Serum Free Thiols Are Superior to Fecal Calprotectin in Reflecting Endoscopic Disease Activity in Inflammatory Bowel Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Measurement of Serum Free Thiols
2.4. Statistical Analysis
2.5. Ethical Considerations
3. Results
3.1. Cohort Demographic and Clinical Characteristics
3.2. Serum Levels of Free Thiols are Markedly Decreased in IBD
3.3. Associations between Serum Albumin-Adjusted Free Thiols and IBD Parameters
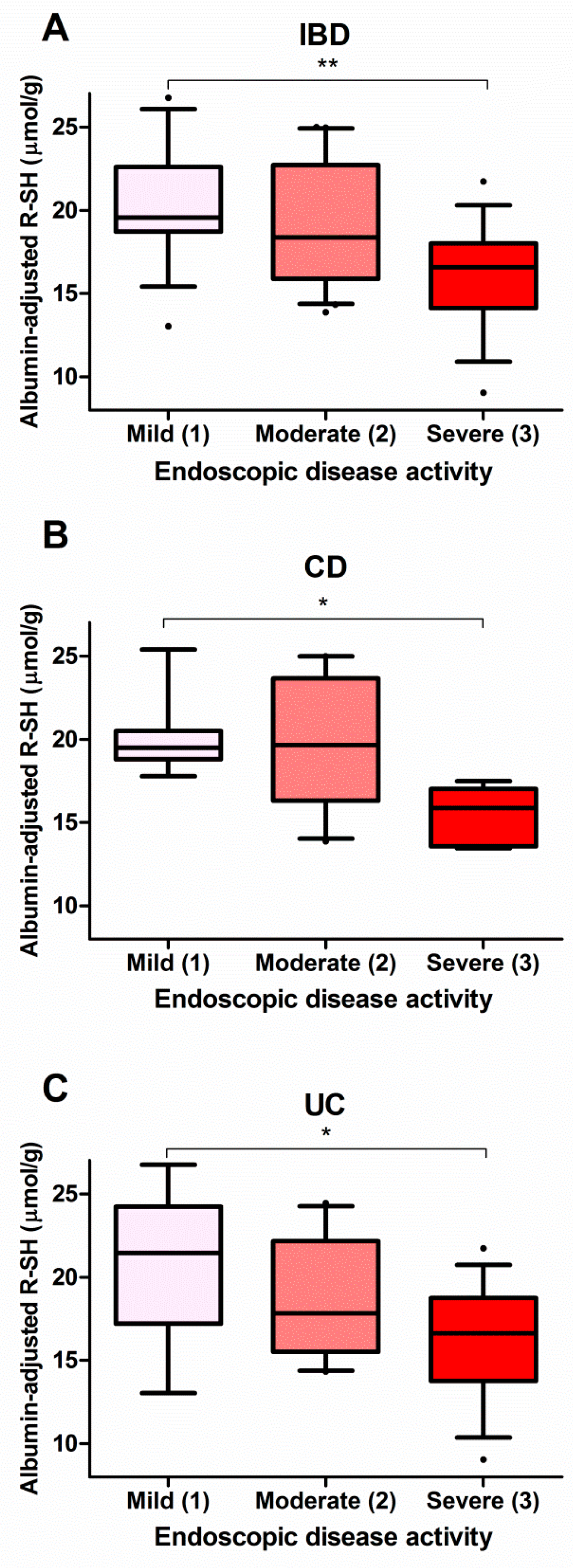
3.4. Serum Free Thiols Strongly Correlate with Endoscopic Disease Activity
3.5. Discriminative Accuracy of Serum Free Thiols regarding Endoscopic Disease Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abraham, C.; Cho, J.H. Inflammatory bowel disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar] [CrossRef] [PubMed]
- Cosnes, J.; Gower-Rousseau, C.; Seksik, P.; Cortot, A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011, 140, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; Loftus, E.V., Jr.; Panaccione, R.; Chen, L.S.; Peterson, S.; McConnell, J.; Baudhuin, L.; Hanson, K.; Feagan, B.G.; Harmsen, S.W.; et al. Relationships between disease activity and serum and fecal biomarkers in patients with Crohn’s disease. Clin. Gastroenterol. Hepatol. 2008, 6, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.F.; Narula, N.; Peyrin-Biroulet, L. Management strategies to improve outcomes of patients with inflammatory bowel diseases. Gastroenterology 2017, 152, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Benitez, J.M.; Meuwis, M.A.; Reenaers, C.; Van Kemseke, C.; Meunier, P.; Louis, E. Role of endoscopy, cross-sectional imaging and biomarkers in Crohn’s disease monitoring. Gut 2013, 62, 1806–1816. [Google Scholar] [CrossRef] [PubMed]
- Langhorst, J.; Elsenbruch, S.; Koelzer, J.; Rueffer, A.; Michalsen, A.; Dobos, G.J. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel disease: Performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am. J. Gastroenterol. 2008, 103, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Cellier, C.; Sahmoud, T.; Froquel, E.; Adenis, A.; Belaiche, J.; Bretagne, J.F.; Florent, C.; Bouvry, M.; Mary, J.Y.; Modigliani, R. Correlations between clinical activity, endoscopic severity, and biological parameters in colonic or ileocolonic Crohn’s disease. A prospective multicentre study of 121 cases. The Groupe d’Études Thérapeutiques des Affections Inflammatoires Digestives. Gut 1994, 35, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.F.; Chen, J.M.; Zuo, J.H.; Yu, A.; Xiao, Z.J.; Deng, F.H.; Nie, B.; Jiang, B. Meta-analysis: Fecal calprotectin for assessment of inflammatory bowel disease activity. Inflamm. Bowel Dis. 2014, 20, 1407–1415. [Google Scholar] [CrossRef]
