Formulation of Broccoli Sprout Powder in Gastro-Resistant Capsules Protects against the Acidic pH of the Stomach In Vitro but Does Not Increase Isothiocyanate Bioavailability In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Broccoli Sprout Powder
2.3. Lymphocyte Isolation and Treatment with Broccoli Sprout Powder Extract
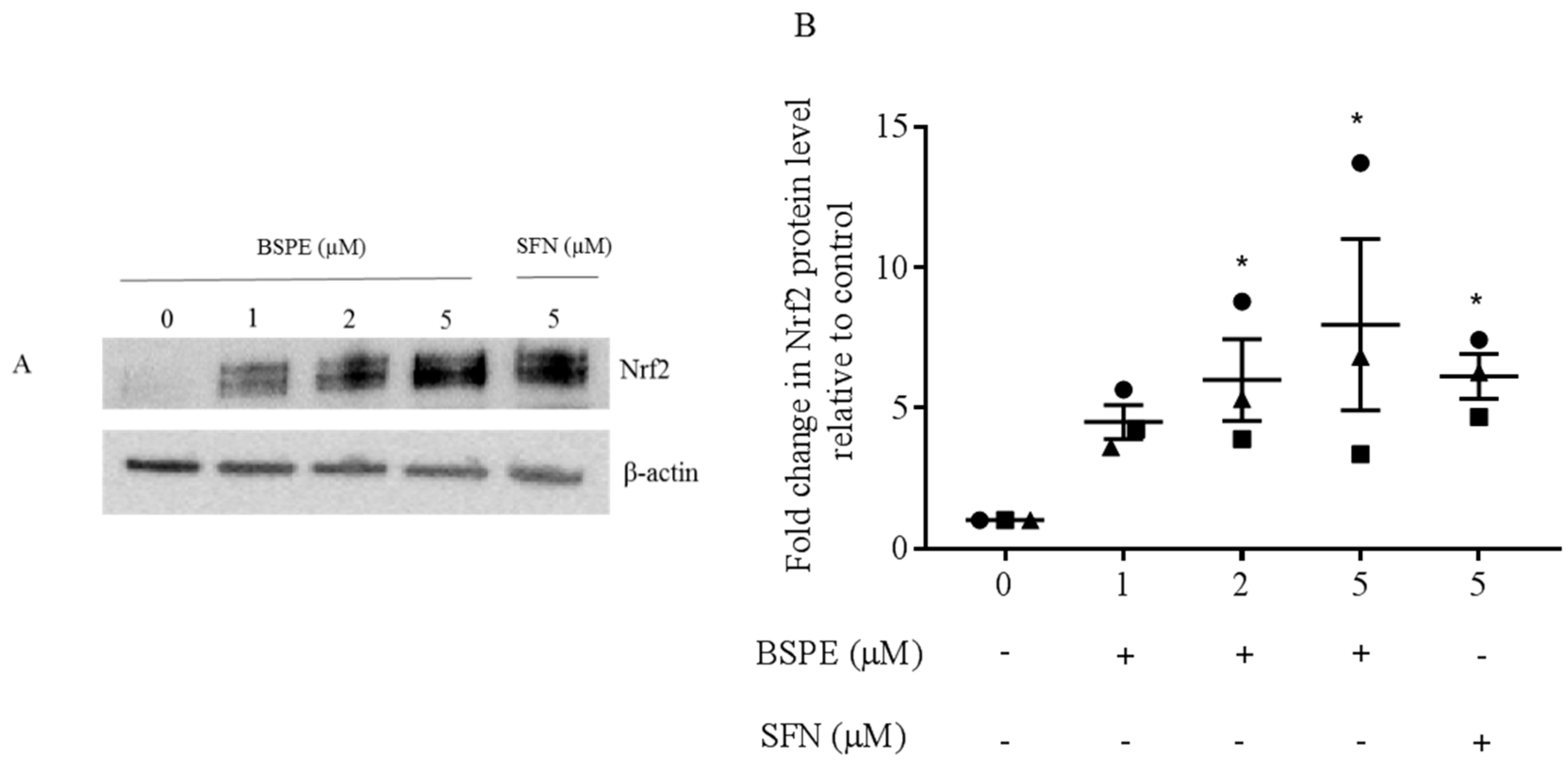
2.4. Nrf2 Immunoblotting
2.5. Preparation of Gastro-Resistant Capsules
2.5.1. Capsule Filling
2.5.2. Capsule Coating
2.6. Evaluation of Gastro-Resistant Capsules
2.6.1. Disintegration Testing
2.6.2. Dissolution Testing
2.7. Pilot Study of Gastro-Resistant Capsules
2.8. Isothiocyanate Derivatisation and Detection by HPLC
2.9. Ascorbic Acid Content of Broccoli Sprout Powder
2.10. Statistical Analyses
3. Results
3.1. Nrf2 Induction in Lymphocytes Exposed to Broccoli Sprout Powder Extract In Vitro and In Vivo
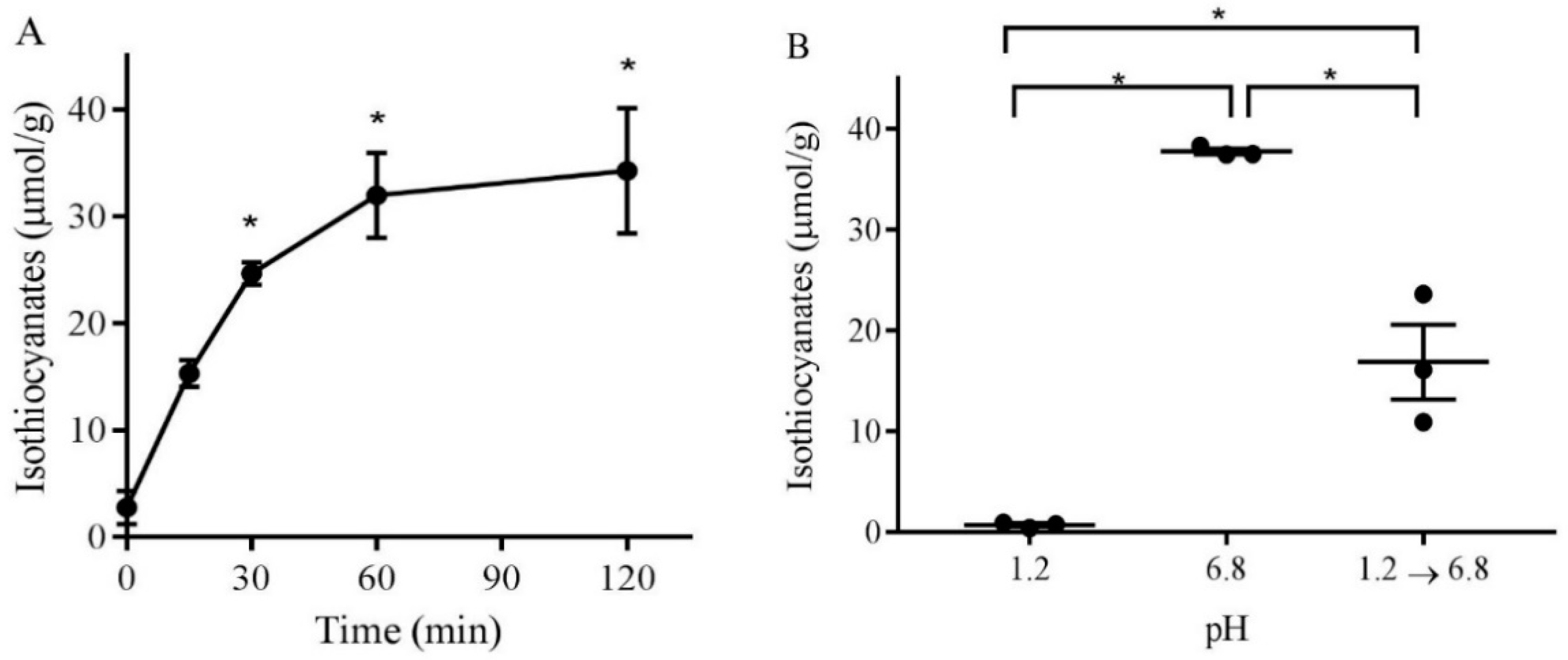
3.2. pH-Dependence of Isothiocyanate Formation from Broccoli Sprout Powder
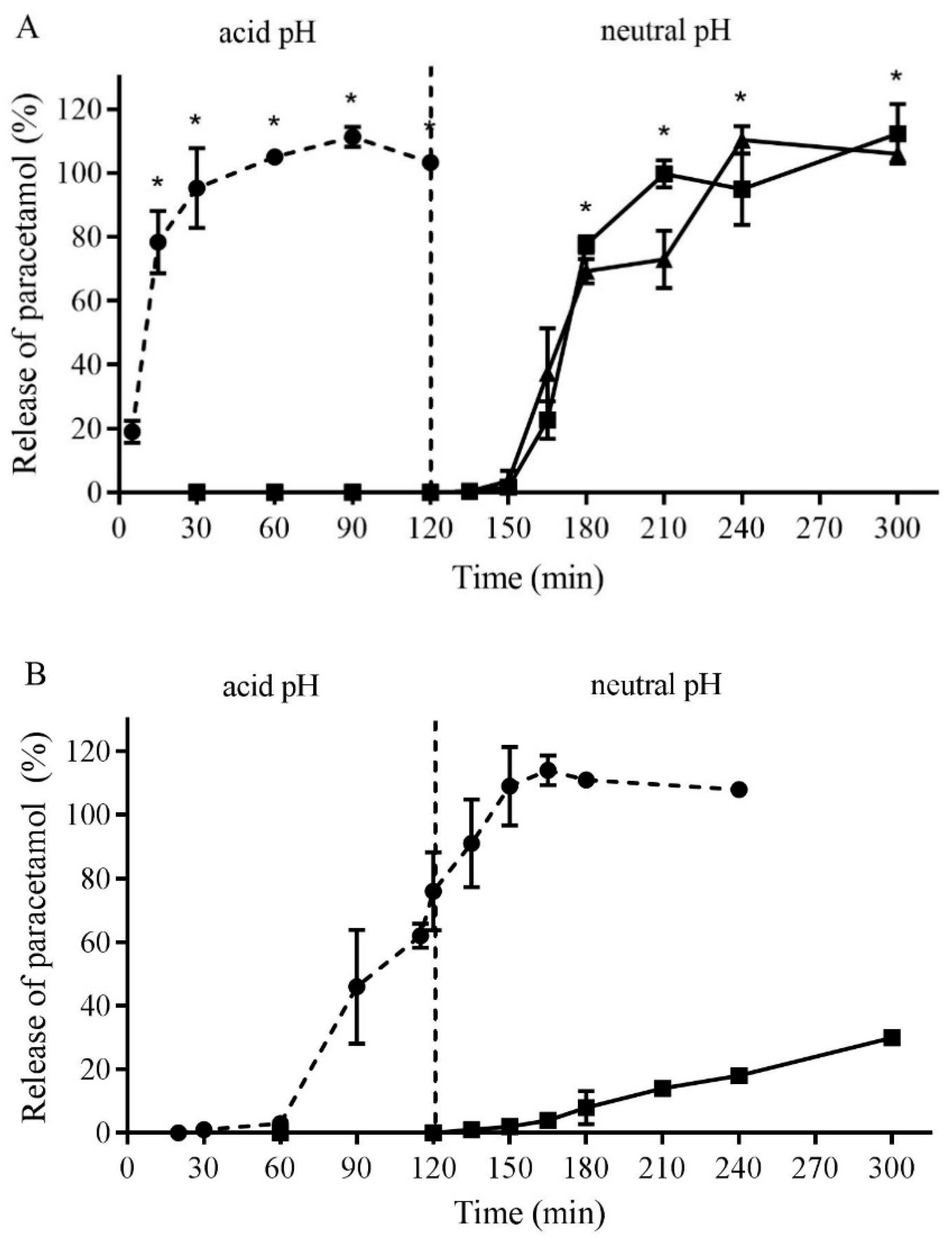
3.3. Formulation and Testing of Gastro-Resistant Capsules
3.4. In Vivo Pharmacokinetic Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dinkova-Kostova, A.T.; Fahey, J.W.; Kostov, R.V.; Kensler, T.W. KEAP1 and done? Targeting the NRF2 pathway with sulforaphane. Trends Food Sci. Technol. 2017, 69, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Halkier, B.A.; Gershenzon, J. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef] [PubMed]
- Kensler, T.W.; Egner, P.A.; Agyeman, A.S.; Visvanathan, K.; Groopman, J.D.; Chen, J.G.; Chen, T.Y.; Fahey, J.W.; Talalay, P. Keap1-nrf2 signaling: A target for cancer prevention by sulforaphane. Top. Curr. Chem. 2013, 329, 163–177. [Google Scholar] [PubMed]
