. Technol. 2006, 97, 614–620. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Bonesi, M.; De Luca, D.; Colica, C.; Menichini, F. Evaluation of Citrus aurantifolia peels and leaf extracts for their chemical composition, antioxidant and anti-cholinesterase activities. J. Sci. Food Agric. 2012, 92, 2960–2967. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol. Res. 2018, 130, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Owira, P.M.; Ojewole, J.A. Grapefruit juice improves glycemic control but exacerbates metformin-induced lactic acidosis in non-diabetic rats. Method Find Exp. Clin. 2009, 31, 563–570. [Google Scholar]
- Mollace, V.; Sacco, I.; Janda, E.; Malara, C.; Ventrice, D.; Colica, C.; Visalli, V.; Muscoli, S.; Ragusa, S.; Muscoli, C.; et al. Hypolipemic and hypoglycaemic activity of bergamot polyphenols: from animal models to human studies. Fitoterapia 2011, 82, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.N.; Shin, M.R.; Shin, S.H.; Lee, A.R.; Lee, J.Y.; Seo, B.I.; Kim, M.Y.; Kim, T.H.; Noh, J.S.; Rhee, M.H.; et al. Study of antiobesity effect through inhibition of pancreatic lipase activity of Diospyros kaki fruit and Citrus unshiu peels. Biomed. Res. Int. 2016, 2016, 1–7. [Google Scholar]
- Jia, S.; Hu, Y.; Zhang, W.; Zhao, X.; Chen, Y.; Sun, C.; Li, X.; Chen, K. Hypoglycemic and hypolipidemic effects of neohesperidin derived from Citrus aurantium L. in diabetic KKA(y) mice. Food Funct. 2015, 6, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.C.; Ko, C.H.; Tsen, S.W.; Tsai, S.H.; Chen, Y.C. Structurally related antitumor effects of flavanones in vitro and in vivo: Involvement of caspase 3 activation, p21 gene expression, and reactive oxygen species production. Toxicol. Appl. Pharmacol. 2004, 197, 84–95. [Google Scholar] [CrossRef]
- Zeng, S.L.; Li, S.Z.; Lai, C.J.; Wei, M.Y.; Chen, B.Z.; Li, P.; Zheng, G.D.; Liu, E.H. Evaluation of anti-lipase activity and bioactive flavonoids in the Citri Reticulatae pericarpium from different harvest time. Phytomedicine 2018, 43, 103–109. [Google Scholar] [CrossRef]
- Bustanji, Y.; Issa, A.; Mohammad, M.; Hudaib, M.; Tawah, K.; Alkhatib, H.; Almasri, I.; Al-Khalidi1, B. Inhibition of hormone sensitive lipase and pancreatic lipase by Rosmarinus officinalis extract and selected phenolic constituents. J. Med. Plants Res. 2019, 4, 2235–2242. [Google Scholar]
- Buchholz, T.; Melzig, M.F. Polyphenolic compounds as pancreatic lipase inhibitors. Planta Med. 2015, 81, 771–783. [Google Scholar] [CrossRef] [Green Version]
- Kamel, H.I.; Salib, J.Y.; El-Toumy, S.A.; Awad, A.H.; Elmenabbawy, M.K. Citrus reticulata peel extract: An Anti-obesity therapy for Adolescents. Middle East J. Appl. Sci. 2019, 9, 117–124. [Google Scholar]
