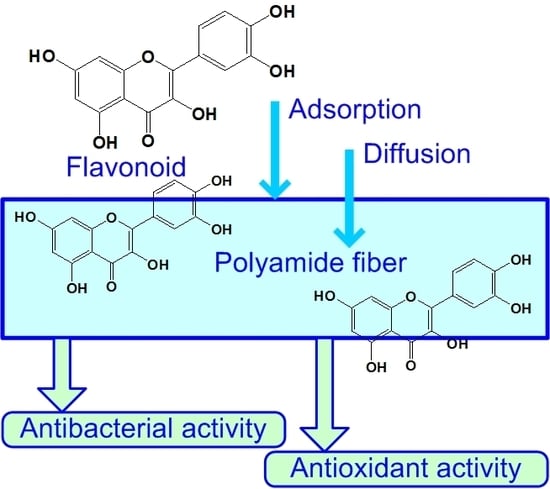
Application of Natural Flavonoids to Impart Antioxidant and Antibacterial Activities to Polyamide Fiber for Health Care Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Flavonoids Adsorption and Polyamide Fabric Treatment
2.3. Measurements
2.3.1. Uptake of Flavonoids
2.3.2. Zeta Potential
2.3.3. Antioxidant Activity
2.3.4. Antibacterial Activity
2.3.5. Durability of Antioxidant and Antibacterial Effects
3. Results and Discussion
3.1. Adsorption Characteristics of Flavonoids
3.1.1. pH Dependence of Flavonoids Adsorption
3.1.2. Adsorption Kinetics of Flavonoids
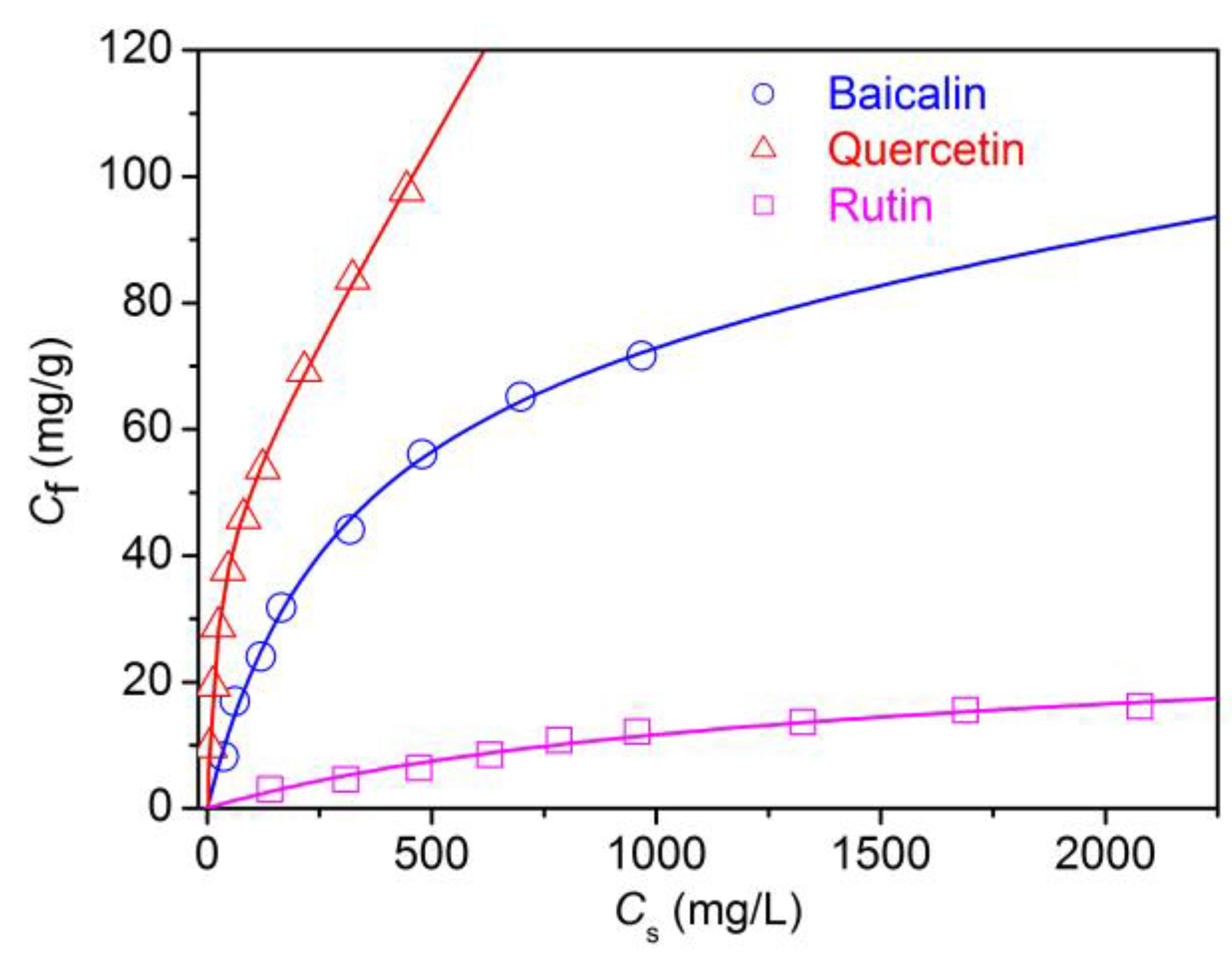
3.1.3. Adsorption Isotherms of Flavonoids
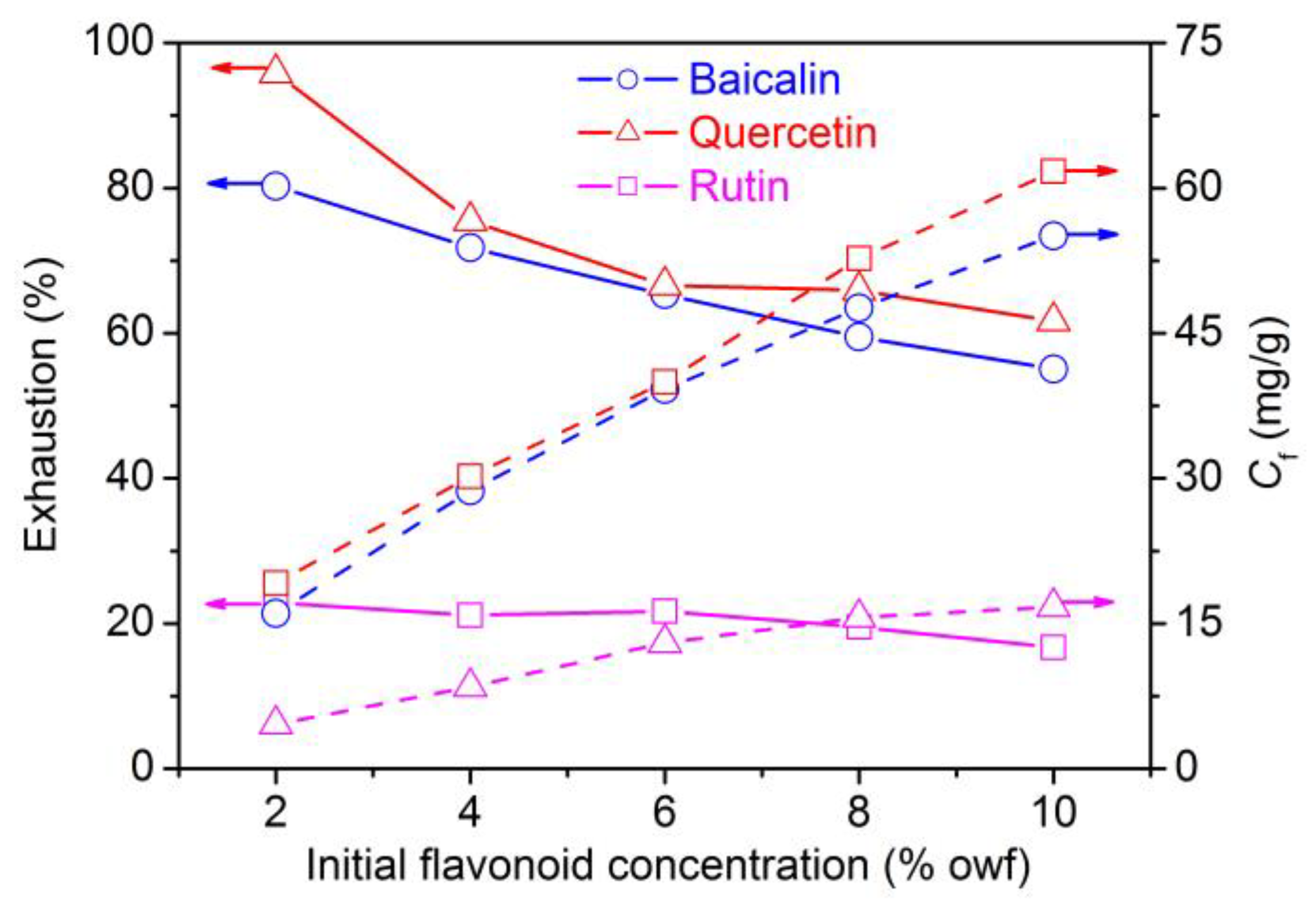
3.1.4. Initial Concentration Dependence of Flavonoids Adsorption
3.2. Antioxidant and Antibacterial Properties of Flavonoids Treated Polyamide Fiber
3.2.1. Antioxidant Property
3.2.2. Antibacterial Property
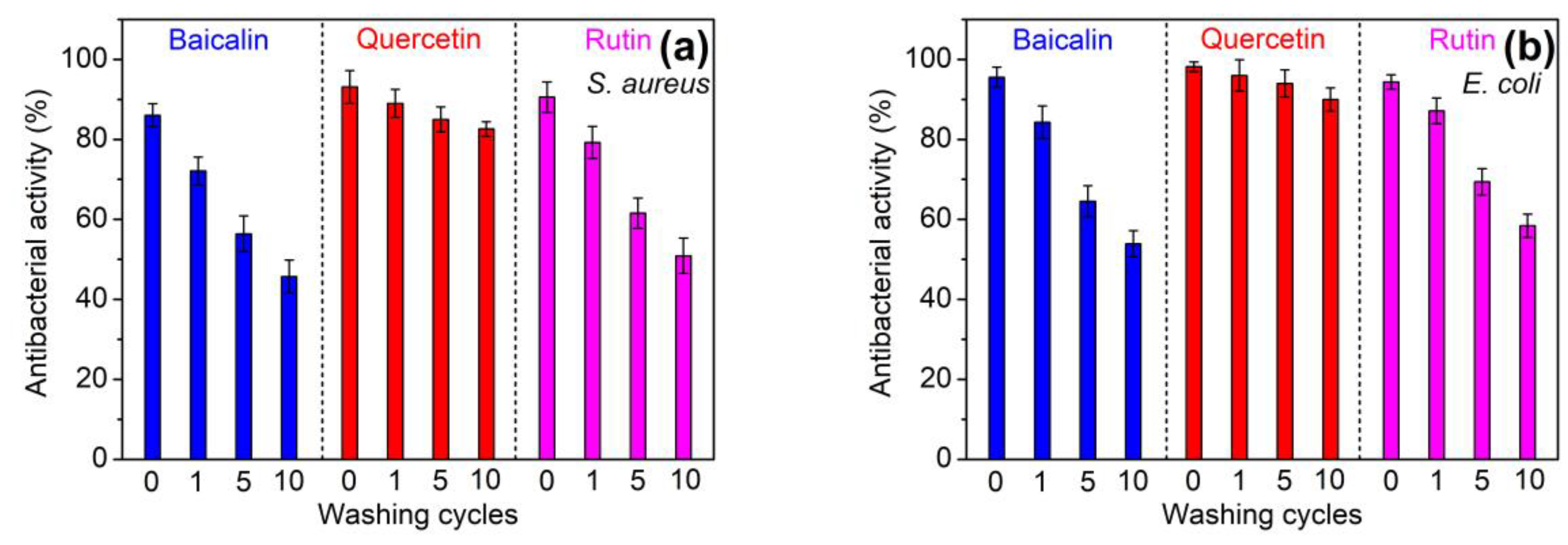
3.2.3. Laundering Durability of Antioxidant and Antibacterial Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shahid, M.; Mohammad, F. Green chemistry approaches to develop antimicrobial textiles based on sustainable biopolymers—A review. Ind. Eng. Chem. Res. 2013, 52, 5245–5260. [Google Scholar] [CrossRef]
- Alonso, C.; Martí, M.; Martínez, V.; Rubio, L.; Parra, J.L.; Coderch, L. Antioxidant cosmeto-textiles: Skin assessment. Eur. J. Pharm. Biopharm. 2013, 84, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, G.; Nichifor, M.; Mihai, D.; Oproiu, L.C. Bioactive cotton fabrics containing chitosan and biologically active substances extracted from plants. Mater. Sci. Eng. C 2013, 33, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Yi, E.; Hong, J.Y.; Yoo, E.S. A novel bioactive fabric dyed with unripe Citrus grandis Osbeck extract part 2: Effects of the Citrus extract and dyed fabric on skin irritancy and atopic dermatitis. Text. Res. J. 2010, 80, 2124–2131. [Google Scholar] [CrossRef]
