Polyphenol Composition of Extracts of the Fruits of Laserpitium Krapffii Crantz and Their Antioxidant and Cytotoxic Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Reagents
2.3. Extraction Method
2.4. Solid Phase Extraction (SPE)
2.5. Total Phenolic, Flavonoid, and Phenolic Acids Content
2.6. LC-ESI-MS/MS Analysis
2.7. Cell Lines and Cell Culture
2.8. Analysis of Cell Viability
2.9. Antioxidant Activity Cell-Free Assays
2.10. Statistical Analysis
3. Results and Discussion
3.1. Polyphenol Composition
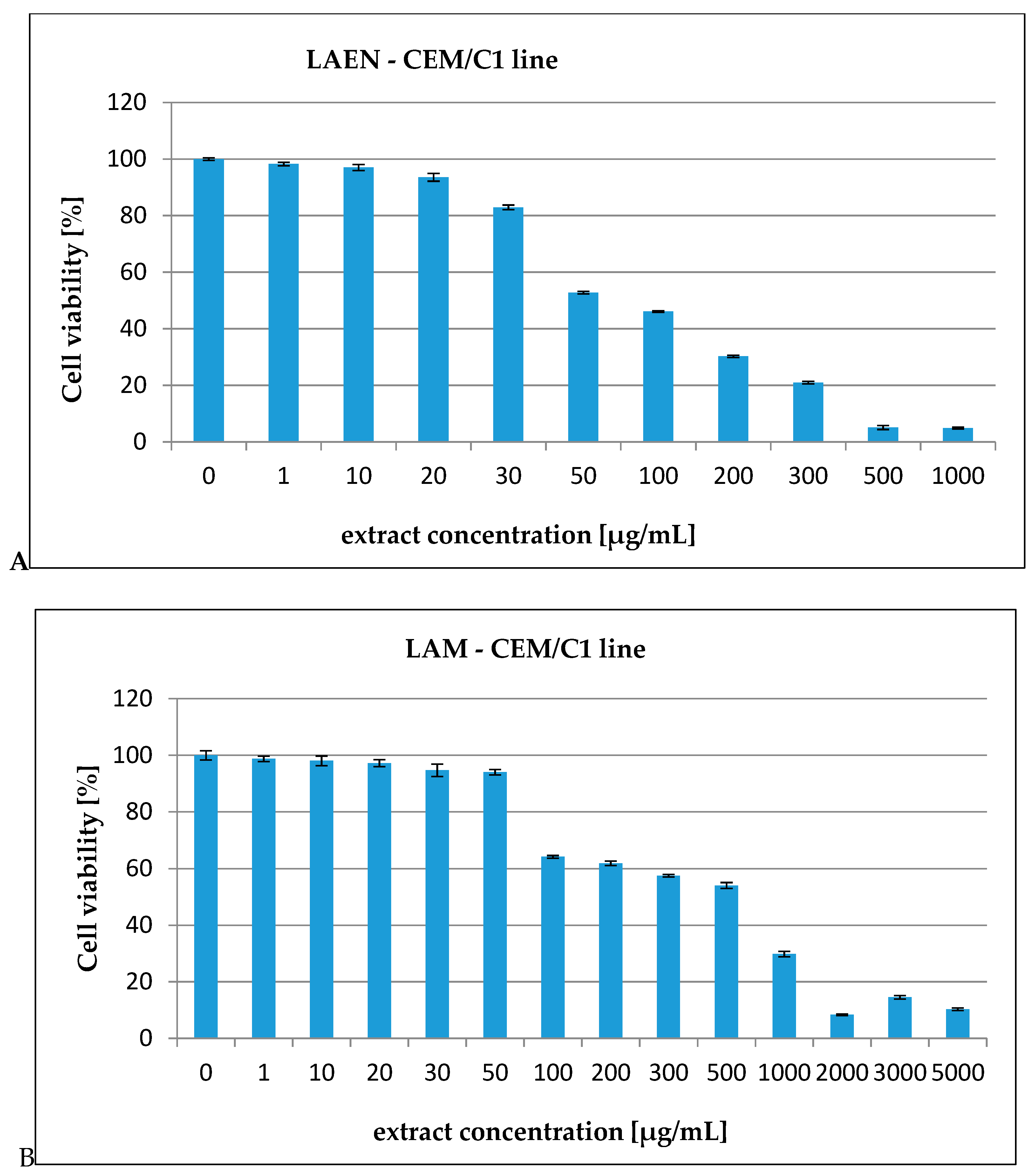
3.2. In Vitro Cytotoxicity Assay
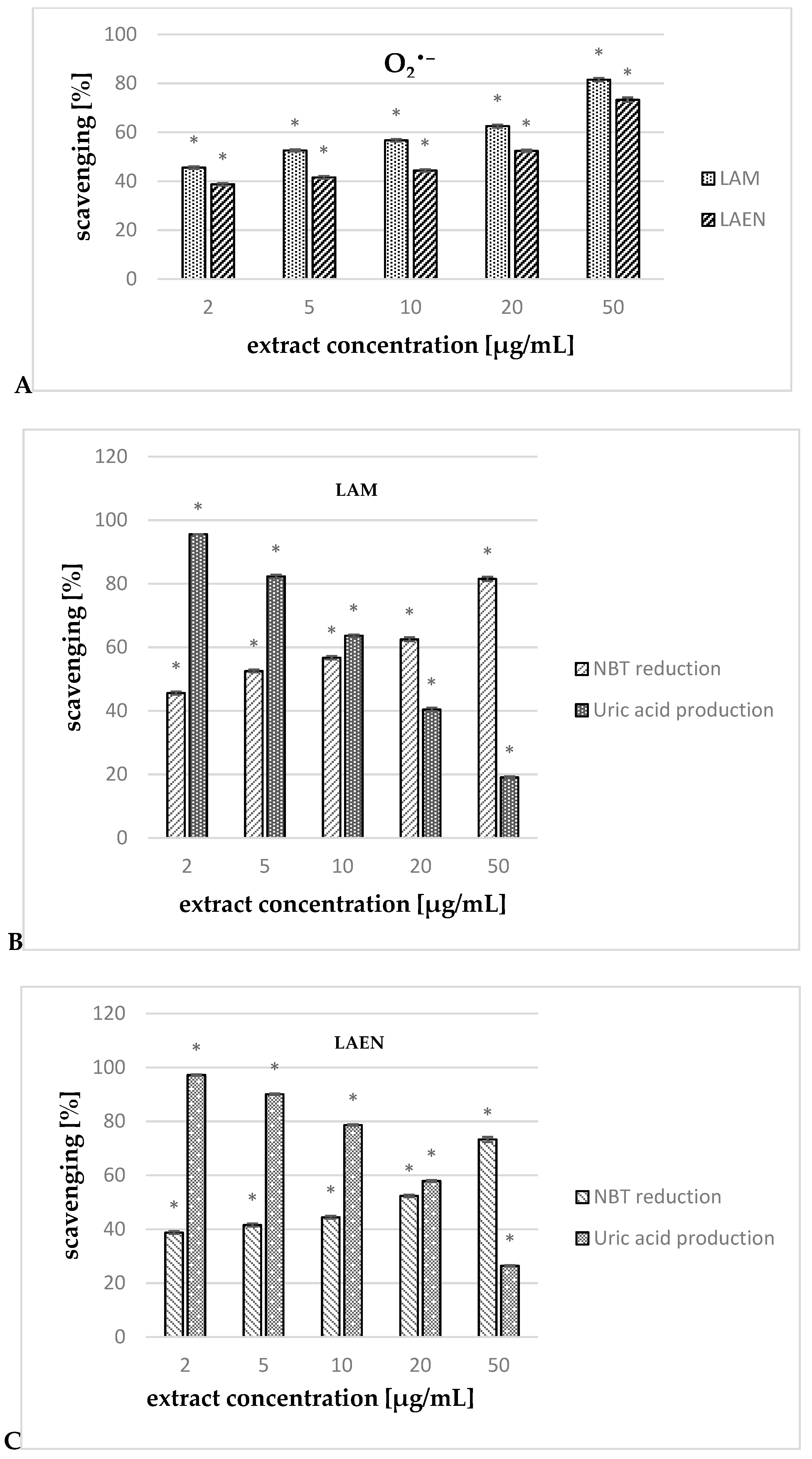
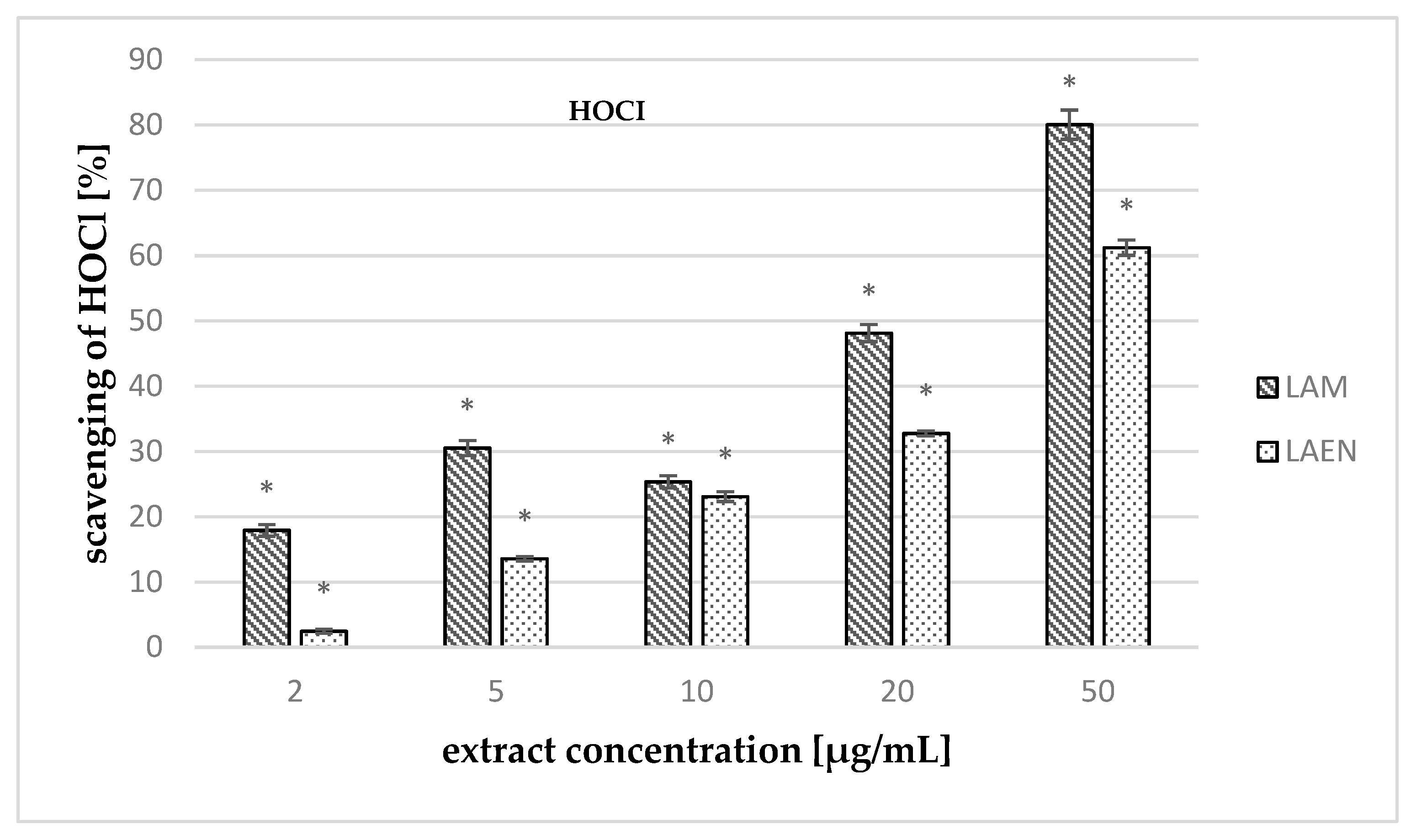
3.3. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tutin, T.G. Laserpitium L. In Flora Europaea 2; Tutin, T.G., Heywood, V.H., Burges, N.A., Moore, D.M., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1968; Volume 2, pp. 368–370. [Google Scholar]
- Djermanovic, M.; Mladenovic, S.; Ristic, N.; Stefanovic, M. Sesquiterpene lactones from Laserpitium alpinum (Umbelliferae). Int. Conf. Chem. Biotechnol. Biol. Act. Nat. Prod. 1981, 3, 317–320. [Google Scholar]
- Milosavljević, S.; Bulatović, V.; Stefanović, M. Sesquiterpene lactones from Yugoslavian wild growing plant families Asteraceae and Apiaceae. J. Serb. Chem. Soc. 1999, 64, 397–442. [Google Scholar] [CrossRef]
- Adcock, J.W.; Betts, T.J. A chemotaxonomic survey of essential oil constituents in the tribe Laserpitieae (F. Umbelliferae). Planta Med. 1974, 26, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Popović, V.; Heyerick, A.; Petrović, S.; Calenbergh, S.V.; Karalić, I.; Niketić, M.; Deforce, D. Sesquiterpene