- Dallas, C.; Gerbi, A.; Elbez, Y.; Caillard, P.; Zamaria, N.; Cloarec, M. Clinical study to assess the efficacy and safety of a Citrus polyphenolic extract of red orange, grapefruit, and orange (Sinetrol-Xpur) on weight management and metabolic parameters in healthy overweight individuals. Phytother. Res. 2014, 28, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Stevens, Y.; Rymenant, E.V.; Grootaert, C.; Camp, J.V.; Possemiers, S.; Masclee, A.; Jonkers, D. The Intestinal Fate of Citrus Flavanones and Their Effects on Gastrointestinal Health. Nutrients 2019, 11, 1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Sample | Site of Collection | Abbreviation | Procedure |
|---|---|---|---|
| Juice | |||
| Cetraro | JD | Sqeezed | |
| Rosarno | JE | Squeezed | |
| Corigliano Calabro | JF | Squeezed | |
| Peel extracts | |||
| Cetraro | BC1 | Soxhlet extractor | |
| BC2 | Ultrasound EtOH/H2O (80:20) | ||
| BC3 | Ultrasound EtOH | ||
| BC4 | Maceration EtOH/H2O (80:20) | ||
| BC5 | Maceration EtOH | ||
| BC6 | Hydrodistillation | ||
| Rosarno | BR1 | Soxhlet extractor | |
| BR2 | Ultrasound EtOH/H2O (80:20) | ||
| BR3 | Ultrasound EtOH | ||
| BR4 | Maceration EtOH/H2O (80:20) | ||
| BR5 | Maceration EtOH | ||
| BR6 | Hydrodistillation | ||
| Corigliano Calabro | BO1 | Soxhlet extractor | |
| BO2 | Ultrasound EtOH/H2O (80:20) | ||
| BO3 | Ultrasound EtOH | ||
| BO4 | Maceration EtOH/H2O (80:20) | ||
| BO5 | Maceration EtOH | ||
| BO6 | Hydrodistillation | ||
| Leaf extracts* | |||
| Corigliano Calabro | CO2 | Ultrasound EtOH/H2O (80:20) | |
| Juice | Enrichment^ |
|---|---|
| JFA | JF + 20% CO2 |
| JFB | JF + 15% CO2 |
| JFC | JF + 10% CO2 |
| JFD | JF + 5% CO2 |
| JFE | JF + 20% BC3 |
| JFF | JF + 15% BC3 |
| JFG | JF + 10% BC3 |
| JFH | JF + 5% BC3 |
| JFI | JF + 20% CO2 +BC3^^ |
| JFL | JF + 15% CO2 +BC3^^ |
| JFM | JF + 10% CO2 + BC3^^ |
| JFN | JF + 5% CO2 + BC3^^ |
| Pasteurized juices | |
| JPFA | JPF + 20% CO2 |
| JPFE | JPF + 20% BC3 |
| JPFI | JPF + 20% CO2 + BC3^^ |
| Parameters | JD | JE | JF | Sign. |
|---|---|---|---|---|
| Juice (pH) | 3.54 ± 0.06b | 3.47 ± 0.05c | 3.72 ± 0.08a | ** |
| Acidity (g/100 mL) | 0.57 ± 0.03c | 0.63 ± 0.05b | 0.70 ± 0.03a | ** |
| °Brix | 10.10 ± 0.02c | 10.41 ± 0.03a | 10.24 ± 0.02b | ** |
| Chroma value (C*) | 30.48 ± 1.12b | 28.22 ± 1.09c | 34.04± 1.14a | ** |
| Ascorbic acid (mg/100 mL) | 65.12 ± 3.23b | 64.48 ± 3.26b | 66.34 ± 3.88a | ** |
| Ash (g/100 g) | 0.45 ± 0.04b | 0.43 ± 0.03b | 0.47 ± 0.05a | ** |
| Fats (g/100 g) | 0.17 ± 0.11a | 0.16 ± 0.19a | 0.15 ± 0.10a | ns |
| Protein (g/100 g) | 0.80 ± 0.09a | 0.83 ± 0.07a | 0.82 ± 0.08a | ns |
| Fiber (g/100 g) | 1.82 ± 1.12a | 1.76 ± 1.04b | 1.80 ± 1.14a | ** |
| Carbohydrates (g/100 g) | 12.31± 1.84a | 12.10 ± 1.80a | 12.01 ± 1.82a | ns |