- Brown, K.K.; Hampton, M.B. Biological targets of isothiocyanates. Biochim. Biophys. Acta 2011, 1810, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of Nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, J.W.; Niture, S.K.; Jaiswal, A.K. Nrf2:INrf2(Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 2009, 47, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohe, R.; Flohe, L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxid. Redox Signal. 2011, 15, 2335–2381. [Google Scholar] [CrossRef]
- Brooks, J.D.; Paton, V.G.; Vidanes, G. Potent induction of phase 2 enzymes in human prostate cells by sulforaphane. Cancer Epidemiol. Prev. Biomark. 2001, 10, 949–954. [Google Scholar]
- Angeloni, C.; Leoncini, E.; Malaguti, M.; Angelini, S.; Hrelia, P.; Hrelia, S. Modulation of phase II enzymes by sulforaphane: Implications for its cardioprotective potential. J. Agric. Food Chem. 2009, 57, 5615–5622. [Google Scholar] [CrossRef]
- Duran, C.G.; Burbank, A.J.; Mills, K.H.; Duckworth, H.R.; Aleman, M.M.; Kesic, M.J.; Peden, D.B.; Pan, Y.; Zhou, H.; Hernandez, M.L. A proof-of-concept clinical study examining the NRF2 activator sulforaphane against neutrophilic airway inflammation. Respir. Res. 2016, 17, 89. [Google Scholar] [CrossRef]
- Atwell, L.L.; Hsu, A.; Wong, C.P.; Stevens, J.F.; Bella, D.; Yu, T.W.; Pereira, C.B.; Lohr, C.V.; Christensen, J.M.; Dashwood, R.H.; et al. Absorption and chemopreventive targets of sulforaphane in humans following consumption of broccoli sprouts or a myrosinase-treated broccoli sprout extract. Mol. Nutr. Food Res. 2015, 59, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Zhang, Y.; Talalay, P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. USA 1997, 94, 10367–10372. