Strategies to Improve Resveratrol Systemic and Topical Bioavailability: An Update
Abstract
:1. Introduction
2. Drug Delivery Systems
2.1. Systemic Delivery
2.1.1. RSV Oral Delivery Systems
2.1.2. RSV Parenteral Delivery Systems
2.2. Topical Delivery
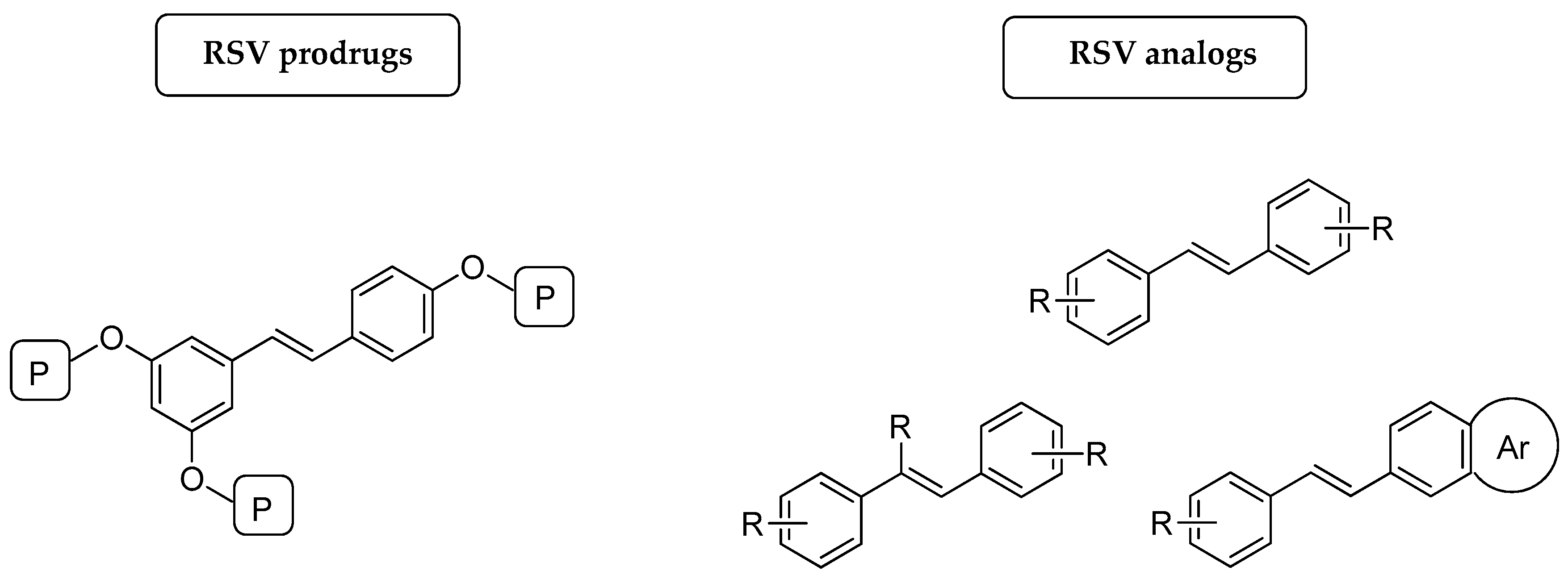
3. Prodrugs
3.1. Prodrugs for Systemic Delivery of RSV
3.1.1. Prodrugs to Improve the Bioavailability of RSV
3.1.2. Prodrugs to Improve the Pharmacological Activity of RSV
3.2. Prodrugs for Dermal Delivery of RSV
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shen, T.; Xie, C.F.; Wang, X.N.; Lou, H.X. Stilbenoids. In Natural Products; Ramawat, K., Mérillon, J.M., Eds.; Springer: Berlin, Germany, 2013. [Google Scholar]
- Gabaston, J.; Cantos-Villar, E.; Biais, B.; Waffo-Teguo, P.; Renouf, E.; Corio-Costet, M.F.; Richard, T.; Merillon, J.M. Stilbenes from Vitis vinifera L. Waste: A Sustainable Tool for Controlling Plasmopara Viticola. J. Agric. Food Chem. 2017, 65, 2711–2718. [Google Scholar] [CrossRef] [PubMed]
- Adrian, M.; Jeandet, P.; Veneau, J.; Weston, L.A.; Bessis, R. Biological Activity of Resveratrol, a Stilbenic Compound from Grapevines, Against Botrytis cinerea, the Causal Agent for Gray Mold. J. Chem. Ecol. 1997, 23, 1689–1702. [Google Scholar] [CrossRef]
- Sobolev, V.S.; Khan, S.I.; Tabanca, N.; Wedge, D.E.; Manly, S.P.; Cutler, S.J.; Coy, M.R.; Becnel, J.J.; Neff, S.A.; Gloer, J.B. Biological Activity of Peanut (Arachis hypogaea) Phytoalexins and Selected Natural and Synthetic Stilbenoids. J. Agric. Food Chem. 2011, 59, 1673–1682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Tuck, T.; Ji, X.; Zhou, X.; Kelly, G.; Cuerrier, A.; Zhang, J. Quality Assessment of Japanese Knotweed (Fallopia japonica) Grown on Prince Edward Island as a Source of Resveratrol. J. Agric. Food Chem. 2013, 61, 6383–6392. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.; Crozier, A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef] [PubMed]
- Stervbo, U.; Vang, O.; Bonnesen, C. A review of the content of the putative chemopreventive phytoalexin resveratrol in red wine. Food Chem. 2007, 101, 449–457. [Google Scholar] [CrossRef]
- Lamuela-Raventos, R.M.; Romero-Perez, A.I.; Waterhouse, A.L.; De la Torre-Boronat, M.C. Direct HPLC Analysis of cis- and trans-Resveratrol and Piceid Isomers in Spanish Red Vitis vinifera Wines. J. Agric. Food Chem. 1995, 43, 281–283. [Google Scholar] [CrossRef]
- Yang, I.; Kim, E.; Kang, J.; Han, H.; Sul, S.; Park, S.B.; Kim, S.K. Photochemical generation of a new, highly fluorescent compound from non-fluorescent resveratrol. Chem Commun. 2012, 48, 3839–3841. [Google Scholar] [CrossRef]
- Adrian, M.; Jeandet, P.; Douillet-Breuil, A.C.; Tesson, L.; Bessis, R. Stilbene Content of Mature Vitis vinifera Berries in Response to UV-C Elicitation. J. Agric. Food Chem. 2000, 48, 6103–6105. [Google Scholar] [CrossRef]
- Jeandet, P.; Delaunois, B.; Conreux, A.; Donnez, D.; Nuzzo, V.; Cordelier, S.; Clement, C.; Courot, E. Biosynthesis, metabolism, molecular engineering, and biological functions of stilbene phytoalexins in plants. Biofactors 2010, 36, 331–341. [Google Scholar] [CrossRef]
- Langcake, P.; Pryce, R.J. The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol. Plant Pathol. 1976, 9, 77–86. [Google Scholar] [CrossRef]
- Kuć, J.; Rush, J.S. Phytoalexins. Arch. Biochem. Biophys. 1985, 236, 455–472. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Suleria, H.A.R.; Ahmad, B.; Peters, D.G.; Mubarak, M.S. A comprehensive review of the health perspectives of resveratrol. Food Funct. 2017, 8, 4284–4305. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Daiber, A.; Forstermann, U.; Li, H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, K.; Cheng, L.; Yan, B.; Qian, W.; Cao, J.; Li, J.; Wu, E.; Ma, Q.; Yang, W. Resveratrol and cancer treatment: Updates. Ann. N. Y. Acad. Sci. 2017, 1403, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhou, J.; Jiang, B.; Miao, M. Resveratrol and inflammatory bowel disease. Ann. N. Y. Acad. Sci. 2017, 1403, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.Y.; Tain, Y.L.; Yu, H.R.; Huang, L.T. The Effects of Resveratrol in the Treatment of Metabolic Syndrome. Int. J. Mol. Sci. 2019, 20, 535. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H. Metabolic benefits of inhibiting cAMP-PDEs with resveratrol. Adipocyte 2012, 1, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Biasutto, L.; Mattarei, A.; Zoratti, M. Resveratrol and health: The starting point. Chembiochem 2012, 13, 1256–1259. [Google Scholar] [CrossRef]
- Nguyen-Hai, N. Naturally Occurring NF-κB Inhibitors. Mini Rev. Med. Chem. 2006, 6, 945–951. [Google Scholar] [CrossRef]
- Gonzalez, R.; Ballester, I.; Lopez-Posadas, R.; Suarez, M.D.; Zarzuelo, A.; Martinez-Augustin, O.; Sanchez de Medina, F. Effects of flavonoids and other polyphenols on inflammation. Crit. Rev. Food Sci. Nutr. 2011, 51, 331–362. [Google Scholar] [CrossRef] [PubMed]