- Rokkas, T.; Portincasa, P.; Koutroubakis, I.E. Fecal calprotectin in assessing inflammatory bowel disease endoscopic activity: A diagnostic accuracy meta-analysis. J. Gastrointestin. Liver Dis. 2018, 27, 299–306. [Google Scholar]
- Lewis, J.D. The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterology 2011, 140, 1817–1826. [Google Scholar] [CrossRef]
- Sands, B.E. Biomarkers of inflammation in inflammatory bowel disease. Gastroenterology 2015, 149, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Goutorbe, F.; Goutte, M.; Minet-Quinard, R.; Boucher, A.L.; Pereira, B.; Bommelaer, G.; Buisson, A. Endoscopic Factors Influencing Fecal Calprotectin Value in Crohn’s Disease. J. Crohns Colitis 2015, 9, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Lan, S. Implications of Antioxidant Systems in Inflammatory Bowel Disease. Biomed. Res. Int. 2018, 2018, 1290179. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, Y.R. Oxidative stress and redox signaling mechanisms of inflammatory bowel disease: Updated experimental and clinical evidence. Exp. Biol. Med. (Maywood) 2012, 237, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Banne, A.F.; Amiri, A.; Pero, R.W. Reduced level of serum thiols in patients with a diagnosis of active disease. J. Anti-Aging Med. 2003, 6, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Cortese-Krott, M.M.; Koning, A.; Kuhnle, G.G.C.; Nagy, P.; Bianco, C.L.; Pasch, A.; Wink, D.A.; Fukuto, J.M.; Jackson, A.A.; van Goor, H.; et al. The Reactive Species Interactome: Evolutionary Emergence, Biological Significance, and Opportunities for Redox Metabolomics and Personalized Medicine. Antioxid. Redox Signal. 2017, 27, 684–712. [Google Scholar] [CrossRef] [PubMed]
- Turell, L.; Radi, R.; Alvarez, B. The thiol pool in human plasma: The central contribution of albumin to redox processes. Free Radic. Biol. Med. 2013, 65, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Koning, A.M.; Meijers, W.C.; Pasch, A.; Leuvenink, H.G.D.; Frenay, A.S.; Dekker, M.M.; Feelisch, M.; de Boer, R.A.; van Goor, H. Serum free thiols in chronic heart failure. Pharmacol. Res. 2016, 111, 452–458. [Google Scholar] [CrossRef]
- Kundi, H.; Ates, I.; Kiziltunc, E.; Cetin, M.; Cicekcioglu, H.; Neselioglu, S.; Erel, O.; Ornek, E. A novel oxidative stress marker in acute myocardial infarction; thiol/disulphide homeostasis. Am. J. Emerg. Med. 2015, 33, 1567–1571. [Google Scholar] [CrossRef]
- Qian, J.; Fang, J.; Zhu, Q.; Ma, S.; Wang, W.; Zheng, Y.; Hao, G.; Deng, B.; Zhao, X.; Ding, F. Serum protein thiol levels in patients with hospital-acquired acute kidney injury. Kidney Blood Press. Res. 2015, 40, 623–629. [Google Scholar] [CrossRef]
- Giustarini, D.; Lorenzini, S.; Rossi, R.; Chindamo, D.; Di Simplicio, P.; Marcolong, R. Altered thiol pattern in plasma of subjects affected by rheumatoid arthritis. Clin. Exp. Rheumatol. 2005, 23, 205–212. [Google Scholar] [PubMed]