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.M.; Jeffery, E.H. Sulforaphane absorption and excretion following ingestion of a semi-purified broccoli powder rich in glucoraphanin and broccoli sprouts in healthy men. Nutr. Cancer 2011, 63, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Hollands, W.; Teucher, B.; Needs, P.W.; Narbad, A.; Ortori, C.A.; Barrett, D.A.; Rossiter, J.T.; Mithen, R.F.; Kroon, P.A. Isothiocyanate concentrations and interconversion of sulforaphane to erucin in human subjects after consumption of commercial frozen broccoli compared to fresh broccoli. Mol. Nutr. Food Res. 2012, 56, 1906–1916. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, M.; Klopping-Ketelaars, I.W.; van den Berg, R.; Vaes, W.H. Bioavailability and kinetics of sulforaphane in humans after consumption of cooked versus raw broccoli. J. Agric. Food Chem. 2008, 56, 10505–10509. [Google Scholar] [CrossRef] [PubMed]
- Conaway, C.C.; Getahun, S.M.; Liebes, L.L.; Pusateri, D.J.; Topham, D.K.; Botero-Omary, M.; Chung, F.L. Disposition of glucosinolates and sulforaphane in humans after ingestion of steamed and fresh broccoli. Nutr. Cancer 2000, 38, 168–178. [Google Scholar] [CrossRef]
- Cramer, J.M.; Teran-Garcia, M.; Jeffery, E.H. Enhancing sulforaphane absorption and excretion in healthy men through the combined consumption of fresh broccoli sprouts and a glucoraphanin-rich powder. Br. J. Nutr. 2012, 107, 1333–1338. [Google Scholar] [CrossRef]