- Sophie, C. Natural Products Triggering Biological Targets—A Review of the Anti-Inflammatory Phytochemicals Targeting the Arachidonic Acid Pathway in Allergy Asthma and Rheumatoid Arthritis. Curr. Drug Targets 2011, 12, 288–301. [Google Scholar] [CrossRef]
- Khanna, D.; Sethi, G.; Ahn, K.S.; Pandey, M.K.; Kunnumakkara, A.B.; Sung, B.; Aggarwal, A.; Aggarwal, B.B. Natural products as a gold mine for arthritis treatment. Curr. Opin. Pharmacol. 2007, 7, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Slusarz, A.; Shenouda, N.S.; Sakla, M.S.; Drenkhahn, S.K.; Narula, A.S.; MacDonald, R.S.; Besch-Williford, C.L.; Lubahn, D.B. Common botanical compounds inhibit the hedgehog signaling pathway in prostate cancer. Cancer Res. 2010, 70, 3382–3390. [Google Scholar] [CrossRef]
- Sara, K.D.; Glenn, A.J.; Anna, S.; Nicholas, J.E.S.; Dennis, B.L. Inhibition of Hedgehog/Gli Signaling by Botanicals: A Review of Compounds with Potential Hedgehog Pathway Inhibitory Activities. Curr. Cancer Drug Targets 2013, 13, 580–595. [Google Scholar] [CrossRef]
- Marie-Helene, T.; Francois, G.; Mario, D.; Marc, D. Targeting the Wingless Signaling Pathway with Natural Compounds as Chemopreventive or Chemotherapeutic Agents. Curr. Pharm. Biotechnol. 2012, 13, 245–254. [Google Scholar] [CrossRef]
- Hardie, D.G. Sensing of energy and nutrients by AMP-activated protein kinase. Am. J. Clin. Nutr. 2011, 93, S891–S896. [Google Scholar] [CrossRef]
- Biasutto, L.; Szabo, I.; Zoratti, M. Mitochondrial effects of plant-made compounds. Antioxid. Redox Signal. 2011, 15, 3039–3059. [Google Scholar] [CrossRef]
- Um, J.H.; Park, S.J.; Kang, H.; Yang, S.; Foretz, M.; McBurney, M.W.; Kim, M.K.; Viollet, B.; Chung, J.H. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes 2010, 59, 554–563. [Google Scholar] [CrossRef]
- Morris, B.J. Seven sirtuins for seven deadly diseases ofaging. Free Radic. Biol. Med. 2013, 56, 133–171. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.M.; Chien, A.; Jialal, I.; Devaraj, S. Resveratrol up-regulates SIRT1 and inhibits cellular oxidative stress in the diabetic milieu: Mechanistic insights. J. Nutr. Biochem. 2012, 23, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Zhang, Y.; Xia, J.; Liu, B.; Zhang, Q.; Liu, J.; Luo, L.; Peng, Z.; Song, Z.; Zhu, R. Resveratrol induces cell cycle arrest via a p53-independent pathway in A549 cells. Mol. Med. Rep. 2015, 11, 2459–2464. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.S.; Cantó, C. The molecular targets of resveratrol. Biochim. Biophys. Acta (BBA) Mol. Bas. Dis. 2015, 1852, 1114–1123. [Google Scholar] [CrossRef] [Green Version]
- Bradamante, S.; Barenghi, L.; Piccinini, F.; Bertelli, A.A.E.; De Jonge, R.; Beemster, P.; De Jong, J.W. Resveratrol provides late-phase cardioprotection by means of a nitric oxide and adenosine-mediated mechanism. Eur. J. Pharmacol. 2003, 465, 115–123. [Google Scholar] [CrossRef]
- Siriwardhana, N.; Kalupahana, N.S.; Cekanova, M.; LeMieux, M.; Greer, B.; Moustaid-Moussa, N. Modulation of adipose tissue inflammation by bioactive food compounds. J. Nutr. Biochem. 2013, 24, 613–623. [Google Scholar] [CrossRef]
- Leiherer, A.; Mündlein, A.; Drexel, H. Phytochemicals and their impact on adipose tissue inflammation and diabetes. Vasc. Pharmacol. 2013, 58, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Cherniack, E.P. Polyphenols: Planting the seeds of treatment for the metabolic syndrome. Nutrition 2011, 27, 617–623. [Google Scholar] [CrossRef]
- Rayalam, S.; Della-Fera, M.A.; Baile, C.A. Synergism between resveratrol and other phytochemicals: Implications for obesity and osteoporosis. Mol. Nutr. Food Res. 2011, 55, 1177–1185. [Google Scholar] [CrossRef]
- Baile, C.A.; Yang, J.Y.; Rayalam, S.; Hartzell, D.L.; Lai, C.Y.; Andersen, C.; Della-Fera, M.A. Effect of resveratrol on fat mobilization. Ann. N. Y. Acad. Sci. 2011, 1215, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Szkudelska, K.; Szkudelski, T. Resveratrol, obesity and diabetes. Eur. J. Pharmacol. 2010, 635, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gehm, B.D.; McAndrews, J.M.; Chien, P.Y.; Jameson, J.L. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc. Natl. Acad. Sci. USA 1997, 94, 14138–14143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowers, J.L.; Tyulmenkov, V.V.; Jernigan, S.C.; Klinge, C.M. Resveratrol Acts as a Mixed Agonist/Antagonist for Estrogen Receptors α and β*. Endocrinology 2000, 141, 3657–3667. [Google Scholar] [CrossRef]
- Yousef, M.; Vlachogiannis, I.A.; Tsiani, E. Effects of Resveratrol against Lung Cancer: In Vitro and In Vivo Studies. Nutrients 2017, 9, 1231. [Google Scholar] [CrossRef] [PubMed]
- Nie, P.; Hu, W.; Zhang, T.; Yang, Y.; Hou, B.; Zou, Z. Synergistic Induction of Erlotinib-Mediated Apoptosis by Resveratrol in Human Non-Small-Cell Lung Cancer Cells by Down-Regulating Survivin and Up-Regulating PUMA. Cell. Physiol. Biochem. 2015, 35, 2255–2271. [Google Scholar] [CrossRef]