- Go, Y.M.; Jones, D.P. Cysteine/cystine redox signaling in cardiovascular disease. Free Radic. Biol. Med. 2011, 50, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Aging-related changes in the thiol/disulfide redox state: Implications for the use of thiol antioxidants. Exp. Gerontol. 2002, 37, 1333–1345. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; von Martels, J.Z.H.; Bulthuis, M.L.C.; van Londen, M.; Faber, K.N.; Dijkstra, G.; van Goor, H. Crohn’s Disease in Clinical Remission Is Marked by Systemic Oxidative Stress. Front. Physiol. 2019, 10, 499. [Google Scholar] [CrossRef] [PubMed]
- Kruidenier, L.; Verspaget, H.W. Review article: Oxidative stress as a pathogenic factor in inflammatory bowel disease—Radicals or ridiculous? Aliment. Pharmacol. Ther. 2002, 16, 1997–2015. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Grácio, D.; Teixeira, J.P.; Magro, F. Oxidative Stress and DNA Damage: Implications in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 2403–2417. [Google Scholar] [CrossRef] [PubMed]
- Lennard-Jones, J.E. Classification of inflammatory bowel disease. Scand. J. Gastroenterol. 1989, 170, 2–6. [Google Scholar] [CrossRef]
- Harvey, R.F.; Bradshaw, J.M. A simple index of Crohn’s-disease activity. Lancet 1980, 1, 514. [Google Scholar] [CrossRef]
- Walmsley, R.S.; Ayres, R.C.; Pounder, R.E.; Allan, R.N. A simple clinical colitis activity index. Gut 1980, 43, 29–32. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., III; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Schroeder, K.W.; Tremaine, W.J.; Ilstrup, D.M. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N. Engl. J. Med. 1987, 317, 1625–1629. [Google Scholar] [CrossRef] [PubMed]
- Daperno, M.; D’Haens, G.; Van Assche, G.; Baert, F.; Bulois, P.; Maunoury, V.; Sostegni, R.; Rocca, R.; Pera, A.; Gevers, A.; et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: The SES-CD. Gastrointest. Endosc. 2004, 60, 505–512. [Google Scholar] [CrossRef]
- Schoepfer, A.M.; Beglinger, C.; Straumann, A.; Trummler, M.; Vavricka, S.R.; Bruegger, L.E.; Seibold, F. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn’s disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am. J. Gastroenterol. 2010, 105, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Hu, M.L.; Louie, S.; Cross, C.E.; Motchnik, P.; Halliwell, B. Antioxidant protection against hypochlorous acid in human plasma. J. Lab. Clin. Med. 1993, 121, 257–262. [Google Scholar] [PubMed]
- D’Agostino, R.B.; Pearson, E.S. Testing for departure from normality. Empirical results for the distributions of b2 and √b1. Biometrika 1973, 60, 613–622. [Google Scholar]
- Luque-Fernandez, M.A.; Redondo-Sanchez, D.; Maringe, C. Cross-Validated Area under the Curve. GitHub Repository. Available online: https://github.com/migariane/cvauroc (accessed on 11 March 2019).