- Clarke, J.D.; Hsu, A.; Riedl, K.; Bella, D.; Schwartz, S.J.; Stevens, J.F.; Ho, E. Bioavailability and inter-conversion of sulforaphane and erucin in human subjects consuming broccoli sprouts or broccoli supplement in a cross-over study design. Pharmacol. Res. 2011, 64, 456–463. [Google Scholar] [CrossRef]
- Clarke, J.D.; Riedl, K.; Bella, D.; Schwartz, S.J.; Stevens, J.F.; Ho, E. Comparison of isothiocyanate metabolite levels and histone deacetylase activity in human subjects consuming broccoli sprouts or broccoli supplement. J. Agric. Food Chem. 2011, 59, 10955–10963. [Google Scholar] [CrossRef]
- Kuchernig, J.C.; Burow, M.; Wittstock, U. Evolution of specifier proteins in glucosinolate-containing plants. BMC Evol. Biol. 2012, 12, 127. [Google Scholar] [CrossRef]
- Shikita, M.; Fahey, J.W.; Golden, T.R.; Holtzclaw, W.D.; Talalay, P. An unusual case of ‘uncompetitive activation’ by ascorbic acid: Purification and kinetic properties of a myrosinase from Raphanus sativus seedlings. Biochem. J. 1999, 341, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Sun, J.; Haytowitz, D.B.; Harnly, J.M.; Chen, P.; Pehrsson, P.R. Challenges of developing a valid dietary glucosinolate database. J. Food Compos. Anal. 2017, 64, 78–84. [Google Scholar] [CrossRef]
- Ludikhuyze, L.; Rodrigo, L.; Hendrickx, M. The activity of myrosinase from broccoli (Brassica oleracea L. cv. Italica): Influence of intrinsic and extrinsic factors. J. Food Prot. 2000, 63, 400–403. [Google Scholar] [PubMed]