- Sun, X.; Peng, B.; Yan, W. Measurement and correlation of solubility of trans-resveratrol in 11 solvents at T=(278.2, 288.2, 298.2, 308.2, and 318.2)K. J. Chem. Thermodyn. 2008, 40, 735–738. [Google Scholar] [CrossRef]
- Cadena, P.G.; Pereira, M.A.; Cordeiro, R.B.; Cavalcanti, I.M.; Barros Neto, B.; Pimentel Mdo, C.; Lima Filho, J.L.; Silva, V.L.; Santos-Magalhaes, N.S. Nanoencapsulation of quercetin and resveratrol into elastic liposomes. Biochim. Biophys. Acta 2013, 1828, 309–316. [Google Scholar] [CrossRef]
- Zupancic, S.; Lavric, Z.; Kristl, J. Stability and solubility of trans-resveratrol are strongly influenced by pH and temperature. Eur. J. Pharm. Biopharm. 2015, 93, 196–204. [Google Scholar] [CrossRef]
- Lopez-Nicolas, J.M.; Garcia-Carmona, F. Aggregation state and pKa values of (E)-resveratrol as determined by fluorescence spectroscopy and UV-visible absorption. J. Agric. Food Chem. 2008, 56, 7600–7605. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings1PII of original article: S0169-409X(96)00423-1. The article was originally published in Advanced Drug Delivery Reviews 23 (1997) 3–25.1. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, E.; Somoza, V. Metabolism and bioavailability of trans-resveratrol. Mol. Nutr. Food Res. 2005, 49, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Jancova, P.; Anzenbacher, P.; Anzenbacherova, E. Phase II drug metabolizing enzymes. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czechoslov. 2010, 154, 103–116. [Google Scholar] [CrossRef]
- Wang, P.; Sang, S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. Biofactors 2018, 44, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.R.; Lucio, M.; Lima, J.L.; Reis, S. Resveratrol in medicinal chemistry: A critical review of its pharmacokinetics, drug-delivery, and membrane interactions. Curr. Med. Chem. 2012, 19, 1663–1681. [Google Scholar] [CrossRef]
- Biasutto, L.; Mattarei, A.; Azzolini, M.; La Spina, M.; Sassi, N.; Romio, M.; Paradisi, C.; Zoratti, M. Resveratrol derivatives as a pharmacological tool. Ann. N. Y. Acad. Sci. 2017, 1403, 27–37. [Google Scholar] [CrossRef]
- Ferré-Filmon, K.; Delaude, L.; Demonceau, A.; Noels, A.F. Catalytic methods for the synthesis of stilbenes with an emphasis on their phytoalexins. Coord. Chem. Rev. 2004, 248, 2323–2336. [Google Scholar] [CrossRef]
- Giacomini, E.; Rupiani, S.; Guidotti, L.; Recanatini, M.; Roberti, M. The Use of Stilbene Scaffold in Medicinal Chemistry and Multi-Target Drug Design. Curr. Med. Chem. 2016, 23, 2439–2489. [Google Scholar] [CrossRef]
- Latruffe, N.; Vervandier-Fasseur, D. Strategic Syntheses of Vine and Wine Resveratrol Derivatives to Explore their Effects on Cell Functions and Dysfunctions. Diseases 2018, 6, 110. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, W.; Zhou, Z.; Deng, S.; Ma, X.; Ma, X.; Li, C.; Shu, X. Therapeutic Versatility of Resveratrol Derivatives. Nutrients 2017, 9, 1188. [Google Scholar] [CrossRef] [PubMed]
- Biasutto, L.; Zoratti, M. Prodrugs of quercetin and resveratrol: A strategy under development. Curr. Drug Metab. 2014, 15, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Chen, H.; Song, C.; Zeng, X.; Zheng, Q.; Zhang, Y.; Lei, X.; Zheng, X. Pharmacological activities and structure-modification of resveratrol analogues. Pharmazie 2015, 70, 765–771. [Google Scholar] [PubMed]
- Liu, Y.; Liu, Y.; Chen, H.; Yao, X.; Xiao, Y.; Zeng, X.; Zheng, Q.; Wei, Y.; Song, C.; Zhang, Y.; et al. Synthetic Resveratrol Derivatives and Their Biological Activities: A Review. Open J. Med. Chem. 2015, 5, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Sorrenti, V.; Raffaele, M.; Vanella, L.; Acquaviva, R.; Salerno, L.; Pittala, V.; Intagliata, S.; Di Giacomo, C. Protective Effects of Caffeic Acid Phenethyl Ester (CAPE) and Novel Cape Analogue as Inducers of Heme Oxygenase-1 in Streptozotocin-Induced Type 1 Diabetic Rats. Int. J. Mol. Sci 2019, 20, 2441. [Google Scholar] [CrossRef] [PubMed]
- Park, K. Controlled drug delivery systems: Past forward and future back. J. Control. Release 2014, 190, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
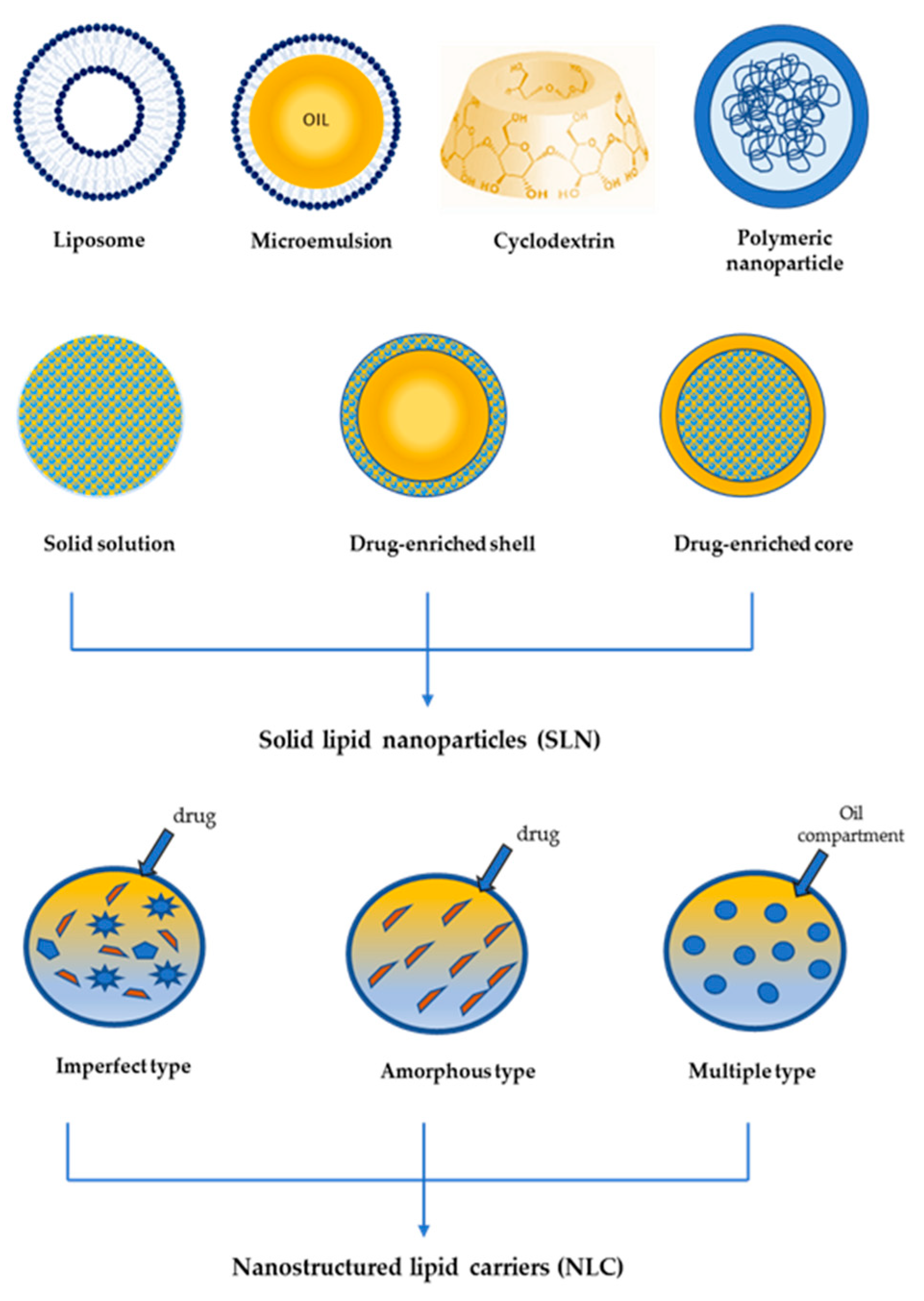