- Eltahir, Y.A.; Saeed, H.A.M.; Xia, Y.; Yong, H.; Wang, Y. Mechanical properties, moisture absorption, and dyeability of polyamide 5,6 fibers. J. Text. Inst. 2016, 107, 208–214. [Google Scholar] [CrossRef]
- Singh, M.K.; Varun, V.K.; Behera, B.K. Cosmetotextiles: State of art. Fibers Text. East. Eur. 2011, 19, 27–33. [Google Scholar] [CrossRef]
- Erem, A.D.; Ozcan, G.; Skrifvars, M.; Cakmak, M. In vitro assesment of antimicrobial activity and characteristics of polyamide 6/silver nanocomposite fibers. Fibers Polym. 2013, 14, 1415–1421. [Google Scholar] [CrossRef]
- Tang, L.; Wang, D.; Xu, Q.; Wang, C.; Wang, H.; Huang, Q. Preparation and characterization of antibacterial nylon 6 fiber. Mater. Sci. Forum 2017, 898, 2254–2262. [Google Scholar] [CrossRef]
- Giménez-Martín, E.; López-Andrade, M.; Moleón-Baca, J.A.; López, M.A.; Ontiveros-Ortega, A. Polyamide fibers covered with chlorhexidine: Thermodynamic aspects. J. Surf. Eng. Mater. Adv. Technol. 2015, 5, 190–206. [Google Scholar] [CrossRef]
- Kim, H.-S.; Hwang, J.-Y.; Lim, S.-H.; Lim, J.-N.; Son, Y.-A. Preparation, physical characteristics and antibacterial finishing of PCM/nylon fibers having sheath/core structure. Text. Color. Finish. 2014, 26, 311–321. [Google Scholar] [CrossRef]
- Shi, Z. Grafting chitosan oxidized by potassium persulfate onto nylon 6 fiber, and characterizing the antibacterial property of the graft. J. Polym. Res. 2014, 21, 534. [Google Scholar] [CrossRef]