- Burmeister, W.P.; Cottaz, S.; Rollin, P.; Vasella, A.; Henrissat, B. High resolution X-ray crystallography shows that ascorbate is a cofactor for myrosinase and substitutes for the function of the catalytic base. J. Biol. Chem. 2000, 275, 39385–39393. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Xu, X.; Hu, H.; Liu, Y.; Pan, S. Optimisation of enzymatic production of sulforaphane in broccoli sprouts and their total antioxidant activity at different growth and storage days. J. Food Sci. Technol. 2017, 54, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Su, G.; Wang, X.; Du, Q.; Wang, K. Endogenous and exogenous enzymolysis of vegetable-sourced glucosinolates and influencing factors. Food Chem. 2010, 119, 987–994. [Google Scholar] [CrossRef]
- Guo, Q.; Guo, L.; Wang, Z.; Zhuang, Y.; Gu, Z. Response surface optimization and identification of isothiocyanates produced from broccoli sprouts. Food Chem. 2013, 141, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Vaughn, S.F.; Berhow, M.A. Glucosinolate hydrolysis products from various plant sources: pH effects, isolation, and purification. Ind. Crops Prod. 2005, 21, 193–202. [Google Scholar] [CrossRef]
- Hayes, J.D.; Kelleher, M.O.; Eggleston, I.M. The cancer chemopreventive actions of phytochemicals derived from glucosinolates. Eur. J. Nutr. 2008, 47, 73–88. [Google Scholar] [CrossRef]
- Fahey, J.W.; Holtzclaw, W.D.; Wehage, S.L.; Wade, K.L.; Stephenson, K.K.; Talalay, P. Sulforaphane bioavailability from glucoraphanin-rich broccoli: Control by active endogenous myrosinase. PLoS ONE 2015, 10, e0140963. [Google Scholar] [CrossRef] [PubMed]
- DRcaps™ Capsules. Performance Characteristics. 2011. Available online: https://www.capsugel.com/quality-responsibility (accessed on 23 August 2018).