- Mishra, B.; Patel, B.B.; Tiwari, S. Colloidal nanocarriers: A review on formulation technology, types and applications toward targeted drug delivery. Nanomedicine 2010, 6, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Lai, F.; Offerta, A.; Sarpietro, M.G.; Micicchè, L.; Maccioni, A.M.; Valenti, D.; Fadda, A.M. From nanoemulsions to nanostructured lipid carriers: A relevant development in dermal delivery of drugs and cosmetics. J. Drug Deliv. Sci. Technol. 2016, 32, 100–112. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Zylberberg, C.; Matosevic, S. Pharmaceutical liposomal drug delivery: A review of new delivery systems and a look at the regulatory landscape. Drug Deliv. 2016, 23, 3319–3329. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.I.; Yeh, M.K. Clinical development of liposome-based drugs: Formulation, characterization, and therapeutic efficacy. Int. J. Nanomed. 2012, 7, 49–60. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Banik, B.L.; Fattahi, P.; Brown, J.L. Polymeric nanoparticles: The future of nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 271–299. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Coll. Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery–A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Montenegro, L. Lipid-Based Nanoparticles as Carriers for Dermal Delivery of Antioxidants. Curr. Drug Metab. 2017, 18, 469–480. [Google Scholar] [CrossRef]
- Montenegro, L. Nanocarriers for skin delivery of cosmetic antioxidants. J. Pharm. Pharmacogn. Res. 2014, 2, 20. [Google Scholar]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54, S131–S155. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Pharmaceutical Applications of Cyclodextrins. 1. Drug Solubilization and Stabilization. J. Pharm. Sci. 1996, 85, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Challa, R.; Ahuja, A.; Ali, J.; Khar, R.K. Cyclodextrins in drug delivery: An updated review. AAPS Pharm. Sci. Tech. 2005, 6, E329–E357. [Google Scholar] [CrossRef] [PubMed]
- Carrier, R.L.; Miller, L.A.; Ahmed, I. The utility of cyclodextrins for enhancing oral bioavailability. J. Control. Release 2007, 123, 78–99. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Pai, R.S. Optimized PLGA nanoparticle platform for orally dosed trans-resveratrol with enhanced bioavailability potential. Expert Opin. Drug Deliv. 2014, 11, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Siu, F.Y.; Ye, S.; Lin, H.; Li, S. Galactosylated PLGA nanoparticles for the oral delivery of resveratrol: Enhanced bioavailability and in vitro anti-inflammatory activity. Int. J. Nanomed. 2018, 13, 4133–4144. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.; Zhang, Y.; Wang, W.; Zhao, X.; Han, X.; Wang, K.; Ge, Y. Preparation and in vitro/in vivo evaluation of resveratrol-loaded carboxymethyl chitosan nanoparticles. Drug Deliv. 2016, 23, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, P.; Ko, Y.T. Improved oral delivery of resveratrol from N-trimethyl chitosan-g-palmitic acid surface-modified solid lipid nanoparticles. Coll. Surf. B Biointerfaces 2016, 139, 52–61. [Google Scholar] [CrossRef]
- Pandita, D. Solid lipid nanoparticles enhance oral bioavailability of resveratrol, a natural polyphenol. Food Res. Int. 2014, 62, 1165–1174. [Google Scholar] [CrossRef]
- Neves, A.R.; Lucio, M.; Martins, S.; Lima, J.L.; Reis, S. Novel resveratrol nanodelivery systems based on lipid nanoparticles to enhance its oral bioavailability. Int. J. Nanomed. 2013, 8, 177–187. [Google Scholar] [CrossRef] [Green Version]
- Santos, A.C.; Veiga, F.J.; Sequeira, J.A.D.; Fortuna, A.; Falcao, A.; Pereira, I.; Pattekari, P.; Fontes-Ribeiro, C.; Ribeiro, A.J. First-time oral administration of resveratrol-loaded layer-by-layer nanoparticles to rats—A pharmacokinetics study. Analyst 2019, 144, 2062–2079. [Google Scholar] [CrossRef]
- Signorelli, P.; Ghidoni, R. Resveratrol as an anticancer nutrient: Molecular basis, open questions and promises. J. Nutr. Biochem. 2005, 16, 449–466. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Shi, Y.; Han, Q.; Dai, G. Endothelial growth factor receptor-targeted and reactive oxygen species responsive lung cancer therapy by docetaxel and resveratrol encapsulated lipid-polymer hybrid nanoparticles. Biomed. Pharmacother. 2018, 105, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Lee, J.H.; Park, J.W.; Quach, C.H.T.; Moon, S.H.; Cho, Y.S.; Lee, K.H. Resveratrol-loaded polymeric nanoparticles suppress glucose metabolism and tumor growth in vitro and in vivo. Int. J. Pharm. 2015, 478, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Li, A.; Jia, Z.; Yuan, Y.; Dai, H.; Li, H. Transferrin modified PEG-PLA-resveratrol conjugates: In vitro and in vivo studies for glioma. Eur. J. Pharmacol. 2013, 718, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Figueiro, F.; Bernardi, A.; Frozza, R.L.; Terroso, T.; Zanotto-Filho, A.; Jandrey, E.H.; Moreira, J.C.; Salbego, C.G.; Edelweiss, M.I.; Pohlmann, A.R.; et al. Resveratrol-loaded lipid-core nanocapsules treatment reduces in vitro and in vivo glioma growth. J. Biomed. Nanotechnol. 2013, 9, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Kumari, P.; Lakhani, P.M.; Ghosh, B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur. J. Pharm. Sci. 2016, 83, 184–202. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhao, Z.; Chen, D.; Qiao, M.; Wan, F.; Cun, D.; Sun, Y.; Yang, M. Co-delivery of resveratrol and docetaxel via polymeric micelles to improve the treatment of drug-resistant tumors. Asian J. Pharm. Sci. 2019, 14, 78–85. [Google Scholar] [CrossRef]