lactones from the extracts of two Balkan endemic Laserpitium species and their cytotoxic activity. Phytochemistry 2013, 87, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Holub, M.; Motl, O.; Samek, Z. Terpenes. CCIV. Structure of two sesquiterpenic lactones, isomontanolide, and acetylisomontanolide from Laserpitium siler. Collect. Czech. Chem. Commun. 1972, 37, 1186–1194. [Google Scholar] [CrossRef]
- Holub, M.; Samek, Z. Structure of archangelolide, a sesquiterpenic lactone from Laserpitium archangelica. Collect. Czech. Chem. Commun. 1973, 38, 731–738. [Google Scholar] [CrossRef]
- Bohlman, F.; Zdero, C. Prutenin, 4-Angeloyloxypruteninone, and 4-Acetoxy-pruteninone und-isopruteninone—4 new sesquiterpene lactones from Laserpitium prutenicum L. Chem. Ber. 1971, 104, 1611–1615. [Google Scholar] [CrossRef]
- Vereskovskii, V.V.; Kuznetsova, Z.P.; Loznukho, I.V.; Sokolov, I.V.; Osokin, D.M. Phenolic compounds of Laserpitium latifolium. Khim. Prir. Soedin. 1992, 6, 724–726. [Google Scholar] [CrossRef]
- Popović, V.; Heyerick, A.; Petrović, S.; Calenbergh, S.; Karalić, I.; Deforce, D.; Niketić, M. Cytotoxic activity of Laserpitium latifolium L. extract and its daucane and phenylpropanoid constituents. Rec. Nat. Prod. 2013, 7, 245–249. [Google Scholar]
- Popović, V.; Goeman, J.; Thommis, J.; Heyerick, A.; Caroen, J.; Van der Eycken, J.; De Bosscher, K. Daucane esters from laserwort (Laserpitium latifolium L.) inhibit cytokine and chemokine production in human lung epithelial cells. Phytomedicine 2017, 26, 28–36. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Duman, H. Composition of the essential oil of Laserpitium petrophilum Boiss. et Heldr. J. Essent. Oil Res. 1997, 9, 707–708. [Google Scholar] [CrossRef]