- Montazer, M.; Shamei, A.; Alimohammadi, F. Stabilized nanosilver loaded nylon knitted fabric using BTCA without yellowing. Prog. Org. Coat. 2012, 74, 270–276. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, Q.; Yang, C.; Pang, L.; Wang, H.; Li, R.; Xing, Z.; Hu, J.; Wu, G. Preparation of antimicrobial MnO4− doped nylon-66 fibers with excellent laundering durability. Appl. Surf. Sci. 2017, 422, 1067–1074. [Google Scholar] [CrossRef]
- Haji, A.; Shoushtari, A.M.; Mirafshar, M. Natural dyeing and antibacterial activity of atmospheric-plasma-treated nylon 6 fabric. Color. Technol. 2014, 130, 37–42. [Google Scholar] [CrossRef]
- Mirjalili, M.; Karimi, L. Antibacterial dyeing of polyamide using turmeric as a natural dye. Autex Res. J. 2013, 13, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Mirjalili, M.; Karimi, L. Extraction and characterization of natural dye from green walnut shells and its use in dyeing polyamide: Focus on antibacterial properties. J. Chem. 2013, 2013, 375352. [Google Scholar] [CrossRef]
- Coman, D.; Vrînceanu, N.; Oancea, S.; Rîmbu, C. Coloristic and antimicrobial behavior of polymeric substrates using bioactive substances. IOP Conf. Ser. Mater. Sci. Eng. 2016, 145, 032003. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; El-Zairy, W.M.; El-Zairy, M.R.; Ghazal, H.A. Enhancing the UV-protection and antibacterial properties of polyamide-6 fabric by natural dyeing. Text. Light Ind. Sci. Technol. 2013, 2, 36–41. [Google Scholar]
- Baaka, N.; Ksibi, I.E.; Mhenni, M.F. Optimization of the recovery of carotenoids from tomato processing wastes: Application on textile dyeing and assessment of its antioxidant activity. Nat. Prod. Res. 2017, 31, 196–203. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- China’s General Administration of Quality Supervision; Inspection and Quarantine and Standardization Administration of China. Textiles—Evaluation for Antibacterial Activity—Part 3: Shake Flask Method; GB/T 20944.3-2008; Beijing, China, 2008.