- Hu, M.; Zhu, J.; Qiu, L. Polymer micelle-based combination therapy of paclitaxel and resveratrol with enhanced and selective antitumor activity. RSC Adv. 2014, 4, 64151–64161. [Google Scholar] [CrossRef]
- Kim, G.; Piao, C.; Oh, J.; Lee, M. Self-assembled polymeric micelles for combined delivery of anti-inflammatory gene and drug to the lungs by inhalation. Nanoscale 2018, 10, 8503–8514. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Rajendra Prasad, N.; Ganamani, A.; Balamurugan, E. Anticancer activity of resveratrol-loaded gelatin nanoparticles on NCI-H460 non-small cell lung cancer cells. Biomed. Prev. Nutr. 2013, 3, 64–73. [Google Scholar] [CrossRef]
- Trotta, V.; Pavan, B.; Ferraro, L.; Beggiato, S.; Traini, D.; Des Reis, L.G.; Scalia, S.; Dalpiaz, A. Brain targeting of resveratrol by nasal administration of chitosan-coated lipid microparticles. Eur. J. Pharm. Biopharm. 2018, 127, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Lin, H.S.; Ho, P.C.; Ng, K.Y. The impact of aqueous solubility and dose on the pharmacokinetic profiles of resveratrol. Pharm. Res. 2008, 25, 2593–2600. [Google Scholar] [CrossRef] [PubMed]
- Carlotti, M.E.; Sapino, S.; Ugazio, E.; Gallarate, M.; Morel, S. Resveratrol in Solid Lipid Nanoparticles. J. Dispers. Sci. Technol. 2012, 33, 465–471. [Google Scholar] [CrossRef]
- Chen, J.; Wei, N.; Lopez-Garcia, M.; Ambrose, D.; Lee, J.; Annelin, C.; Peterson, T. Development and evaluation of resveratrol, Vitamin E, and epigallocatechin gallate loaded lipid nanoparticles for skin care applications. Eur. J. Pharm. Biopharm. 2017, 117, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Shrotriya, S.N.; Ranpise, N.S.; Vidhate, B.V. Skin targeting of resveratrol utilizing solid lipid nanoparticle-engrossed gel for chemically induced irritant contact dermatitis. Drug Deliv. Transl Res. 2017, 7, 37–52. [Google Scholar] [CrossRef]
- Rigon, R.B.; Fachinetti, N.; Severino, P.; Santana, M.H.; Chorilli, M. Skin Delivery and in Vitro Biological Evaluation of Trans-Resveratrol-Loaded Solid Lipid Nanoparticles for Skin Disorder Therapies. Molecules 2016, 21, 116. [Google Scholar] [CrossRef]
- Montenegro, L.; Parenti, C.; Turnaturi, R.; Pasquinucci, L. Resveratrol-Loaded Lipid Nanocarriers: Correlation between In Vitro Occlusion Factor and In Vivo Skin Hydrating Effect. Pharmaceutics 2017, 9, 58. [Google Scholar] [CrossRef]
- Gokce, E.H.; Korkmaz, E.; Dellera, E.; Sandri, G.; Bonferoni, M.C.; Ozer, O. Resveratrol-loaded solid lipid nanoparticles versus nanostructured lipid carriers: Evaluation of antioxidant potential for dermal applications. Int. J. Nanomed. 2012, 7, 1841–1850. [Google Scholar] [CrossRef]
- Park, S.N.; Jo, N.R.; Jeon, S.H. Chitosan-coated liposomes for enhanced skin permeation of resveratrol. J. Ind. Eng. Chem. 2014, 20, 1481–1485. [Google Scholar] [CrossRef]
- Jøraholmen, M.W.; Škalko-Basnet, N.; Acharya, G.; Basnet, P. Resveratrol-loaded liposomes for topical treatment of the vaginal inflammation and infections. Eur. J. Pharm. Sci. 2015, 79, 112–121. [Google Scholar] [CrossRef] [Green Version]
- Joraholmen, M.W.; Basnet, P.; Tostrup, M.J.; Moueffaq, S.; Skalko-Basnet, N. Localized Therapy of Vaginal Infections and Inflammation: Liposomes-In-Hydrogel Delivery System for Polyphenols. Pharmaceutics 2019, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Doppalapudi, S.; Mahira, S.; Khan, W. Development and in vitro assessment of psoralen and resveratrol co-loaded ultradeformable liposomes for the treatment of vitiligo. J. Photochem. Photobiol. B Biol. 2017, 174, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Cosco, D.; Paolino, D.; Maiuolo, J.; Marzio, L.D.; Carafa, M.; Ventura, C.A.; Fresta, M. Ultradeformable liposomes as multidrug carrier of resveratrol and 5-fluorouracil for their topical delivery. Int. J. Pharm. 2015, 489, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Walther, R.; Rautio, J.; Zelikin, A.N. Prodrugs in medicinal chemistry and enzyme prodrug therapies. Adv. Drug Deliv. Rev. 2017, 118, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Rautio, J.; Meanwell, N.A.; Di, L.; Hageman, M.J. The expanding role of prodrugs in contemporary drug design and development. Nat. Rev. Drug Discov. 2018, 17, 559–587. [Google Scholar] [CrossRef] [PubMed]
- Rautio, J.; Kumpulainen, H.; Heimbach, T.; Oliyai, R.; Oh, D.; Jarvinen, T.; Savolainen, J. Prodrugs: Design and clinical applications. Nat. Rev. Drug Discov. 2008, 7, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Jornada, D.H.; Dos Santos Fernandes, G.F.; Chiba, D.E.; De Melo, T.R.; Dos Santos, J.L.; Chung, M.C. The Prodrug Approach: A Successful Tool for Improving Drug Solubility. Molecules 2015, 21, 42. [Google Scholar] [CrossRef]