| Energy kcal/100 g | 54 ± 2.03a | 53 ± 2.04a | 52 ± 2.02a | ns |
| Phosphorus (mg/100 g) | 20 ± 1.2a | 21 ± 1.3a | 22 ± 1.4a | ns |
| Potassium (mg/100 g) | 181 ± 4.52b | 178 ± 3.33c | 183 ± 3.56a | ** |
| Calcium (mg/100 g) | 31 ± 1.24a | 30 ± 1.27a | 31 ± 1.25a | ns |
| Magnesium (mg/100 g) | 12 ± 0.83b | 12 ± 0.91b | 13 ± 0.94a | ** |
| Sample | Yields | Total Phenol Content | Total Flavonoid Content | Total Carotenoid Content |
|---|---|---|---|---|
| Juice | (% L/Kg) | (mg CAE)/100 mL) | (mg QE)/100 mL) | (mg β-caroteneE)/100 mL) |
| JD | 48.19 ± 6.07b | 29.46 ± 1.11b | 30.28 ± 1.17b | 31.12 ± 1.19b |
| JE | 47.13 ± 6.01c | 17.58 ± 0.93c | 18.16 ± 0.99c | 18.23 ± 0.92c |
| JF | 48.37 ± 6.42b | 54.65 ± 2.92a | 51.48 ± 2.84a | 53.54 ± 2.89a |
| Sign. | ** | ** | ** | ** |
| Peels | (% g/g) | (mg CAE)/g FW) | (mg QE)/g FW) | (mg β-caroteneE)/g FW) |
| BC1 | 14.42 ± 1.41a | 8.75 ± 0.83a | 6.05 ± 0.67a | 39.84 ± 3.47a |
| BC2 | 12.91 ± 1.24c | 6.30 ± 0.64c | 4.02 ± 0.42d | 17.89 ± 1.76c |
| BC3 | 12.07 ± 1.23e | 6.27 ± 0.68c | 5.08 ± 0.58b | 16.66 ± 1.63d |
| BC4 | 12.86 ± 1.27c | 5.34 ± 0.56f | 4.02 ± 0.44d | 15.60 ± 1.57g |
| BC5 | 12.48 ± 1.20d | 5.49 ± 0.51e | 3.89 ± 0.46e | 12.14 ± 1.25i |
| BR1 | 14.28 ± 1.42b | 7.13 ± 0.78b | 4.64 ± 0.47c | 19.62 ± 1.98b |
| BR2 | 9.48 ± 0.97n | 4.43 ± 0.45h | 3.13 ± 0.33l | 10.88 ± 1.11m |
| BR3 | 10.62 ± 1.01i | 4.27 ± 0.43l | 3.39 ± 0.31h | 10.42 ± 1.05o |
| BR4 | 9.52 ± 0.94n | 4.38 ± 0.44i | 3.44 ± 0.36h | 15.97 ± 1.69f |
| BR5 | 10.49 ± 1.00l | 3.99 ± 0.45m | 3.20 ± 0.34i | 11.68 ± 1.11l |
| BO1 | 14.99 ± 1.47a | 5.91 ± 0.56d | 4.62 ± 0.49c | 16.49 ± 1.68e |
| BO2 | 11.50 ± 1.11e | 4.50 ± 0.41g | 3.56 ± 0.34g | 9.66 ± 0.92q |
| BO3 | 10.88 ± 1.04g | 3.80 ± 0.38o | 3.80 ± 0.36f | 10.28 ± 1.05p |
| BO4 | 9.70 ± 0.93m | 3.45 ± 0.36p | 2.47 ± 0.22n | 10.48 ± 1.02n |
| BO5 | 10.74 ± 1.07h | 3.85 ± 0.37n | 2.78 ± 0.28m | 13.60 ± 1.37h |
| Sign. | ** | ** | ** | ** |
| Compounds | RI1 | Relative Amount (%) | I.M2 | Sign. | ||
|---|---|---|---|---|---|---|
| BC6 | BR6 | BO6 | ||||
| α-Pinene | 938 | 1.10 ± 0.12c | 1.55 ± 0.21b | 3.13 ± 0.33a | 1,2,3 | ** |
| Sabinene | 973 | 2.55 ± 0.70a | 0.97 ± 0.12c | 1.52 ± 0.21b | 1,2,3 | ** |
| β-Pinene | 980 | tr | tr | tr | 1,2,3 | ** |
| Myrcene | 993 | 3.56 ± 0.31c | 4.94 ± 0.46b | 9.10 ± 0.91a | 1,2,3 | ** |
| +/- Limonene | 1030 | 75.11± 4.55b | 83.09 ± 5.12a | 61.31 ± 4.02c | 1,2,3 | ** |
| (E)- β-Ocimene | 1049 | 3.31 ± 0.37a | nd | nd | 1,2 | ** |
| γ-Terpinene | 1057 | 0.33 ± 0.03a | tr | 0.32 ± 0.03a | 1,2,3 | ** |
| Terpinolene | 1086 | tr | tr | 0.30 ± 0.03a | 1,2,3 | ** |
| Linalool | 1098 | 5.30 ± 0.55b | 3.29 ± 0.36c | 6.64 ± 0.61a | 1,2,3 | ** |
| Nonanal | 1100 | 1.84 ± 0.23b | 1.62 ± 0.24c | 3.64 ± 0.34a | 1,2 | ** |