- Zhou, Y.; Yang, Z.-Y.; Tang, R.-C. Bioactive and UV protective silk materials containing baicalin—The multifunctional plant extract from Scutellaria baicalensis Georgi. Mater. Sci. Eng. C 2016, 67, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Pan, B.C.; Xiong, Y.; Su, Q.; Li, A.M.; Chen, J.L.; Zhang, Q.X. Role of amination of a polymeric adsorbent on phenol adsorption from aqueous solution. Chemosphere 2003, 51, 953–962. [Google Scholar] [CrossRef]
- Pan, B.C.; Zhang, X.; Zhang, W.M.; Zheng, J.Z.; Pan, B.J.; Chen, J.L.; Zhang, Q.X. Adsorption of phenolic compounds from aqueous solution onto a macroporous polymer and its aminated derivative: Isotherm analysis. J. Hazard. Mater. 2005, 121, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.-C.; Tang, H.; Yang, C. Adsorption isotherms and mordant dyeing properties of tea polyphenols on wool, silk, and nylon. Ind. Eng. Chem. Res. 2010, 49, 8894–8901. [Google Scholar] [CrossRef]
- Wang, H.; Chen, J.M.; Zhang, Q.M. Determination of the physical chemistry constants of baicalin. J. Shenyang Pharm. Univ. 2000, 17, 105–106. (In Chinese) [Google Scholar]
- Liang, R.; Han, R.-M.; Fu, L.-M.; Ai, X.-C.; Zhang, J.-P.; Skibsted, L.H. Baicalin in radical scavenging and its synergistic effect with β-carotene in antilipoxidation. J. Agric. Food Chem. 2009, 57, 7118–7124. [Google Scholar] [CrossRef]
- Ramešová, Š.; Sokolová, R.; Degano, I.; Bulíčková, J.; Žabka, J.; Gál, M. On the stability of the bioactive flavonoids quercetin and luteolin under oxygen-free conditions. Anal. Bioanal. Chem. 2012, 402, 975–982. [Google Scholar] [CrossRef]