- Koutroubakis, I.E.; Malliaraki, N.; Dimoulios, P.D.; Karmiris, K.; Castanas, E.; Kouroumalis, E.A. Decreased total and corrected antioxidant capacity in patients with inflammatory bowel disease. Dig. Dis. Sci. 2004, 49, 1433–1437. [Google Scholar] [CrossRef]
- Maor, I.; Rainis, T.; Lanir, A.; Lavy, A. Oxidative stress, inflammation and neutrophil superoxide release in patients with Crohn’s disease: Distinction between active and non-active disease. Dig. Dis. Sci. 2008, 53, 2208–2214. [Google Scholar] [CrossRef]
- Akman, T.; Akarsu, M.; Akpinar, H.; Resmi, H.; Taylan, E. Erythrocyte deformability and oxidative stress in inflammatory bowel disease. Dig. Dis. Sci. 2012, 57, 458–464. [Google Scholar] [CrossRef]
- Krzystek-Korpacka, M.; Neubauer, K.; Berdowska, I.; Zielinski, B.; Paradowski, L.; Gamian, A. Impaired erythrocyte antioxidant defense in active inflammatory bowel disease: Impact of anemia and treatment. Inflamm. Bowel Dis. 2010, 16, 1467–1475. [Google Scholar] [CrossRef]
- Yuksel, M.; Ates, I.; Kaplan, M.; Arikan, M.F.; Ozin, Y.O.; Kilic, Z.M.Y.; Topcuoglu, C.; Kayacetin, E. Is Oxidative Stress Associated with Activation and Pathogenesis of Inflammatory Bowel Disease? J. Med. Biochem. 2017, 36, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Krzystek-Korpacka, M.; Neubauer, K.; Matusiewicz, M. Platelet-derived growth factor-BB reflects clinical, inflammatory and angiogenic disease activity and oxidative stress in inflammatory bowel disease. Clin. Biochem. 2009, 42, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Hortin, G.L.; Sviridov, D.; Anderson, N.L. High-abundance polypeptides of the human plasma proteome comprising the top 4 logs of polypeptide abundance. Clin. Chem. 2008, 54, 1608–1616. [Google Scholar] [CrossRef] [PubMed]
- Anraku, M.; Chuang, V.T.; Maruyama, T.; Otagiri, M. Redox properties of serum albumin. Biochim. Biophys. Acta 2013, 1830, 5465–5472. [Google Scholar] [CrossRef] [PubMed]
- D’Incà, R.; Cardin, R.; Benazzato, L.; Angriman, L.; Martines, D.; Sturniolo, G.C. Oxidative DNA damage in the mucosa of ulcerative colitis increases with disease duration and dysplasia. Inflamm. Bowel Dis. 2004, 10, 23–27. [Google Scholar] [CrossRef]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxid. Med. Cell. Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef]
- Langholz, E. Current trends in inflammatory bowel disease: The natural history. Ther. Adv. Gastroenterol. 2010, 3, 77–86. [Google Scholar] [CrossRef]
- Voudoukis, E.; Karmiris, K.; Koutroubakis, I.E. Multipotent role of platelets in inflammatory bowel diseases: A clinical approach. World J. Gastroenterol. 2014, 20, 3180–3190. [Google Scholar] [CrossRef]
- Minderhoud, I.M.; Steyerberg, E.W.; van Bodegraven, A.A.; van der Woude, C.J.; Hommes, D.W.; Dijkstra, G.; Fidder, H.H.; Schwartz, M.P.; Oldenburg, B. Predicting Endoscopic Disease Activity in Crohn’s Disease: A New and Validated Noninvasive Disease Activity Index (The Utrecht Activity Index). Inflamm. Bowel Dis. 2015, 21, 2453–2459. [Google Scholar]
- Vaghari-Tabari, M.; Moein, S.; Qujeq, D.; Kashifard, M.; Hajian-Tilaki, K. Positive Correlation of Fecal Calprotectin with Serum Antioxidant Enzymes in Patients with Inflammatory Bowel Disease: Accidental Numerical Correlation or a New Finding? Am. J. Med. Sci. 2018, 355, 449–455. [Google Scholar] [CrossRef]