| Citronellal | 1148 | 0.40 ± 0.04b | tr | 0.75 ± 0.07a | 1,2 | ** |
| Terpinen-4-ol | 1176 | 0.56 ± 0.05b | 0.20 ± 0.02c | 0.88 ± 0.08a | 1,2 | ** |
| α-Terpineol | 1189 | 0.47 ± 0.04b | 0.49 ± 0.04b | 1.55 ± 0.12c | 1,2,3 | ** |
| Decanal | 1205 | 1.05 ± 0.10b | 1.04 ± 0.10b | 2.20 ± 0.21a | 1,2 | ** |
| trans-Caryophyllene | 1415 | tr | tr | tr | 1,2,3 | ** |
| trans-β-Farnesene | 1441 | tr | tr | 0.35 ± 0.03a | 1,2 | ** |
| α-Humulene | 1454 | tr | tr | tr | 1,2,3 | ** |
| δ-cadinene | 1526 | 0.31 ± 0.02a | 0.22 ± 0.02c | 0.26 ± 0.02b | 1,2 | ** |
| β-Sinensal | 1697 | 0.29 ± 0.02a | tr | 0.13 ± 0.02b | 1,2 | ** |
| α-Sinensal | 1750 | 0.70 ± 0.07a | 0.37 ± 0.04c | 0.45 ± 0.05b | 1,2 | ** |
| Total identified | 96.88 | 97.78 | 92.53 | |||
| Selected Markers | JD | JE | JF | Sign. |
|---|---|---|---|---|
| Apigenin | 0.06 ± 0.04c | 0.05 ± 0.03b | 0.09 ± 0.08a | ** |
| Caffeic acid | 7.48 ± 1.61b | 3.65 ± 1.30c | 8.52 ± 1.81a | ** |
| Chlorogenic acid | 2.59 ± 1.22a | 2.06 ± 1.20b | 2.56 ± 1.26a | ** |
| Didymin | 3.85 ± 1.26c | 4.17 ± 1.33b | 5.51 ± 0.04a | ** |
| Eriocitrin | 1.84 ± 0.19c | 2.33 ± 1.34a | 0.91 ± 0.11b | ** |
| Gallic acid | 1.02 ± 0.09b | 0.62 ± 0.04c | 1.67 ± 0.18a | ** |
| Hesperidin | 81.08 ± 4.94a | 40.06 ± 3.04c | 65.3 ± 3.54b | ** |
| Naringin | 1.73 ± 0.18c | 2.12 ± 1.19b | 3.14 ± 1.32a | ** |
| Narirutin | 8.50 ± 1.83a | 6.25 ± 1.32c | 7.88 ± 1.81b | ** |
| Neoeriocitrin | 2.69 ± 0.72c | 3.14 ± 1.04b | 3.41 ± 1.39a | ** |
| Neohesperidin | 110.63 ± 5.52b | 80.26 ± 4.83c | 112.32 ± 5.63a | ** |
| Nobiletin | 0.06 ± 0.01c | 0.12 ± 0.01b | 0.14 ± 0.01a | ** |
| p-Coumaric acid | 7.67 ± 1.69b | 6.47 ± 1.58c | 8.29 ± 1.72a | ** |
| Poncirin | 1.52 ± 0.19c | 1.74 ± 0.21b | 2.63 ± 0.51a | ** |
| Protocatechuic acid | 1.02 ± 0.74b | 0.62 ± 0.07c | 1.67 ± 0.96a | ** |
| Quercetin | 0.42 ± 0.03b | 0.56 ± 0.05c | 1.32 ± 0.13a | ** |
| Sinensetin | 0.005 ± 0.01b | 0.006 ± 0.01b | 0.01 ± 0.03a | ** |
| Tangeretin | 0.06 ± 0.07a | 0.05 ± 0.06b | 0.07 ± 0.08a | ** |
| Vanillic acid | 0.69 ± 0.07b | 2.16 ± 1.30a | 0.43 ± 0.03c | ** |
| ∑ Indentified phenols | 232.92 | 156.44 | 226.01 |
| Sample | Tangeretin | Sinensetin | Luteolin | Quercetin | Quercetin-3-O-glucoside | Poncirin | Hesperidin | Neoeriocitrin | Eriocitrin | Caffeic Acid |
|---|---|---|---|---|---|---|---|---|---|---|
| BC1 | 7.47 ± 0.78cd | 32.86 ± 3.23c | 6.17 ± 0.68d | 8.98 ± 0.93a | 11.26 ± 1.27a | 1.98 ± 0.12b | 778.70 ± 15.91d | 4.89 ± 0.57a | 3.75 ± 0.33a | 0.22± 0.02h |
| BC2 | 7.92 ± 0.80bc | 29.36 ± 2.94f | 5.16 ± 0.50h | 7.98 ± 0.72b | 8.49 ± 0.96d | 0.57 ± 0.05g | 1007.86 ± 8.12b | 3.29 ± 0.32b | 2.12 ± 0.21b | 6.98 ± 0.64d |
| BC3 | 6.57 ± 0.77fg | 27.07 ± 2.76g | 4.05 ± 0.47m | 6.22 ± 0.61g | 6.61 ± 0.68h | 2.29 ± 0.34a | 1093.36 ± 8.13a | 2.43 ± 0.26d | 1.56 ± 0.14c | 0.15 ± 0.01h |