- Musialik, M.; Kuzmicz, R.; Pawłowski, T.S.; Litwinienko, G. Acidity of hydroxyl groups: An overlooked influence on antiradical properties of flavonoids. J. Org. Chem. 2009, 74, 2699–2709. [Google Scholar] [CrossRef]
- Jovanovic, S.V.; Steenken, S.; Tosic, M.; Marjanovic, B.; Simic, M.G. Flavonoids as antioxidants. J. Am. Chem. Soc. 1994, 116, 4846–4851. [Google Scholar] [CrossRef]
- Sun, S.-S.; Tang, R.-C. Adsorption and UV protection properties of the extract from honeysuckle onto wool. Ind. Eng. Chem. Res. 2011, 50, 4217–4224. [Google Scholar] [CrossRef]
- Samanta, A.K.; Agarwal, P. Application of natural dyes on textiles. Indian J. Fiber Text. Res. 2009, 34, 384–399. [Google Scholar]
- Pietta, P.-G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Liu, I.X.; Durham, D.G.; Richards, R.M.E. Baicalin synergy with β-lactam antibiotics against methicillin-resistant Staphylococcus aureus and other β-lactam-resistant strains of S. Aureus. J. Pharm. Pharmacol. 2000, 52, 361–366. [Google Scholar] [CrossRef]
- Li, M.; Xu, Z. Quercetin in a lotus leaves extract may be responsible for antibacterial activity. Arch. Pharm. Res. 2008, 31, 640–644. [Google Scholar] [CrossRef]
- Vaquero, M.J.R.; Alberto, M.R.; de Nadra, M.C.M. Antibacterial effect of phenolic compounds from different wines. Food Control 2007, 18, 93–101. [Google Scholar] [CrossRef]
- Baliarsingh, S.; Panda, A.K.; Jena, J.; Das, T.; Das, N.B. Exploring sustainable technique on natural dye extraction from native plants for textile: Identification of colorants, colorimetric analysis of dyed yarns and their antimicrobial evaluation. J. Clean. Prod. 2012, 37, 257–264. [Google Scholar] [CrossRef]
- Ghaheh, F.S.; Mortazavi, S.M.; Alihosseini, F.; Fassihi, A.; Nateri, A.S.; Abedi, D. Assessment of antibacterial activity of wool fabrics dyed with natural dyes. J. Clean. Prod. 2014, 72, 139–145. [Google Scholar] [CrossRef]