- Kramer, P.A.; Chacko, B.K.; Ravi, S.; Johnson, M.S.; Mitchell, T.; Darley-Usmar, V.M. Bioenergetics and the oxidative burst: Protocols for the isolation and evaluation of human leukocytes and platelets. J. Vis. Exp. 2014, 85, e51301. [Google Scholar] [CrossRef] [PubMed]
- Chung, F.L.; Jiao, D.; Getahun, S.M.; Yu, M.C. A urinary biomarker for uptake of dietary isothiocyanates in humans. Cancer Epidemiol. Prev. Biomark. 1998, 7, 103–108. [Google Scholar]
- Ye, L.; Dinkova-Kostova, A.T.; Wade, K.L.; Zhang, Y.; Shapiro, T.A.; Talalay, P. Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: Pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin. Chim. Acta 2002, 316, 43–53. [Google Scholar] [CrossRef]
- Carr, A.C.; Pullar, J.M.; Moran, S.; Vissers, M.C. Bioavailability of vitamin C from kiwifruit in non-smoking males: Determination of ‘healthy’ and ‘optimal’ intakes. J. Nutr. Sci. 2012, 1, e14. [Google Scholar] [CrossRef] [PubMed]
- U.S. Pharmacopeia. Disintegration. Available online: http://www.pharmacopeia.cn/v29240/usp29nf24s0_c701h.html (accessed on 1 April 2006).
- Zhang, Y. Role of glutathione in the accumulation of anticarcinogenic isothiocyanates and their glutathione conjugates by murine hepatoma cells. Carcinogenesis 2000, 21, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Bones, A.M.; Rossiter, J.T. The enzymic and chemically induced decomposition of glucosinolates. Phytochemistry 2006, 67, 1053–1067. [Google Scholar] [CrossRef]
- Gu, Z.-X.; Guo, Q.-H.; Gu, Y.-J. Factors influencing glucoraphanin and sulforaphane formation in brassica plants: A review. J. Integr. Agric. 2012, 11, 1804–1816. [Google Scholar] [CrossRef]
- Van Etten, C.H.; Daxenbichler, M.E.; Peters, J.E.; Tookey, H.L. Variation in enzymatic degradation products from major thioglucosides in crambe abyssinica and brassica napus seed meals. J. Agric. Food Chem. 1966, 14, 426–430. [Google Scholar] [CrossRef]
- Gil, V.; MacLeod, A.J. The effects of pH on glucosinolate degradation by a thioglucoside glucohydrolase preparation. Phytochemistry 1980, 19, 2547–2551. [Google Scholar] [CrossRef]