- Trela, B.C.; Waterhouse, A.L. Resveratrol: Isomeric Molar Absorptivities and Stability. J. Agric. Food Chem. 1996, 44, 1253–1257. [Google Scholar] [CrossRef]
- Francioso, A.; Mastromarino, P.; Masci, A.; D’erme, M.; Mosca, L. Chemistry, stability and bioavailability of resveratrol. Med. Chem. 2014, 10, 237–245. [Google Scholar] [CrossRef]
- Biasutto, L.; Marotta, E.; Mattarei, A.; Beltramello, S.; Caliceti, P.; Salmaso, S.; Bernkop-Schnurch, A.; Garbisa, S.; Zoratti, M.; Paradisi, C. Absorption and metabolism of resveratrol carboxyesters and methanesulfonate by explanted rat intestinal segments. Cell Physiol. Biochem. 2009, 24, 557–566. [Google Scholar] [CrossRef]
- Liang, L.; Liu, X.; Wang, Q.; Cheng, S.; Zhang, S.; Zhang, M. Pharmacokinetics, tissue distribution and excretion study of resveratrol and its prodrug 3,5,4′-tri-O-acetylresveratrol in rats. Phytomedicine 2013, 20, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Biasutto, L.; Marotta, E.; Bradaschia, A.; Fallica, M.; Mattarei, A.; Garbisa, S.; Zoratti, M.; Paradisi, C. Soluble polyphenols: Synthesis and bioavailability of 3,4’,5-tri(alpha-d-glucose-3-O-succinyl) resveratrol. Bioorg. Med. Chem. Lett. 2009, 19, 6721–6724. [Google Scholar] [CrossRef] [PubMed]
- Basavaraj, S.; Benson, H.A.; Cruickshank, C.; Brown, D.H.; Chen, Y. Development of a liquid chromatography/mass spectrometry methodology to separate, detect, characterize and quantify PEG-resveratrol prodrugs and the conjugation reaction precursors and intermediates. Rapid Commun. Mass Spectrom. 2011, 25, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, H.; Shang, Z.; Chen, A.; Huang, D.; Zhao, H.; Du, H. Amino acid-PEGylated resveratrol and its influence on solubility and the controlled release behavior. Biol. Pharm. Bull. 2014, 37, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; Tomé-Carneiro, J.; Yáñez-Gascón, M.J.; Alcántara, D.; Selma, M.V.; Beltrán, D.; García-Conesa, M.T.; Urbán, C.; Lucas, R.; Tomás-Barberán, F.; et al. Preventive Oral Treatment with Resveratrol Pro-prodrugs Drastically Reduce Colon Inflammation in Rodents. J. Med. Chem. 2010, 53, 7365–7376. [Google Scholar] [CrossRef] [PubMed]
- Penalver, P.; Belmonte-Reche, E.; Adan, N.; Caro, M.; Mateos-Martin, M.L.; Delgado, M.; Gonzalez-Rey, E.; Morales, J.C. Alkylated resveratrol prodrugs and metabolites as potential therapeutics for neurodegenerative diseases. Eur. J. Med. Chem. 2018, 146, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Mattarei, A.; Azzolini, M.; Carraro, M.; Sassi, N.; Zoratti, M.; Paradisi, C.; Biasutto, L. Acetal Derivatives as Prodrugs of Resveratrol. Mol. Pharm. 2013, 10, 2781–2792. [Google Scholar] [CrossRef] [PubMed]
- Mattarei, A.; Carraro, M.; Azzolini, M.; Paradisi, C.; Zoratti, M.; Biasutto, L. New water-soluble carbamate ester derivatives of resveratrol. Molecules 2014, 19, 15900–15917. [Google Scholar] [CrossRef] [PubMed]
- Azzolini, M.; Mattarei, A.; La Spina, M.; Marotta, E.; Zoratti, M.; Paradisi, C.; Biasutto, L. Synthesis and Evaluation as Prodrugs of Hydrophilic Carbamate Ester Analogues of Resveratrol. Mol. Pharm. 2015, 12, 3441–3454. [Google Scholar] [CrossRef] [PubMed]
- Mattarei, A.; Azzolini, M.; Zoratti, M.; Biasutto, L.; Paradisi, C. N-Monosubstituted Methoxy-oligo(ethylene glycol) Carbamate Ester Prodrugs of Resveratrol. Molecules 2015, 20, 16085–16102. [Google Scholar] [CrossRef] [PubMed]
- Mattarei, A.; Azzolini, M.; La Spina, M.; Zoratti, M.; Paradisi, C.; Biasutto, L. Amino Acid Carbamates As Prodrugs Of Resveratrol. Sci. Rep. 2015, 5, 15216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azzolini, M.; Mattarei, A.; La Spina, M.; Fanin, M.; Chiodarelli, G.; Romio, M.; Zoratti, M.; Paradisi, C.; Biasutto, L. New natural amino acid-bearing prodrugs boost pterostilbene’s oral pharmacokinetic and distribution profile. Eur. J. Pharm. Biopharm. 2017, 115, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Biasutto, L.; Mattarei, A.; Marotta, E.; Bradaschia, A.; Sassi, N.; Garbisa, S.; Zoratti, M.; Paradisi, C. Development of mitochondria-targeted derivatives of resveratrol. Bioorg. Med. Chem. Lett. 2008, 18, 5594–5597. [Google Scholar] [CrossRef] [PubMed]
- Sassi, N.; Mattarei, A.; Azzolini, M.; Bernardi, P.; Szabo, I.; Paradisi, C.; Zoratti, M.; Biasutto, L. Mitochondria-targeted resveratrol derivatives act as cytotoxic pro-oxidants. Curr. Pharm. Des. 2014, 20, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Sassi, N.; Mattarei, A.; Azzolini, M.; Szabo, I.; Paradisi, C.; Zoratti, M.; Biasutto, L. Cytotoxicity of mitochondria-targeted resveratrol derivatives: Interactions with respiratory chain complexes and ATP synthase. Biochim. Biophys. Acta 2014, 1837, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, M.L. Harnessing Polypharmacology with Medicinal Chemistry. ACS Med. Chem. Lett. 2019, 10, 273–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorrenti, V.; Pittala, V.; Romeo, G.; Amata, E.; Dichiara, M.; Marrazzo, A.; Turnaturi, R.; Prezzavento, O.; Barbagallo, I.; Vanella, L.; et al. Targeting heme Oxygenase-1 with hybrid compounds to overcome Imatinib resistance in chronic myeloid leukemia cell lines. Eur. J. Med. Chem. 2018, 158, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Panico, A.M.; Santagati, L.M.; Siciliano, E.A.; Intagliata, S.; Modica, M.N. Solid Lipid Nanoparticles Loading Idebenone Ester with Pyroglutamic Acid: In Vitro Antioxidant Activity and In Vivo Topical Efficacy. Nanomaterials 2018, 9, 43. [Google Scholar] [CrossRef]