- Chizolla, R.; Novak, J.; Franz, C. Fruit of Laserpitium siler grown in France. J. Essen. Oil Res. 1999, 11, 197–198. [Google Scholar] [CrossRef]
- Popović, V.; Petrović, S.; Milenković, M.; Drobaca, M. Composition and antimicrobial activity of the essential oils of Laserpitium latifolium L. and L. ochridanum Micevski (Apiaceae). Chem. Biodivers. 2015, 12, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Hegi, G. Siler Miller, Laserpitium, L. In Illustrierte Flora von Mitteleuropa, Band V (2); Beger, H., Ed.; A. Pichler’s Witwe & Sohn: Wien, Austria, 1906; pp. 1467–1501. [Google Scholar]
- Harmatha, J.; Buděšínský, M.; Vokáč, K.; Kostecká, P.; Kmoníčková, E.; Zídek, Z. Trilobolide and related sesquiterpene lactones from Laser trilobum possessing immunobiological properties. Fitoterapia 2013, 89, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Tirillini, B.; Pagiotti, R.; Angelini, P.; Pintore, G.; Chessa, M.; Menghini, L. Chemical composition and fungicidal activity of the essential oil of Laserpitium garganicum from Italy. Chem. Nat. Comp. 2009, 45, 103–105. [Google Scholar] [CrossRef]
- Popović, V.; Petrović, S.; Tomić, M.; Stepanović-Petrović, R.; Micov, A.; Pavlović-Drobac, M.; Couladis, M.; Niketić, M. Antinociceptive and anti-edematous activities of the essential oils of two Balkan endemic Laserpitium species. Nat. Prod. Commun. 2014, 9, 125–128. [Google Scholar] [PubMed]
- Polish Pharmacopoeia IX. PTFarm; Polish Pharmaceutical Society: Warsaw, Poland, 2011; p. 150.