- Fahey, J.W.; Wehage, S.L.; Holtzclaw, W.D.; Kensler, T.W.; Egner, P.A.; Shapiro, T.A.; Talalay, P. Protection of humans by plant glucosinolates: Efficiency of conversion of glucosinolates to isothiocyanates by the gastrointestinal microflora. Cancer Prev. Res. 2012, 5, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, T.A.; Fahey, J.W.; Wade, K.L.; Stephenson, K.K.; Talalay, P. Human metabolism and excretion of cancer chemoprotective glucosinolates and isothiocyanates of cruciferous vegetables. Cancer Epidemiol. Prev. Biomark. 1998, 7, 1091–1100. [Google Scholar]
- Aburub, A.; Fischer, M.; Camilleri, M.; Semler, J.R.; Fadda, H.M. Comparison of pH and motility of the small intestine of healthy subjects and patients with symptomatic constipation using the wireless motility capsule. Int. J. Pharm. 2018, 544, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Al-Gousous, J.; Tsume, Y.; Fu, M.; Salem, I.I.; Langguth, P. Unpredictable performance of pH-dependent coatings accentuates the need for improved predictive in vitro test systems. Mol. Pharm. 2017, 14, 4209–4219. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K. Small intestine transit time in the normal small bowel study. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1968, 104, 522–524. [Google Scholar] [CrossRef] [PubMed]
- Mrsny, R.J. The colon as a site for drug delivery. J. Control. Releas. 1992, 22, 15–34. [Google Scholar] [CrossRef]
- Graff, J.; Brinch, K.; Madsen, J.L. Gastrointestinal mean transit times in young and middle-aged healthy subjects. Clin. Physiol. 2001, 21, 253–259. [Google Scholar] [CrossRef]
- Oliviero, T.; Lamers, S.; Capuano, E.; Dekker, M.; Verkerk, R. Bioavailability of isothiocyanates from broccoli sprouts in protein, lipid, and fiber gels. Mol. Nutr. Food Res. 2018, 62, e1700837. [Google Scholar] [CrossRef]
- Rouge, N.; Buri, P.; Doelker, E. Drug absorption sites in the gastrointestinal tract and dosage forms for site-specific delivery. Int. J. Pharm. 1996, 136, 117–139. [Google Scholar] [CrossRef]
- Geus, W.P.; Mathôt, R.A.; Mulder, P.G.; Lamers, C.B. Pharmacodynamics and kinetics of omeprazole MUPS 20 mg and pantoprazole 40 mg during repeated oral administration in Helicobacter pylori-negative subjects. Aliment. Pharmacol. Ther. 2000, 14, 1057–1064. [Google Scholar] [CrossRef]