| BC4 | 5.43 ± 0.53l | 26.22 ± 2.62h | 5.02 ± 0.54i | 7.72 ± 0.77c | 8.20 ± 0.83e | 2.53 ± 0.28a | 228.63 ± 8.13l | 2.99 ± 0.37c | 2.02 ± 0.26b | 6.40 ± 0.61e |
| BC5 | 7.19 ± 0.79def | 32.56 ± 3.21c | 5.39 ± 0.05g | 7.95 ± 0.08b | 8.45 ± 0.08d | 2.65 ± 0.02a | 238.53 ± 8.22i | 0.68 ± 0.077e | 3.62 ± 0.36a | 0.20 ± 0.02h |
| BR1 | 8.31 ± 0.85b | 37.99 ± 3.74a | 5.53 ± 0.53f | 6.86 ± 0.64e | 7.29 ± 0.71g | 1.33 ± 0.17de | 977.23 ± 18.11c | nd | nd | nd |
| BR2 | 6.47 ± 0.66gh | 25.59 ± 2.11i | 3.77 ± 0.48n | 8.02 ± 0.84b | 7.27 ± 0.73g | 1.35 ± 0.18de | 558.14 ± 15.24f | nd | nd | 5.32 ± 0.51f |
| BR3 | 7.0± 0.77ef | 26.39± 2.63h | 3.53 ± 0.39o | 5.78 ± 0.52h | nd | 1.39 ± 0.17cd | 667.18 ± 15.54e | nd | nd | nd |
| BR4 | 9.6 ± 0.97a | 37.2 ± 3.40b | 8.2 ± 0.87c | nd | nd | nd | 100.26 ± 8.15o | nd | nd | 8.99 ± 0.94b |
| BR5 | 7.37 ± 0.76de | 31.41± 3.12d | 5.74 ± 0.57e | nd | nd | n.d | 173.52 ± 8.13m | nd | nd | 10.87 ± 1.22a |
| BO1 | 6.75 ± 0.62gf | 31.51± 3.14d | 6.11 ± 0.63d | 7.13 ± 0.77d | 7.58 ± 0.71f | 1.51 ± 0.19cd | 243.98 ± 10.21hi | nd | nd | 2.68 ± 0.31g |
| BO2 | 6.06 ± 0.61hi | 30.74 ± 3.07e | 4.72 ± 0.43l | 7.76 ± 0.88c | 6.37± 0.66i | 1.21 ± 0.17ef | 364.52 ± 11.33g | nd | 0.73 ± 0.07e | 8.0 ± 0.82c |
| BO3 | 6.2 ± 0.63hi | 19.56 ± 2.19m | 3.02 ± 0.32p | 7.7 ± 0.87c | 6.66 ± 0.63h | 1.61 ± 0.17c | 253.8 ± 10.15h | nd | 0.88 ± 0.08d | nd |
| BO4 | 5.8 ± 0.68li | 25.7 ± 2.56i | 8.58 ± 0.94a | 7.07 ± 0.72d | 10.54 ± 1.26b | 1.08 ± 0.17f | 155.28 ± 8.12n | nd | nd | nd |
| BO5 | 5.55 ± 5.01l | 21.2 ± 2.13l | 8.36 ± 0.87b | 6.51 ± 0.65f | 9.65 ± 0.92c | 1.05 ± 0.18f | 179.42 ± 8.16m | nd | 0.78 ± 0.08d | nd |
| Sign. | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| Sample | DPPH Test IC50 (μg/mL) | ABTS Test IC50 (μg/mL) | β-Carotene Bleaching test IC50 (μg/mL) | FRAP Test μM Fe (II)/g | RACI Values | |
|---|---|---|---|---|---|---|
| t 30 min | t 60 min | |||||
| JD | 82.43 ± 2.96**** | 33.63 ± 2.01**** | 25.90% | 27.50% | 2.8 ± 0.96**** | −0.13 |
| JE | 84.02 ± 2.92**** | 40.32 ± 2.54**** | 27.50% | 28.47% | 3.01 ± 0.98**** | 0.72 |
| JF | 81.13 ± 2.73**** | 24.82 ± 1.96**** | 31.33% | 34.20% | 5.70 ± 1.00**** | −0.59 |
| Positive control | ||||||
| Ascorbic acid | 5.01 ± 0.80 | 1.72 ± 0.06 | ||||
| Propyl gallate | 0.09 ± 0.004 | 0.09 ± 0.004 | ||||
| BHT | 63.23 ± 4.31 | |||||
| Sample | DPPH Test IC50 (µg/mL) | ABTS Test IC50 µg/mL) | β-Carotene Bleaching Test IC50 (µg/mL) | FRAP μM Fe (II)/g | RACI | |
|---|---|---|---|---|---|---|
| t = 30 min | t = 60 min | |||||
| BC1 | 105.66 ± 4.01**** | 14.82 ± 1.22**** | 32.52 ± 1.93**** | 11.22 ± 1.08**** | 23.51 ± 1.77**** | −0.55 |