- Olech, M.; Nowak, R. Influence of different extraction procedures on the antiradical activity and phenolic profile of Rosa rugosa petals. Acta Pol. Pharm. Drug Res. 2012, 69, 501–507. [Google Scholar]
- Nowacka, N.; Nowak, R.; Drozd, M.; Olech, M.; Łoś, R.; Malm, A. Antibacterial, antiradical potential and phenolic compounds of thirty-one polish mushrooms. PLoS ONE 2015, 10, e0140355. [Google Scholar] [CrossRef]
- Kubrak, T.; Bogucka-Kocka, A.; Komsta, Ł.; Załuski, D.; Bogucki, J.; Gałkowski, D.; Kaczmarczyk, R.; Feldo, M.; Cioch, M.; Kocki, J. Modulation of multidrug resistance gene expression by coumarin derivatives in human leukemic cells. Oxid. Med. Cell. Longev. 2017, 2017, 5647281. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.W.; Kim, S.C.; Hwang, S.S.; Choi, B.K.; Ahn, H.J.; Lee, M.Y.; Park, S.H.; Kim, S.K. Antioxidant activity free radical scavenging capacity between Korean medicinal plants flavonoids by assay-guided comparison. Plant Sci. 2002, 163, 1161–1168. [Google Scholar] [CrossRef]
- Olech, M.; Nowacka-Jechalke, N.; Masłyk, M.; Martyna, A.; Pietrzak, W.; Kubiński, K.; Załuski, D.; Nowak, R. Polysaccharide-rich fractions from Rosa rugosa Thunb.—Composition and chemopreventive Potential. Molecules 2019, 24, 1354. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS· radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Czerwińska, M.; Kiss, A.K.; Naruszewicz, M. A comparison of antioxidant activities of oleuropein its dialdehydic derivative from olive oil, oleacein. Food Chem. 2012, 131, 940–947. [Google Scholar] [CrossRef]