- Khan, M.I.; Ahmad, A.; Khan, S.A.; Yusuf, M.; Shahid, M.; Manzoor, N.; Mohammad, F. Assessment of antimicrobial activity of catechu and its dyed substrate. J. Clean. Prod. 2011, 19, 1385–1394. [Google Scholar] [CrossRef]







| Flavonoid | ri (mg/[g·min]) | t1/2 (min) | k (×10−2g/[mg·min]) | Ce (mg/g) | R2 |
|---|---|---|---|---|---|
| Baicalin | 3.43 | 4.826 | 1.251 | 16.56 | 0.9995 |
| Quercetin | 5.03 | 3.954 | 1.272 | 19.88 | 0.9998 |
| Rutin | 0.34 | 15.100 | 1.303 | 5.08 | 0.9987 |
| Flavonoid | ND (%) | ||
|---|---|---|---|
| Langmuir | Freundlich | Langmuir–Nernst | |
| Baicalin | 4.59 | 12.81 | 5.04 |
| Quercetin | 15.76 | 7.55 | 3.13 |
| Rutin | 7.13 | 9.07 | 6.64 |
| Flavonoid | S (mg/g) | KL (10−3 L/mg) | KP (10−3 L/mg) |
|---|---|---|---|
| Baicalin | 82.43 | 3.39 | 9.23 |
| Quercetin | 46.48 | 46.53 | 121.67 |
| Rutin | 22.10 | 0.91 | 1.13 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-D.; Guan, J.-P.; Tang, R.-C.; Qiao, Y.-F. Application of Natural Flavonoids to Impart Antioxidant and Antibacterial Activities to Polyamide Fiber for Health Care Applications. Antioxidants 2019, 8, 301. https://doi.org/10.3390/antiox8080301
Li Y-D, Guan J-P, Tang R-C, Qiao Y-F. Application of Natural Flavonoids to Impart Antioxidant and Antibacterial Activities to Polyamide Fiber for Health Care Applications. Antioxidants. 2019; 8(8):301. https://doi.org/10.3390/antiox8080301
Chicago/Turabian StyleLi, Ya-Dong, Jin-Ping Guan, Ren-Cheng Tang, and Yi-Fan Qiao. 2019. "Application of Natural Flavonoids to Impart Antioxidant and Antibacterial Activities to Polyamide Fiber for Health Care Applications" Antioxidants 8, no. 8: 301. https://doi.org/10.3390/antiox8080301