- Bøyum, A.; Skrede, K.K.; Myhre, O.; Tennfjord, V.A.; Neurauter, C.G.; Tolleshaug, H.; Knudsen, E.; Opstad, P.K.; Bjøras, M.; Benestad, H.B. Calprotectin (S100A8/S100A9) and myeloperoxidase: Co-regulators of formation of reactive oxygen species. Toxins (Basel) 2010, 2, 95–115. [Google Scholar] [CrossRef] [PubMed]
- Foell, D.; Wittkowski, H.; Roth, J. Monitoring disease activity by stool analyses: From occult blood to molecular markers of intestinal inflammation and damage. Gut 2009, 58, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F.; Travis, S.P. Mucosal healing in inflammatory bowel disease: A systemic review. Gut 2012, 61, 1619–1635. [Google Scholar] [CrossRef] [PubMed]
- Walsh, A.; Palmer, R.; Travis, S. Mucosal healing as a target of therapy for colonic inflammatory bowel disease and methods to score disease activity. Gastrointest. Endosc. Clin. N. Am. 2014, 24, 367–378. [Google Scholar] [CrossRef] [PubMed]


| Variables | HC | IBD | P-Value |
|---|---|---|---|
| n = 50 | n = 78 | ||
| Serum free thiols per gram of albumin (µmol/g) | 21.1 ± 1.9 | 18.5 ± 3.4 | <0.001 |
| Age (years) | 50.7 ± 8.5 | 43.3 ± 8.5 | <0.01 |
| Female, n (%) | 23 (46.0) | 36 (46.2) | 0.99 |
| BMI (kg/m2) | 26.9 ± 3.1 | 24.8 ± 4.9 | <0.01 |
| Current smoking, n (%) | 13 (26.0) | 13 (18.8) | 0.35 |
| Laboratory Measurements | |||
| Hemoglobin (g/dL) | 14.8 ± 1.3 | 12.3 ± 1.8 | <0.001 |
| CRP (mg/L) * | 1.0 (0.6–1.5) | 4.1 (1.2–10.3) | <0.001 |
| WBC (×109/L) * | 6.7 (5.4–7.8) | 7.6 (6.0–10.3) | <0.01 |
| Platelets (×109/L) * | 244 (207–296) | 308 (259–376) | <0.001 |
| Albumin (g/L) | 45.6 ± 2.3 | 40.1 ± 3.9 | <0.001 |
| eGFR (mL/min * 1.73m2) | 87.7 ± 14.5 | 102.4 ± 26.4 | <0.01 |
| Creatinine (µmol/L) | 81.5 ± 15.9 | 73.1 ± 21.1 | <0.05 |
| Variables | Total IBD Cohort | Below-Average Thiols | Above-Average Thiols | P-value |
|---|---|---|---|---|
| n = 78 | n = 40 | n = 38 | ||
| Serum free thiols per gram of albumin (µmol/g) | 18.5 ± 3.4 | 15.9 ± 2.1 | 21.1 ± 2.2 | <0.001 |
| Age (years) | 43.3 ± 8.5 | 44.4 ± 16.7 | 42.1 ± 13.8 | 0.51 |
| Female, n (%) | 36 (46.2) | 17 (42.5) | 19 (50.0) | 0.51 |
| BMI (kg/m2) | 24.8 ± 4.9 | 24.4 ± 4.9 | 25.2 ± 4.9 | 0.49 |
| Current smoking, n (%) | 13 (18.8) | 7 (21.1) | 6 (16.7) | 0.63 |
| Prior surgery, n (%) | 21 (26.9) | 7 (17.5) | 14 (36.8) | 0.06 |
| Disease duration (years)* | 7.0 (3.0–12.3) | 6.0 (2.0–11.0) | 9.0 (3.8–13.0) | <0.05 |
| Prior anti-TNF, n (%) | 67 (85.6) | 32 (80.0) | 35 (92.1) | 0.28 |
| HBI/SCCAI* | 6 (5–10) | 6 (4.8–10.0) | 6.5 (4.8–10.3) | 0.93 |
| Disease Location (CD), n (%) | 0.05 | |||
| n = 31 | n = 12 | n = 19 | ||
| L1 (ileal) | 6 (19.4) | - | 6 (31.6) | |
| L2 (colonic) | 4 (12.9) | 3 (25.0) | 1 (5.3) | |
| L3 (ileocolonic) | 21 (67.7) | 9 (75.0) | 12 (63.2) | |
| Disease Extent (UC), n (%) | 0.54 | |||
| n = 47 | n = 28 | n = 19 | ||
| E1 (proctitis) | - | - | - | |
| E2 (left-sided colitis) | 15 (31.9) | 10 (35.7) | 5 (26.3) | |
| E3 (pancolitis) | 32 (68.1) | 18 (64.3) | 14 (73.7) | |
| Age at Diagnosis (Montreal A) | 0.32 | |||
| A1 (< 16 years) | 10 (12.8) | 5 (12.5) | 5 (13.2) | |
| A2 (17 – 40 years) | 50 (64.1) | 23 (57.5) | 27 (71.1) | |