- Kiss, A.K.; Filipek, A.; Czerwińska, M.; Naruszewicz, M. Oenothera paradoxa defatted seeds extract its bioactive component penta-O-galloyl-β-d-glucose decreased production of reactive oxygen species inhibited release of leukotriene B4, interleukin-8, elastase, myeloperoxidase in human neutrophils. J. Agric. Food Chem. 2010, 58, 9960–9966. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Kirpotina, L.N.; Jakiw, L.; Khlebnikov, A.I.; Blaskovich, C.L.; Jutila, M.A.; Quinn, M.T. Immunomodulatory activity of oenothein B isolated from Epilobium angustifolium. J. Immunol. 2009, 83, 6754–6766. [Google Scholar] [CrossRef]
- Farhoosh, R.; Johnny, S.; Asnaashari, M.; Molaahmadibahraseman, N.; Sharif, A. Structure-antioxidant activity relationships of o-hydroxyl, o-methoxy, and alkyl ester derivatives of p-hydroxybenzoic acid. Food Chem. 2016, 194, 128–134. [Google Scholar] [CrossRef]
- Middleton, E.J.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar]
- Szewczyk, K.; Grzywa-Celińska, A. Antioxidant and cytotoxic activities of phenolic acida and their role in the anticancer therapies. In Phenolic Acids: Properties, Food Sources and Health Effects, 1st ed.; Flores, A., Ed.; Nova Publishers: New York, NY, USA, 2016; pp. 61–104. [Google Scholar]
- Stańczyk, M.; Majsterek, I. Apoptoza-cel ukierunkowanej terapii przeciwnowotworowej. Postepy Biol. Komorki 2008, 35, 467–484. [Google Scholar]
- Jaouad, A.; Romero-Jiménez, M.; Fernández-Bedmar, Z.; Villatoro-Pulido, M.; Analla, M.; Alonso-Moraga, A.; Muñoz-Serrano, A. Antigenotoxicity, cytotoxicity, and apoptosis induction by apigenin, bisabolol, and protocatechuic acid. J. Med. Food. 2011, 14, 276–283. [Google Scholar]
- Nenadis, N.; Lazaridou, O.; Tsimidou, M.Z. Use of reference compounds in antioxidant activity assessment. J. Agric. Food Chem. 2007, 55, 5452–5460. [Google Scholar] [CrossRef]
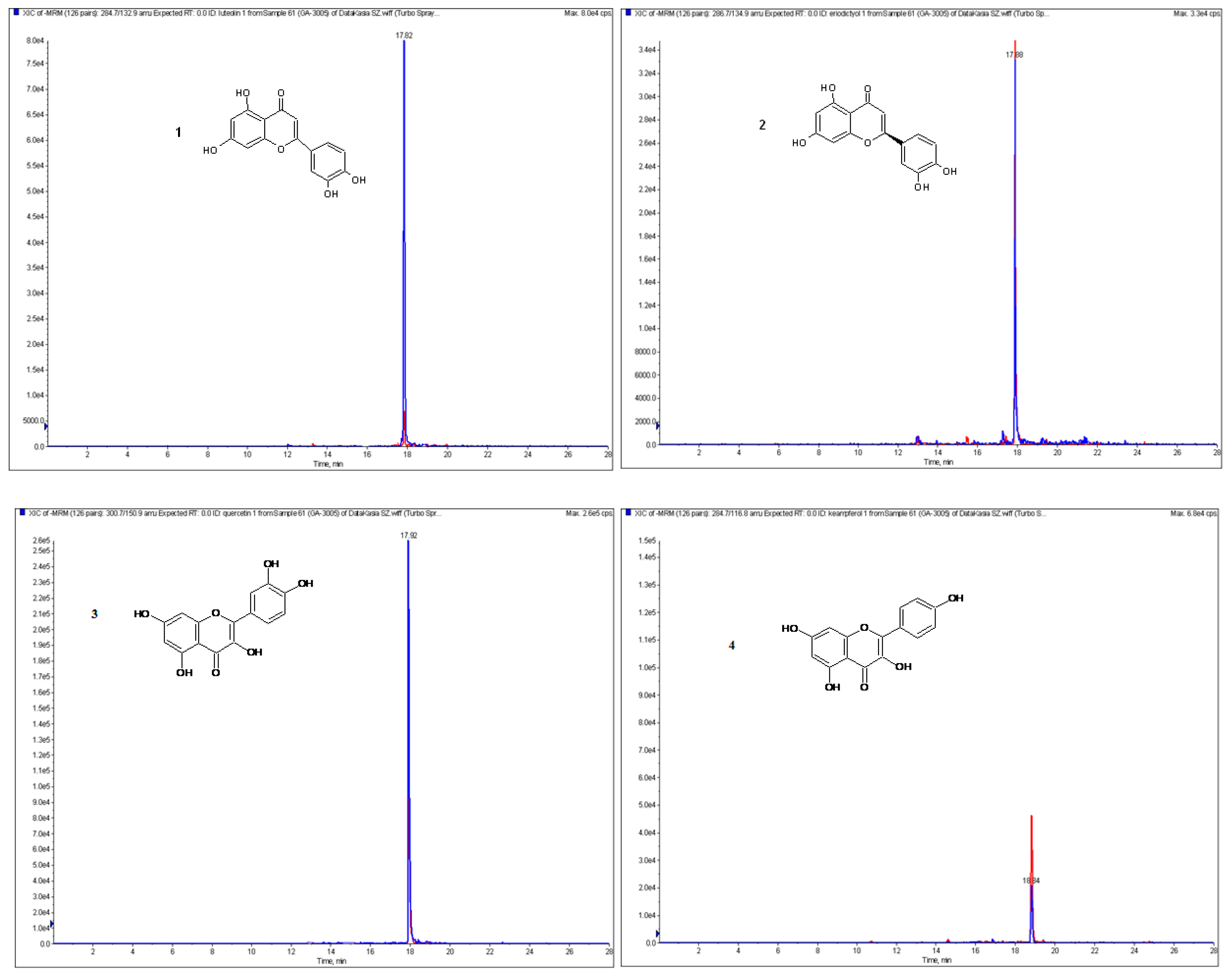
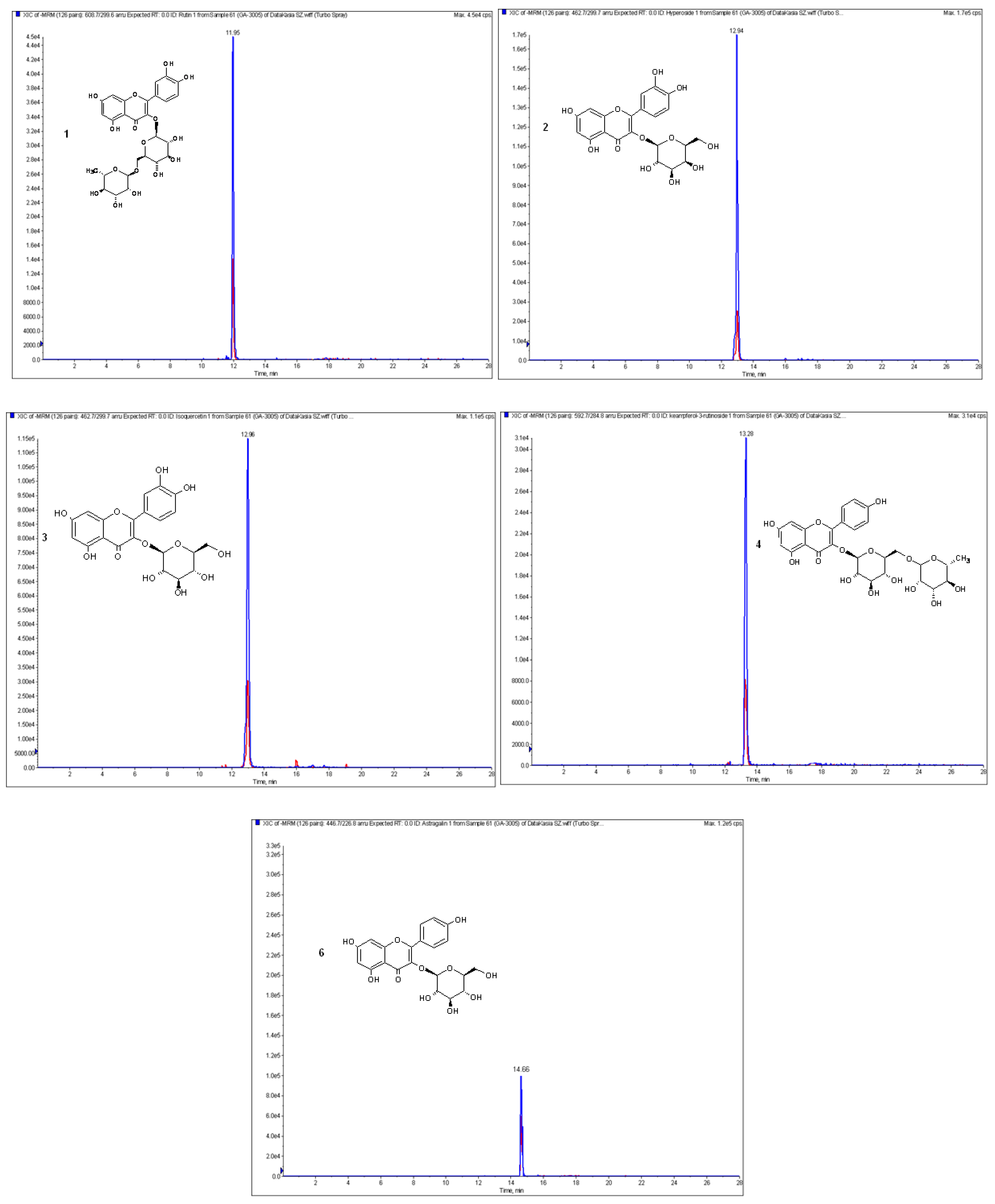




| Compound | Retention Time [min] | [M − H]− [m/z] | Fragment Ions [m/z] | Collision Energy [eV] |
|---|---|---|---|---|