- Intagliata, S.; Modica, M.N.; Pittala, V.; Salerno, L.; Siracusa, M.A.; Cagnotto, A.; Salmona, M.; Romeo, G. Design and synthesis of new homo and hetero bis-piperazinyl-1-propanone derivatives as 5-HT7R selective ligands over 5-HT1AR. Bioorg. Med. Chem. Lett. 2016, 26, 4052–4056. [Google Scholar] [CrossRef]
- Bernhaus, A.; Fritzer-Szekeres, M.; Grusch, M.; Saiko, P.; Krupitza, G.; Venkateswarlu, S.; Trimurtulu, G.; Jaeger, W.; Szekeres, T. Digalloylresveratrol, a new phenolic acid derivative induces apoptosis and cell cycle arrest in human HT-29 colon cancer cells. Cancer Lett. 2009, 274, 299–304. [Google Scholar] [CrossRef]
- Tsai, C.L.; Chiu, Y.M.; Ho, T.Y.; Hsieh, C.T.; Shieh, D.C.; Lee, Y.J.; Tsay, G.J.; Wu, Y.Y. Gallic Acid Induces Apoptosis in Human Gastric Adenocarcinoma Cells. Anticancer Res. 2018, 38, 2057–2067. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Suzuki, R.; Sakaguchi, N.; Li, Z.; Takeda, T.; Ogihara, Y.; Jiang, B.Y.; Chen, Y. Selective induction of cell death in cancer cells by gallic acid. Biol. Pharm. Bull. 1995, 18, 1526–1530. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, K.; Kataoka, T.; Hayashi, T.; Hasegawa, M.; Ishi, Y.; Hibasami, H. Induction of apoptosis by gallic acid in human stomach cancer KATO III and colon adenocarcinoma COLO 205 cell lines. Oncol. Rep. 2000, 7, 1221–1223. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Fu, J.; Shurlknight, K.L.; Soroka, D.N.; Hu, Y.; Chen, X.; Sang, S. Novel Resveratrol-Based Aspirin Prodrugs: Synthesis, Metabolism, and Anticancer Activity. J. Med. Chem. 2015, 58, 6494–6506. [Google Scholar] [CrossRef] [PubMed]
- Aldawsari, F.S.; Elshenawy, O.H.; El Gendy, M.A.; Aguayo-Ortiz, R.; Baksh, S.; El-Kadi, A.O.; Velazquez-Martinez, C.A. Design and synthesis of resveratrol-salicylate hybrid derivatives as CYP1A1 inhibitors. J. Enzym. Inhib. Med. Chem. 2015, 30, 884–895. [Google Scholar] [CrossRef] [PubMed]
- Aldawsari, F.S.; Aguayo-Ortiz, R.; Kapilashrami, K.; Yoo, J.; Luo, M.; Medina-Franco, J.L.; Velazquez-Martinez, C.A. Resveratrol-salicylate derivatives as selective DNMT3 inhibitors and anticancer agents. J. Enzym. Inhib. Med. Chem. 2016, 31, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Goldhahn, K.; Handler, N.; Loebsch, S.; Schmetterer, K.; Steiner, G.; Kloesch, B.; Erker, T. Antiproliferative and Pro-apoptotic Activities of a Novel Resveratrol Prodrug Against Jurkat CD4+T-Cells. Anticancer Res. 2016, 36, 683–689. [Google Scholar] [PubMed]
- Acerson, M.J.; Fabick, K.M.; Wong, Y.; Blake, C.; Lephart, E.D.; Andrus, M.B. A new synthesis of 4′-resveratrol esters and evaluation of the potential for anti-depressant activity. Bioorg. Med. Chem. Lett. 2013, 23, 2941–2944. [Google Scholar] [CrossRef]
- Peterson, J.A.; Doughty, H.P.; Eells, A.J.; Johnson, T.A.; Hastings, J.P.; Crowther, C.M.; Andrus, M.B.; Kenealey, J.D. The Effects of 4′-Esterified Resveratrol Derivatives on Calcium Dynamics in Breast Cancer Cells. Molecules 2017, 22, 1968. [Google Scholar] [CrossRef]
- Oh, W.Y.; Shahidi, F. Lipophilization of Resveratrol and Effects on Antioxidant Activities. J. Agric. Food Chem. 2017, 65, 8617–8625. [Google Scholar] [CrossRef]
- Oh, W.Y.; Shahidi, F. Antioxidant activity of resveratrol ester derivatives in food and biological model systems. Food Chem. 2018, 261, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D. Resveratrol, 4’ Acetoxy Resveratrol, R-equol, Racemic Equol or S-equol as Cosmeceuticals to Improve Dermal Health. Int. J. Mol. Sci. 2017, 18, 1193. [Google Scholar] [CrossRef] [PubMed]
- Chedea, V.S.; Vicas, S.I.; Sticozzi, C.; Pessina, F.; Frosini, M.; Maioli, E.; Valacchi, G. Resveratrol: From diet to topical usage. Food Funct. 2017, 8, 3879–3892. [Google Scholar] [CrossRef] [PubMed]