- Tirpude, R.N.; Puranik, P.K. Rabeprazole sodium delayed-release multiparticulates: Effect of enteric coating layers on product performance. J. Adv. Pharm. Technol. Res. 2011, 2, 184–191. [Google Scholar] [CrossRef] [PubMed]
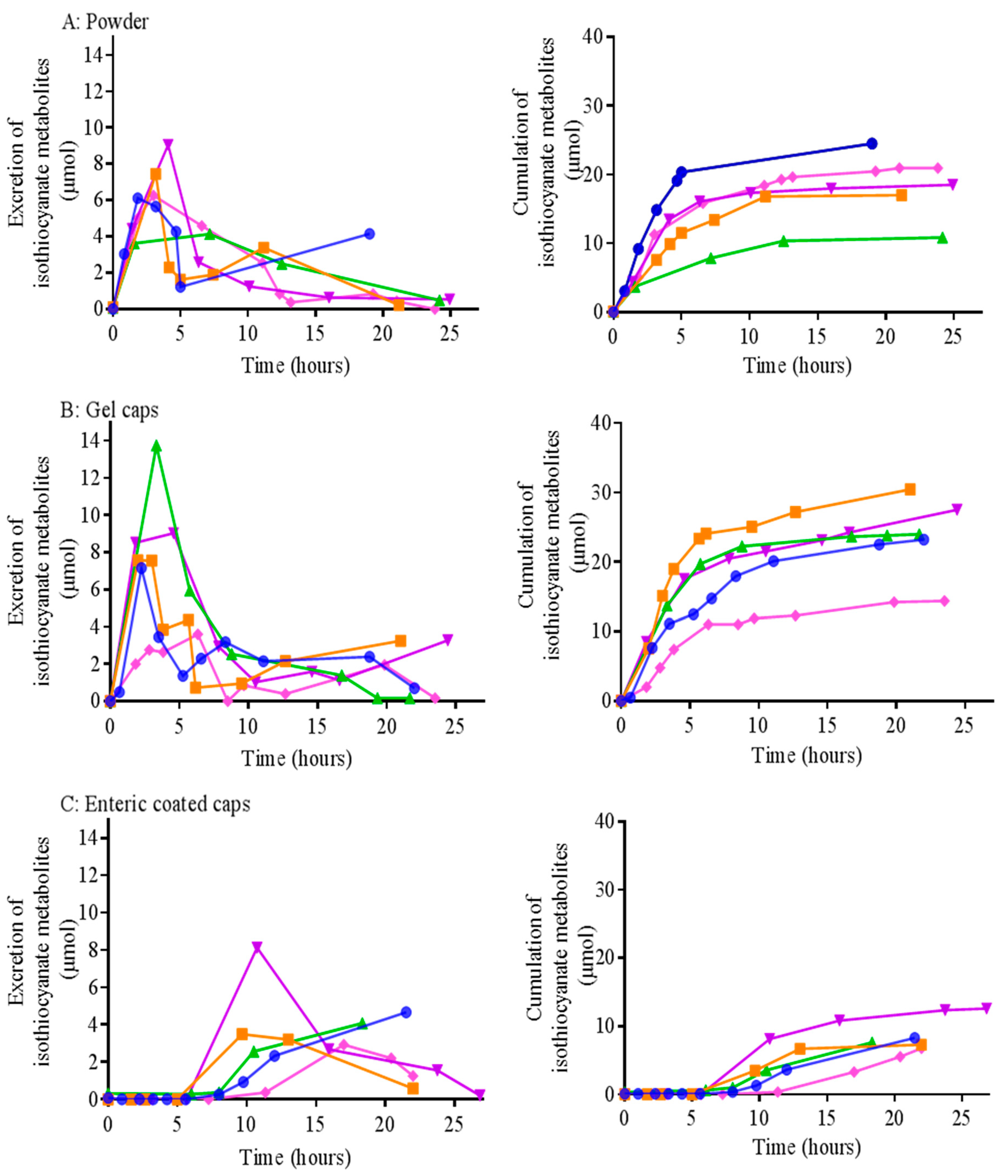
| Tmax (h) | Cmax (µmol) | Total Isothiocyanate Metabolites (µmol) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Subject No. | Powder | Gel-Cap | Enteric-Cap | Powder | Gel-Cap | Enteric-Cap | Powder | Gel-Cap | Enteric-Cap |
| 1 | 1.8 | 2.3 | 21.5 | 6.1 | 7.2 | 4.7 | 24.5 | 23.2 | 8.3 |
| 2 | 3.2 | 2.0 | 9.7 | 7.5 | 7.6 | 3.5 | 17.0 | 30.5 | 7.3 |
| 3 | 7.2 | 3.3 | 18.3 | 4.1 | 13.8 | 4.1 | 10.9 | 24.0 | 7.6 |
| 4 | 4.1 | 4.6 | 10.8 | 9.1 | 9.0 | 8.1 | 18.5 | 27.5 | 12.6 |
| 5 | 3.0 | 6.3 | 17.0 | 6.3 | 3.6 | 2.9 | 20.9 | 14.4 | 6.8 |
| mean | 3.9 | 3.7 | 15.4 * | 6.6 | 8.2 | 4.7 | 18.4 | 23.9 | 8.5 * |
| SEM | 0.9 | 0.7 | 2.3 | 0.8 | 1.6 | 0.9 | 2.3 | 2.7 | 1.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zawari, M.; Poller, B.; Walker, G.; Pearson, A.; Hampton, M.; Carr, A.C. Formulation of Broccoli Sprout Powder in Gastro-Resistant Capsules Protects against the Acidic pH of the Stomach In Vitro but Does Not Increase Isothiocyanate Bioavailability In Vivo. Antioxidants 2019, 8, 359. https://doi.org/10.3390/antiox8090359
Zawari M, Poller B, Walker G, Pearson A, Hampton M, Carr AC. Formulation of Broccoli Sprout Powder in Gastro-Resistant Capsules Protects against the Acidic pH of the Stomach In Vitro but Does Not Increase Isothiocyanate Bioavailability In Vivo. Antioxidants. 2019; 8(9):359. https://doi.org/10.3390/antiox8090359
Chicago/Turabian StyleZawari, Masuma, Bettina Poller, Greg Walker, Andree Pearson, Mark Hampton, and Anitra C. Carr. 2019. "Formulation of Broccoli Sprout Powder in Gastro-Resistant Capsules Protects against the Acidic pH of the Stomach In Vitro but Does Not Increase Isothiocyanate Bioavailability In Vivo" Antioxidants 8, no. 9: 359. https://doi.org/10.3390/antiox8090359
APA StyleZawari, M., Poller, B., Walker, G., Pearson, A., Hampton, M., & Carr, A. C. (2019). Formulation of Broccoli Sprout Powder in Gastro-Resistant Capsules Protects against the Acidic pH of the Stomach In Vitro but Does Not Increase Isothiocyanate Bioavailability In Vivo. Antioxidants, 8(9), 359. https://doi.org/10.3390/antiox8090359