| Gallic acid | 5.07 | 168.7 | 78.9 124.9 | −36 −14 |
| Protocatechuic acid | 8.23 | 152.9 | 80.9 107.8 | −26 −38 |
| 4-Hydroxybenzoic acid | 11.27 | 136.8 | 92.9 107.9 | −18 −18 |
| Syringic acid | 11.41 | 196.9 | 122.8 181.9 | −24 −12 |
| Gentisic acid | 11.43 | 352.9 | 80.0 96.9 | −110 −52 |
| Vanilic acid | 11.49 | 166.8 | 107.9 123.0 | −18 −12 |
| Caffeic acid | 11.68 | 178.7 | 88.9 134.9 | −46 −16 |
| Rutin | 11.99 | 608.7 | 299.6 270.9 | −46 −60 |
| 3-Hydroxybenzoic acid | 12.08 | 136.9 | 93.0 75.0 | −16 −48 |
| Hyperoside | 12.80 | 462.7 | 299.7 254.7 | −28 −42 |
| Isoquercetin | 13.00 | 462.7 | 299.7 270.7 | −30 −44 |
| Nicotiflorin | 13.31 | 592.7 | 284.8 226.7 | −38 −68 |
| p-Coumaric acid | 14.18 | 162.8 | 93.0 119.0 | −44 −14 |
| Astragalin | 14.66 | 446.7 | 226.8 254.8 | −54 −40 |
| Ferulic acid | 14.84 | 192.8 | 133.9 177.9 | −16 −12 |
| o-Coumaric acid | 17.17 | 162.8 | 119.0 93.0 | −14 −46 |
| Luteolin | 17.54 | 284.7 | 132.9 150.9 | −38 −26 |
| Eriodictyol | 17.87 | 286.7 | 134.9 150.9 | −32 −18 |
| Quercetin | 17.91 | 300.7 | 150.9 178.8 | −26 −20 |
| Salicylic acid | 18.06 | 136.9 | 75.0 93.0 | −48 −16 |
| Kaempferol | 18.56 | 284.7 | 116.8 93.0 | −46 −52 |
| Compound | LOD [ng/mL] | LOQ [ng/mL] | R2 | Linearity Range [ng/mL] |
|---|---|---|---|---|
| Gallic acid | 33.3 | 95.0 | 0.9987 | 167–3300 |
| Protocatechuic acid | 17.0 | 34.0 | 0.9997 | 34–3470 |
| 4-Hydroxybenzoic acid | 17.4 | 34.7 | 0.9993 | 69.4–3470 |
| Syringic acid | 167.0 | 666.0 | 0.9993 | 666–11,100 |
| Gentisic acid | 1.7 | 3.3 | 0.9997 | 3.3–330 |
| Vanillic acid | 100.0 | 250.0 | 0.9997 | 330–33,000 |
| Caffeic acid | 60.0 | 160.0 | 0.9990 | 175–3500 |
| Rutin | 120.0 | 300.0 | 0.9985 | 2000–25,000 |
| 3-Hydroxybenzoic acid | 33.3 | 334.0 | 0.9994 | 334–6670 |
| Hyperoside | 150.0 | 200.0 | 0.9987 | 1000–25,000 |