| BC2 | 52.58 ± 2.32**** | 8.22 ± 0.84**** | 75.03 ± 2.77**** | 22.86 ± 1.93**** | 28.48 ± 1.83**** | −0.37 |
| BC3 | 45.79 ± 2.14**** | 15.06 ± 1.62 | 16.47 ± 1.85**** | 12.53 ± 1.17**** | 34.28 ± 1.95**** | −0.59 |
| BC4 | 117.86 ± 4.07**** | 17.54 ± 1.91**** | 90.99 ± 3.93**** | 12.53 ± 1.26**** | 26.26 ± 1.77**** | −0.43 |
| BC5 | 140.39 ± 4.35**** | 21.03 ± 1.79**** | 18.26 ± 1.81**** | 10.47 ± 1.08**** | 23.19 ± 1.63**** | 0.02 |
| BC6 | 308.55 ± 6.12**** | 24.13 ± 2.03**** | 91.92 ± 3.98 | 47.22 ± 2.21**** | 6.13 ± 0.61**** | 0.51 |
| BR1 | 68.13 ± 2.43**** | 15.21 ± 1.23**** | 36.84 ± 1.94**** | 11.45 ± 1.16**** | 30.97 ± 1.97**** | −0.46 |
| BR2 | 81.26 ± 3.41**** | 10.97 ± 1.05**** | 68.75 ± 2.96**** | 58.52 ± 2.57**** | 25.78 ± 1.73**** | −0.05 |
| BR3 | 113.17 ± 4.03 | 9.47 ± 0.97**** | 8.78 ± 0.83**** | 19.38 ± 1.83**** | 54.95 ± 2.14 | −0.51 |
| BR4 | 83.14 ± 3.61**** | 20.25± 1.94**** | 96.8 ± 3.18**** | 89.39 ± 3.82**** | 40.46 ± 2.47**** | −0.10 |
| BR5 | 125.37 ± 4.11**** | 11.76 ± 1.13**** | 43.22 ± 2.11**** | 72.4 ± 2.71**** | 21.13 ± 1.03**** | 1.20 |
| BR6 | 370.3 ± 6.74**** | 26.30 ± 2.07**** | 55.7 ± 2.53**** | 10.37 ± 0.93**** | 23.91 ± 1.74**** | 0.48 |
| BO1 | 259.57 ± 5.31**** | 31.50 ± 2.11**** | 71.00 ± 2.64**** | 11.41 ± 0.94**** | 6.48 ± 0.62**** | 0.20 |
| BO2 | 174.17 ± 4.43**** | 28.37 ± 2.07**** | 72.78 ± 2.75**** | 64.15 ± 2.76**** | 21.26 ± 1.71**** | 0.63 |
| BO3 | 212.65 ± 3.83**** | 15.21 ± 1.13**** | 87.98 ± 2.83 | 39.09 ± 2.17**** | 27.39 ± 1.37**** | −0.18 |
| BO4 | 169.48 ± 4.87**** | 14.05 ± 1.25**** | 87.98 ± 3.61**** | 39.09 ± 2.16**** | 26.1 ± 1.83**** | 0.15 |
| BO5 | 212.65 ± 4.52**** | 8.22 ± 0.89**** | 58.33 ± 2.59**** | 12.90 ± 0.95**** | 27.39 ± 1.85**** | −0.21 |
| BO6 | 333.7 ± 6.01**** | 18.31 ± 1.91**** | 61.8 ± 2.62**** | 11.78 ± 1.02**** | 25.38 ± 1.71**** | 0.25 |
| Positivecontrol | ||||||
| Ascorbic acid 5.0 ± 0.8 | 1.7 ± 0.06 | |||||
| Propyl gallate | 0.0 9 ± 0.004 | 0.09 ± 0.004 | ||||
| BHT | 82.43± 1.52 | |||||
| Sample | α-Amylase IC50 (μg/mL) | α-Glucosidase IC50 (μg/mL) | Lipase IC50 (μg/mL) |
|---|---|---|---|
| JD | 189.81 ± 2.09**** | 89.37 ± 2.07**** | 192.14 ± 2.47**** |
| BC1 | 210.68 ± 4.95**** | 141.32 ± 4.38**** | 186.14 ± 4.24**** |
| BC2 | 132.00 ± 4.22**** | 152.15 ± 4.47**** | 145.59 ± 3.71**** |
| BC3 | 79.73 ± 3.64**** | 71.97 ± 2.61**** | 112.06 ± 3.64**** |
| BC4 | 146.89 ± 4.39**** | 101.91 ± 3.92**** | 186.54 ± 4.2**** |
| BC5 | 258.13 ± 5.18**** | 126.75 ± 4.14**** | 189.37 ± 4.31**** |
| BC6 | 228.35 ± 4.94**** | 225.35 ± 4.90**** | 181.48 ± 4.04**** |
| JE | 194.33 ± 2.15**** | 103.43 ± 2.43**** | 197.69 ± 2.68**** |
| BR1 | 154.77 ± 4.42**** | 152.15 ± 4.42**** | 171.12 ± 3.82**** |
| BR2 | 146.02 ± 4.34**** | 202.07 ± 4.80*** | 181.37 ± 4.05**** |