| A3 (> 40 years) | 18 (23.1) | 12 (30.0) | 6 (15.8) | |
| Medication, n (%) | 0.28 | |||
| None | 34 (43.6) | 20 (50.0) | 14 (36.8) | |
| Thiopurines | 19 (24.4) | 7 (17.5) | 12 (31.6) | |
| Mesalamine | 18 (23.1) | 8 (20.0) | 10 (26.3) | |
| Combination | 7 (9.0) | 5 (12.5) | 2 (5.3) | |
| Laboratory Measurements | ||||
| Hemoglobin (g/dL) | 12.3 ± 1.8 | 12.1 ± 1.6 | 12.4 ± 1.9 | 0.32 |
| CRP (mg/L)* | 4.1 (1.2–10.3) | 4.1 (1.0–12.5) | 3.9 (1.2–9.2) | 0.77 |
| ESR (mm/h)* | 17.0 (6.0–41.3) | 22.5 (7.0–40.1) | 13.5 (4.0–44.3) | 0.42 |
| WBC (×109/L)* | 7.6 (6.0–10.3) | 8.7 (6.5–12.5) | 6.5 (5.3–9.2) | <0.01 |
| Platelets (×109/L)* | 308 (259–376) | 339 (283–402) | 290 (236–331) | <0.05 |
| Albumin (g/L) | 40.1 ± 3.9 | 40.6 ± 3.4 | 39.6 ± 4.3 | 0.270 |
| eGFR (mL/min/1.73 m2) | 102.4 ± 26.4 | 95.8 ± 22.9 | 109.0 ± 28.4 | <0.05 |
| Creatinine (µmol/L) | 73.1 ± 21.1 | 77.1 ± 24.1 | 69.0 ± 16.7 | 0.091 |
| Fecal calprotectin (µg/g)*,† | 1220 (610–1885) | 1465 (929–2420) | 600 (255–1220) | <0.01 |
| Serum Free Thiols/Gram of Albumin | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| Variables | B Coefficient # | P-Value | B Coefficient # | P-Value |
| Age | −0.237 | 0.04† | −0.500 | <0.01† |
| Female sex | 0.093 | 0.42 | ||
| Current smoker | −0.054 | 0.66 | ||
| BMI | −0.042 | 0.73 | ||
| Disease duration * | 0.288 | <0.05 † | 0.364 | <0.05† |
| Prior anti-TNF | 0.180 | 0.11 | ||
| HBI/SCCAI * | −0.177 | 0.19 | ||
| Medication | ||||
| Thiopurines | 0.070 | 0.55 | ||
| Mesalamine | 0.119 | 0.30 | ||
| Combination | −0.105 | 0.36 | ||
| Laboratory Measurements | ||||
| Hemoglobin | 0.102 | 0.37 | ||
| CRP * | −0.007 | 0.95 | ||
| ESR * | −0.128 | 0.27 | ||
| WBC * | −0.338 | <0.01 † | ||
| Platelets * | −0.223 | 0.05 † | −0.278 | <0.05† |
| Albumin | −0.127 | 0.27 | ||
| eGFR | 0.370 | <0.01 † | ||
| Creatinine | −0.235 | 0.04 † | ||
| Fecal calprotectin * | −0.422 | 0.01 † | −0.345 | <0.05† |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourgonje, A.R.; Gabriëls, R.Y.; de Borst, M.H.; Bulthuis, M.L.C.; Faber, K.N.; van Goor, H.; Dijkstra, G. Serum Free Thiols Are Superior to Fecal Calprotectin in Reflecting Endoscopic Disease Activity in Inflammatory Bowel Disease. Antioxidants 2019, 8, 351. https://doi.org/10.3390/antiox8090351
Bourgonje AR, Gabriëls RY, de Borst MH, Bulthuis MLC, Faber KN, van Goor H, Dijkstra G. Serum Free Thiols Are Superior to Fecal Calprotectin in Reflecting Endoscopic Disease Activity in Inflammatory Bowel Disease. Antioxidants. 2019; 8(9):351. https://doi.org/10.3390/antiox8090351
Chicago/Turabian StyleBourgonje, Arno R., Ruben Y. Gabriëls, Martin H. de Borst, Marian L. C. Bulthuis, Klaas Nico Faber, Harry van Goor, and Gerard Dijkstra. 2019. "Serum Free Thiols Are Superior to Fecal Calprotectin in Reflecting Endoscopic Disease Activity in Inflammatory Bowel Disease" Antioxidants 8, no. 9: 351. https://doi.org/10.3390/antiox8090351
APA StyleBourgonje, A. R., Gabriëls, R. Y., de Borst, M. H., Bulthuis, M. L. C., Faber, K. N., van Goor, H., & Dijkstra, G. (2019). Serum Free Thiols Are Superior to Fecal Calprotectin in Reflecting Endoscopic Disease Activity in Inflammatory Bowel Disease. Antioxidants, 8(9), 351. https://doi.org/10.3390/antiox8090351