| Isoquercetin | 150.0 | 300.0 | 0.9987 | 2000–25,000 |
| Nicotiflorin | 60.0 | 120.0 | 0.9991 | 120–50,000 |
| p-Coumaric acid | 7.3 | 18.1 | 0.9996 | 18.1–1820 |
| Astragalin | 100.0 | 200.0 | 0.9978 | 1200–24,000 |
| Ferulic acid | 17.4 | 34.7 | 0.9994 | 69.4–11,600 |
| o-Coumaric acid | 7.3 | 18.1 | 0.9996 | 18.1–1820 |
| Luteolin | 25.0 | 40.0 | 0.9989 | 40–4000 |
| Eriodictyol | 10.0 | 15.0 | 0.9982 | 15–5000 |
| Quercetin | 5.0 | 10.0 | 0.9980 | 20–3000 |
| Salicylic acid | 3.3 | 16.5 | 0.9989 | 16.5–1650 |
| Kaempferol | 20.0 | 33.0 | 0.9989 | 33–20,000 |
| Extract | TPC [mg GAE/g DE] | TFC [mg QE/g DE] | TPAC [mg CAE/g DE] |
|---|---|---|---|
| LAEN | 5.53 ± 0.58 | 0.41 ± 0.17 | 1.19 ± 0.06 |
| LAM | 14.53 ± 0.60 | 1.22 ± 0.38 | 7.98 ± 0.07 |
| Compound | LAEN | LAM |
|---|---|---|
| Phenolic Acid/Flavonoid (μg per g of Dry Extract) | ||
| Gallic acid | BQL | 1.19 ± 0.05 |
| Protocatechuic acid | 58.10 ± 0.67 | 195.93 ± 0.27 |
| 4-Hydroxybenzoic acid | 5.76 ± 0.17 | 24.4 ± 0.02 |
| Syringic acid | 1.60 ± 0.05 | 9.02 ± 0.02 |
| Gentisic acid | 1.53 ± 0.13 | 8.33 ± 0.14 |
| Vanillic acid | 12.45 ± 0.12 | 34.13 ± 0.05 |
| Caffeic acid | 5.34 ± 0.08 | 20.55 ± 0.22 |
| Rutin | 3.17 ± 0.02 | 11.05 ± 0.42 |
| 3-Hydroxybenzoic acid | nd | 0.73 ± 0.08 |
| Hyperoside | 1.89 ± 0.00 | 5.12 ± 0.08 |
| Isoquercetin | 3.05 ± 0.06 | 22.53 ± 0.01 |
| Nicotiflorin | BQL | 15.36 ± 0.02 |
| p-Coumaric acid | 6.65 ± 0.18 | 24.3 ± 0.22 |
| Astragalin | 0.74 ± 0.03 | 15.51 ± 0.01 |
| Ferulic acid | 0.75 ± 0.05 | 2.91 ± 0.08 |
| o-Coumaric acid | 2.56 ± 0.05 | 9.58 ± 0.32 |
| Luteolin | 0.16 ± 0.00 | 0.86 ± 0.08 |
| Eriodictyol | BQL | 0.17 ± 0.02 |
| Quercetin | 13.92 ± 0.01 | 22.18 ±0.05 |
| Salicylic acid | 3.83 ± 0.24 | 13.35 ± 0.25 |
| Kaempferol | BQL | 0.04 ± 0.00 |
| - | IC50 [µg/mL] | |