| BR3 | 237.98 ± 4.97**** | 239.73 ± 5.16**** | 191.91 ± 4.80**** |
| BR4 | 128.50 ± 4.12**** | 256.07 ± 5.27**** | 174.15 ± 3.81**** |
| BR5 | 181.93 ± 4.36**** | 197.69 ± 4.85**** | 179.63 ± 3.92**** |
| BR6 | 185.43 ± 4.11**** | 287.91 ± 5.24**** | 182.74± 4.13**** |
| JF | 139.89 ± 1.81**** | 67.19 ± 1.31**** | 179.32 ± 2.19**** |
| BO1 | 207.33 ± 4.83**** | 130.25 ± 4.25**** | 173.42 ± 4.02**** |
| BO2 | 167.91 ± 4.55**** | 129.37 ± 4.27**** | 142.06 ± 3.85**** |
| BO3 | 160.91 ± 4.52**** | 143.39 ± 4.45**** | 132.37 ± 3.56**** |
| BO4 | 280.03 ± 5.23**** | 138.13 ± 4.35**** | 165.18 ± 3.95**** |
| BO5 | 186.13 ± 4.60**** | 224.84 ± 2.97**** | 179.83 ± 4.01**** |
| BO6 | 252.00 ± 5.12**** | 260.76 ± 5.13**** | 198.36 ± 4.83**** |
| Positive control | |||
| Acarbose | 50.01 ± 0.92 | 35.52 ± 1.23 | |
| Orlistat | 37.63 ± 1.01 | ||
| Sample | DPPH Test % Inhibition | ABTS Test % Inhibition | β-carotene Bleaching Test % Inhibition | FRAP μM Fe (II)/g | |
|---|---|---|---|---|---|
| t = 30 min | t = 60 min | ||||
| JFA | 122.71 ± 2.74**** | 115.46 ± 2.21**** | 52.47 ± 1.26**** | 64.56 ± 1.38**** | 100.43 ± 2.47**** |
| JFB | 118.23 ± 2.61**** | 111.78 ± 2.19**** | 50.32 ± 1.20**** | 60.29 ± 1.33**** | 98.36 ± 1.73**** |
| JFC | 105.21 ± 2.55**** | 110.77± 2.17**** | 49.83 ± 1.22**** | 53.24 ± 1.27**** | 97.95 ± 1.65**** |
| JFD | 97.78 ± 2.51* | 107.64 ± 1.79**** | 43.89 ± 1.14**** | 49.73 ± 1.26**** | 91.93 ± 1.67**** |
| JFE | 108.21 ± 2.83**** | 112.32 ± 1.93**** | 49.52 ± 1.21**** | 59.37 ± 1.28**** | 89.58 ± 1.63**** |
| JFF | 101.77 ± 2.34**** | 110.84 ± 1.95**** | 48.43 ± 1.20**** | 57.22 ± 1.21**** | 88.56 ± 1.61*** |
| JFG | 98.40 ± 2.26** | 109.77± 1.97**** | 46.14 ± 1.18**** | 45.22 ± 1.16**** | 86.33 ± 1.60* |
| JFH | 95.15 ± 2.21ns | 107.43 ± 1.97**** | 41.59 ± 1.14**** | 39.47 ± 1.17*** | 83.37 ± 1.57ns |
| JFI | 106.23 ± 2.55**** | 118.87± 1.94**** | 49.73 ± 1.33**** | 62.63 ± 1.33**** | 99.28 ± 1.75**** |
| JFL | 101.78 ± 2.37**** | 113.92 ± 1.93**** | 49.02 ± 1.28**** | 60.14 ± 1.32**** | 96.72 ± 1.67**** |
| JFM | 98.57 ± 2.34** | 109..57 ± 1.83**** | 48.61 ± 1.11**** | 53.62 ± 1.31**** | 87.04 ± 1.53** |
| JFN | 96.57 ± 2.20ns | 97.21 ± 1.65**** | 42.16 ± 1.13**** | 48.23 ± 1.26**** | 84.21 ± 1.54ns |
| Negative control | |||||
| JF | 94.68 ± 2.2 | 96.87 ± 0.06 | 34.20 ± 1.06 | 34.25 ± 1.08 | 82.43± 1.52 |
| Sample | α-Amylase | α-Glucosidase | Lipase |
|---|---|---|---|
| JFA | 120.53 ± 2.91**** | 134.36 ± 3.01**** | 89.36 ± 2.22**** |
| JFB | 117.83 ± 2.82**** | 122.12 ± 2.95**** | 81.41 ± 2.12**** |
| JFC | 111.01 ± 2.69**** | 118.73 ± 2.90**** | 77.71 ± 2.03*** |
| JFD | 98.46 ± 2.63ns | 112.52 ± 2.60**** | 75.48 ± 2.02**** |
| JFE | 112.64 ± 2.61**** | 110.68 ± 2.59**** | 83.11 ± 2.33**** |