|---|---|---|
| LAEN | LAM | |
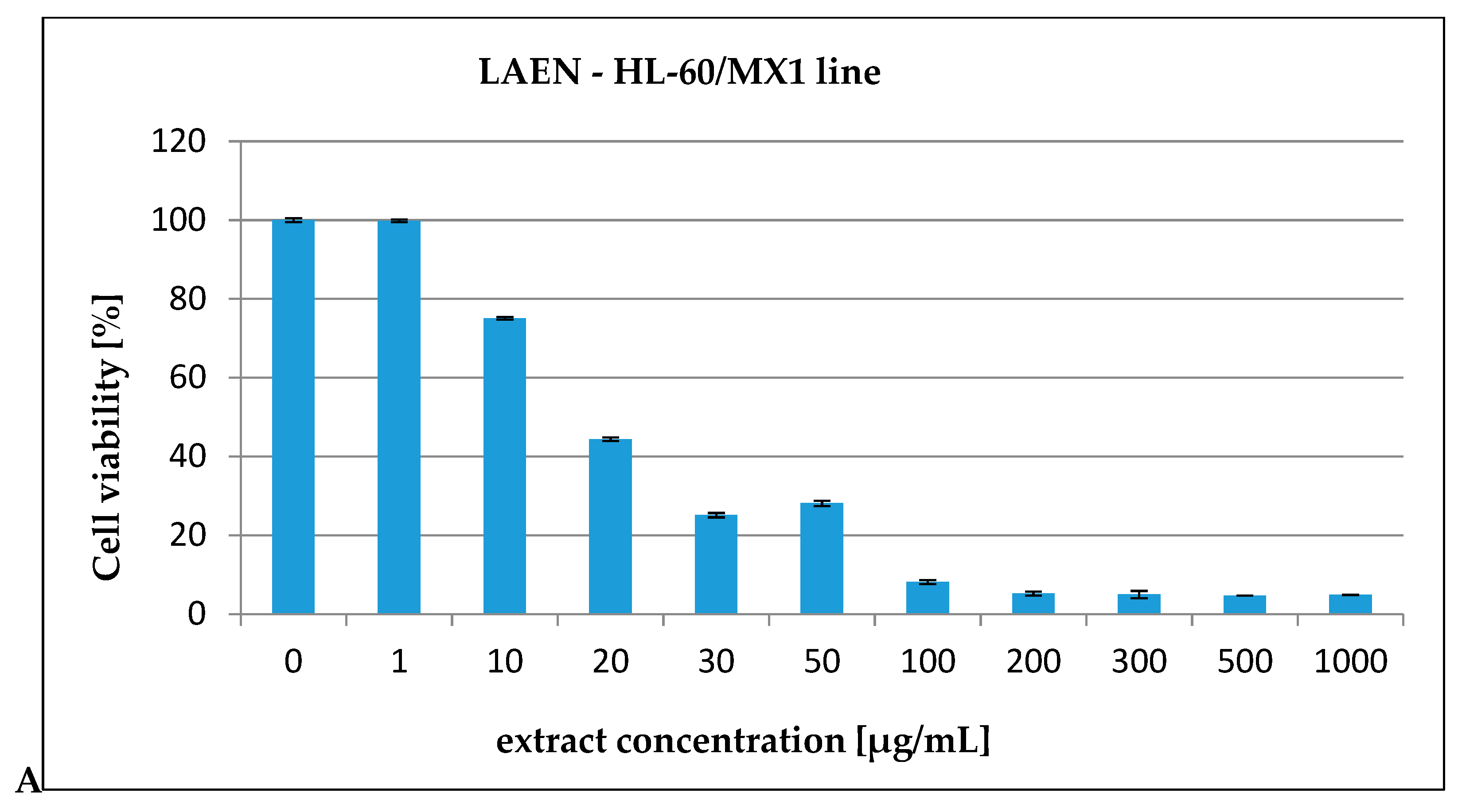
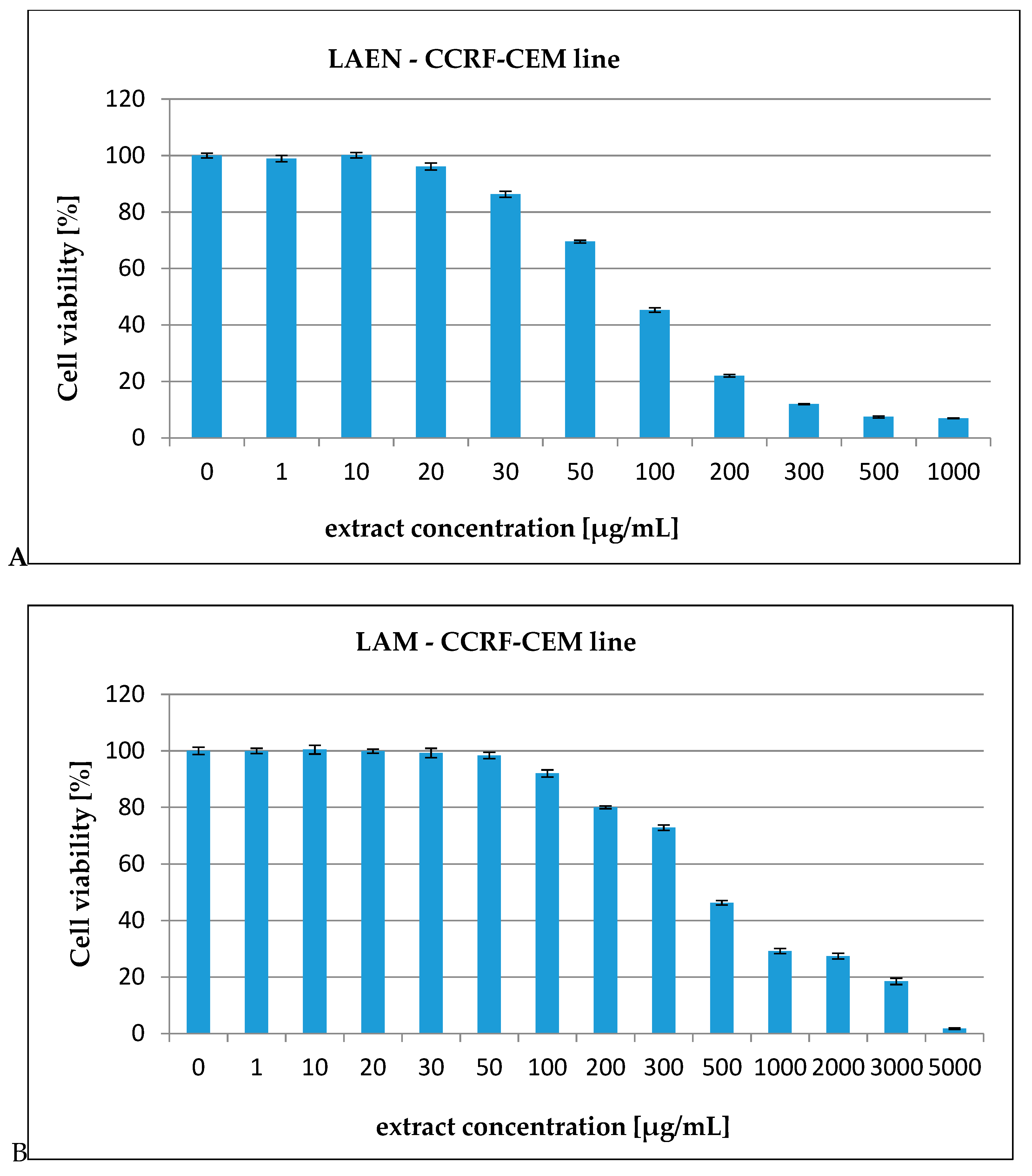
| HL-60 | 72.19 | 757.77 |
| HL-60/MX1 | 31.08 | 205.72 |
| HL-60/MX2 | 248.43 | 2848.31 |
| CEM/C1 | 99.40 | 513.03 |
| CCRF/CEM | 172.58 | 671.90 |
| Assay | Antioxidant Activity [IC50 in μg/mL of Extract] | ||||
|---|---|---|---|---|---|
| LAEN | LAM | AA | GA | Q | |
| DPPH• | 22.70 ± 0.23 | 18.93 ± 0.34 | 25.01 ± 0.14 | - | - |
| ABTS•+ | 30.45 ± 0.47 | 20.69 ± 0.43 | - | 0.75 ± 0.01 | - |
| O2•− | 18.35 ± 0.16 | 3.28 ± 0.12 | - | - | 5.72 ± 0.02 |
| HOCl | 38.17 ± 0.02 | 25.09 ± 0.10 | 49.56 ± 1.35 | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogucka-Kocka, A.; Vorobets, N.; Chrząszcz, M.; Pietrzak, W.; Szewczyk, K. Polyphenol Composition of Extracts of the Fruits of Laserpitium Krapffii Crantz and Their Antioxidant and Cytotoxic Activity. Antioxidants 2019, 8, 363. https://doi.org/10.3390/antiox8090363
Bogucka-Kocka A, Vorobets N, Chrząszcz M, Pietrzak W, Szewczyk K. Polyphenol Composition of Extracts of the Fruits of Laserpitium Krapffii Crantz and Their Antioxidant and Cytotoxic Activity. Antioxidants. 2019; 8(9):363. https://doi.org/10.3390/antiox8090363
Chicago/Turabian StyleBogucka-Kocka, Anna, Natalia Vorobets, Małgorzata Chrząszcz, Wioleta Pietrzak, and Katarzyna Szewczyk. 2019. "Polyphenol Composition of Extracts of the Fruits of Laserpitium Krapffii Crantz and Their Antioxidant and Cytotoxic Activity" Antioxidants 8, no. 9: 363. https://doi.org/10.3390/antiox8090363
APA StyleBogucka-Kocka, A., Vorobets, N., Chrząszcz, M., Pietrzak, W., & Szewczyk, K. (2019). Polyphenol Composition of Extracts of the Fruits of Laserpitium Krapffii Crantz and Their Antioxidant and Cytotoxic Activity. Antioxidants, 8(9), 363. https://doi.org/10.3390/antiox8090363