| JFF | 102.34 ± 2.53*** | 106.76 ± 2.52**** | 78.93 ± 2.06**** |
| JFG | 99.83 ± 2.68ns | 102.41 ± 2.54**** | 76.24 ± 2.08*** |
| JFH | 98.12 ± 2.64ns | 99.34 ± 2.58*** | 73.56 ± 2.02*** |
| JFI | 118.67 ± 2.80**** | 118.12 ± 2.90**** | 86.42 ± 2.28**** |
| JFL | 116.43 ± 2.71**** | 115.43 ± 2.60**** | 81.02 ± 2.18**** |
| JFM | 110.91 ± 2.62**** | 106.24 ± 2.53**** | 78.93 ± 1.98**** |
| JFN | 98.03 ± 2.61ns | 100.01 ± 2.50**** | 74.57 ± 1.91*** |
| Negative control | |||
| JF | 96.08 ± 2.42 | 97.05 ± 1.23 | 72.36 ± 1.01 |
| Sample | Appearance | Colour | Odour | Aroma | Sweetness | Aidity | Astringency | Mouthfeel |
|---|---|---|---|---|---|---|---|---|
| JF | 8.12ed | 8.52ab | 8.01c | 8.14ab | 8.23a | 7.98a | 8.03a | 8.26a |
| JFA | 8.01f | 8.23f | 7.87e | 7.96e | 8.01c | 7.93a | 7.86de | 7.98i |
| JFB | 8.04f | 8.28ef | 7.93d | 7.98de | 8.12abc | 7.95a | 7.89cd | 8.01h |
| JFC | 8.08e | 8.33def | 7.98d | 8.02dc | 8.14abc | 7.96a | 7.91cb | 8.15cb |
| JFD | 8.11e | 8.49ab | 8.00c | 8.09b | 8.18ab | 7.98a | 7.96ab | 8.17b |
| JFE | 8.32a | 8.55a | 8.11a | 8.03c | 8.03bc | 7.95a | 7.87de | 8.06fg |
| JFF | 8.30a | 8.54a | 8.07b | 8.09b | 8.14abc | 7.95a | 7.92cb | 8.10cde |
| JFG | 8.25b | 8.53b | 8.06b | 8.12ab | 8.17ab | 7.96a | 7.98b | 8.12cd |
| JFH | 8.22b | 8.52ab | 8.02c | 8.16ab | 8.21a | 7.97a | 8.02a | 8.16cb |
| JFI | 8.11ed | 8.38cde | 8.07b | 8.10b | 8.05abc | 7.94a | 7.85e | 8.04gh |
| JFL | 8.13cd | 8.42bcd | 8.04b | 8.12ab | 8.13abc | 7.96a | 7.89cd | 8.08ef |
| JFM | 8.14c | 8.46abcd | 8.05b | 8.13ab | 8.16abc | 7.98a | 7.92cb | 8.12de |
| JFN | 8.12ed | 8.52ab | 8.00c | 8.15a | 8.20a | 7.98a | 7.98b | 8.13bcd |
| Sign. | ** | ** | ** | ** | ** | ** | ** | ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leporini, M.; Loizzo, M.R.; Sicari, V.; Pellicanò, T.M.; Reitano, A.; Dugay, A.; Deguin, B.; Tundis, R. Citrus × Clementina Hort. Juice Enriched with Its By-Products (Peels and Leaves): Chemical Composition, In Vitro Bioactivity, and Impact of Processing. Antioxidants 2020, 9, 298. https://doi.org/10.3390/antiox9040298
Leporini M, Loizzo MR, Sicari V, Pellicanò TM, Reitano A, Dugay A, Deguin B, Tundis R. Citrus × Clementina Hort. Juice Enriched with Its By-Products (Peels and Leaves): Chemical Composition, In Vitro Bioactivity, and Impact of Processing. Antioxidants. 2020; 9(4):298. https://doi.org/10.3390/antiox9040298
Chicago/Turabian StyleLeporini, Mariarosaria, Monica Rosa Loizzo, Vincenzo Sicari, Teresa Maria Pellicanò, Antonella Reitano, Annabelle Dugay, Brigitte Deguin, and Rosa Tundis. 2020. "Citrus × Clementina Hort. Juice Enriched with Its By-Products (Peels and Leaves): Chemical Composition, In Vitro Bioactivity, and Impact of Processing" Antioxidants 9, no. 4: 298. https://doi.org/10.3390/antiox9040298