- Manevski, N.; Swart, P.; Balavenkatraman, K.K.; Bertschi, B.; Camenisch, G.; Kretz, O.; Schiller, H.; Walles, M.; Ling, B.; Wettstein, R.; et al. Phase II metabolism in human skin: Skin explants show full coverage for glucuronidation, sulfation, N-acetylation, catechol methylation, and glutathione conjugation. Drug Metab. Dispos. 2015, 43, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Seok, J.K.; An, S.M.; Baek, J.H.; Koh, J.S.; Boo, Y.C. A study of the human skin-whitening effects of resveratryl triacetate. Arch. Dermatol. Res. 2015, 307, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D.; Acerson, M.J.; Andrus, M.B. Synthesis and skin gene analysis of 4′-acetoxy-resveratrol (4AR), therapeutic potential for dermal applications. Bioorg. Med. Chem. Lett. 2016, 26, 3258–3262. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D.; Andrus, M.B. Human skin gene expression: Natural (trans) resveratrol versus five resveratrol analogs for dermal applications. Exp. Biol. Med. 2017, 242, 1482–1489. [Google Scholar] [CrossRef] [Green Version]
- Jo, D.J.; Seok, J.K.; Kim, S.Y.; Park, W.; Baek, J.H.; Kim, Y.M.; Boo, Y.C. Human skin-depigmenting effects of resveratryl triglycolate, a hybrid compound of resveratrol and glycolic acid. Int. J. Cosmet. Sci. 2018, 40, 256–262. [Google Scholar] [CrossRef]
- Zhang, G.; Flach, C.R.; Mendelsohn, R. Tracking the dephosphorylation of resveratrol triphosphate in skin by confocal Raman microscopy. J. Control. Release 2007, 123, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Park, J.H.; Suh, H.J.; Lee, I.C.; Koh, J.; Boo, Y.C. Effects of resveratrol, oxyresveratrol, and their acetylated derivatives on cellular melanogenesis. Arch. Dermatol. Res. 2014, 306, 475–487. [Google Scholar] [CrossRef]
- Park, S.; Seok, J.K.; Kwak, J.Y.; Choi, Y.H.; Hong, S.S.; Suh, H.J.; Park, W.; Boo, Y.C. Anti-melanogenic effects of resveratryl triglycolate, a novel hybrid compound derived by esterification of resveratrol with glycolic acid. Arch. Dermatol. Res. 2016, 308, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D.; Sommerfeldt, J.M.; Andrus, M.B. Resveratrol: Influences on gene expression in human skin. J. Funct. Foods 2014, 10, 377–384. [Google Scholar] [CrossRef]
- Mohammadi, F.; Tavasoli, E. Synthetic Methods for the Preparation of Resveratrol Glycolate and Tartrate Derivatives for Use in Skin Formulations. WO2017155875A1, 14 September 2017. [Google Scholar]
- Kang, N.J.; Bu, Y.C.; Lee, J.E.; Oh, J.J. Resveratrol Derivatives for Preventing Skin Aging. KR2017038719A, 29 October 2017. [Google Scholar]
- Cheilian, S.; Therouin-Koely, S.; Socroun-Metivier, C. Cosmetic Compositions Comprising Trans-Resveratrol Its Derivative. FR3034987A1, 21 October 2016. [Google Scholar]
- Bu, Y.C. Resveratrol Derivatives for Skin Lightening. KR2014094394A, 30 July 2014. [Google Scholar]
- Cho, W.G.; Kang, N.G.; Park, H.W. Synthesis of Resveratrol Derivative via Polyethoxylation of Resveratrol and Cosmetic Compositions Containing It. KR2005011174A, 29 January 2005. [Google Scholar]
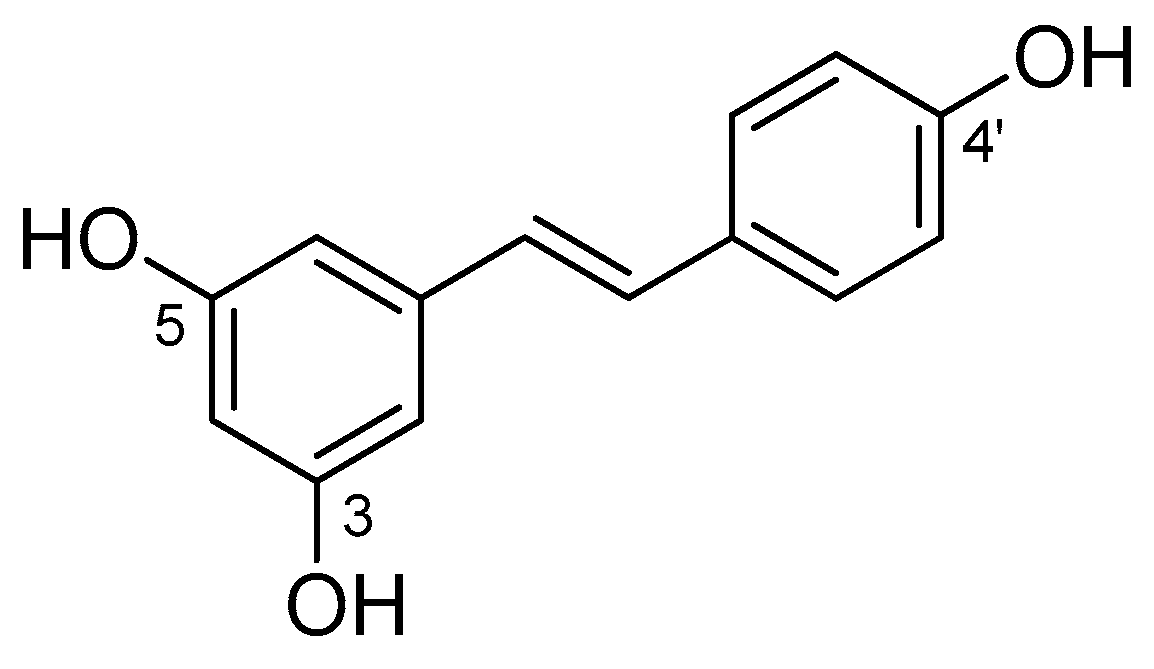
| Molecular Descriptors 1 | |
|---|---|
| Molecular weight (MW) | 228.25 |
| Calculated LogP (cLogP) | 3.40 |
| Hydrogen Bond Donors (HBD) | 3 |
| Hydrogen Bond Acceptors (HBA) | 3 |
| Rotatable Bonds Number (RBN) | 2 |
| Topological Polar Surface Area (TPSA) | 60.69 |
| Nanocarriers | Study | Animal Model | Outcomes | Ref. |
|---|---|---|---|---|
| PLGA NP | RSV pharmacokinetics, in vivo biodistribution, single-pass intestinal perfusion | rats | ↑ RSV oral bioavailability in comparison to the free drug | [85] |
| Galactosylated PLGA NP (GNPs) | oral bioavailability | rats | ↑ RSV oral bioavailability, ↑ intestinal permeability and transcellular transport of RSV, ↑ anti-inflammatory activity in RAW 264.7 cells model | [86] |
| NP based on chitosan derivatives | antioxidant activity and in vivo bioavailability | rats | ↑ RSV water solubility, ↑ antioxidant activity, ↑ bioavailability | [87] |
| SLN N-trimethyl chitosan conjugated with palmitic acid | oral bioavailability | Balb/c mice | ↑ RSV bioavailability, ↑ ability to prevent RSV enzymatic and/or chemical degradation | [88] |
| SLN coated with poloxamer 188 | oral bioavailability | in rats | ↑ RSV effectiveness after oral dosing, ↑ RSV bioavailability | [89] |
| SLN and NLC | oral bioavailability | in vitro study | RSV controlled release Prevention of RSV degradation | [90] |
| Layer by layer (LbL) NP | pharmacokinetic study | Wistar rats | ↑ RSV bioavailability and chemical stability | [91] |
| Nanocarriers | Study | Animal Model | Outcomes | Ref. |
|---|---|---|---|---|
| RSV and DTX co-encapsulated into LPNs | treatment of lung cancer | i.v. injection in mice in vitro (cells HCC827 and NCIH2135) | ↓ tumor growth and size, ↓ the viability of tumor cells | [93] |
| PEG-PLA NP | cancer treatment | in vitro assays on CT26 colon cancer cell i.v. administration in tumor-bearing mice | ↓ reduction of cell number and colony forming, ↓ tumor growth, ↑ survival time of mice, ↑ RSV stability | [94] |
| PEG-PLA NP including transferrin (Tf) | treatment of glioma | i.p. administration in C6 glioma-bearing rat models | ↑ anti-cancer activity, ↓ tumor volume with a concomitant increase of survival time | [95] |
| lipid core nanocapsules | treatment of glioma | i.p. administration in rats bearing brain-implanted C6 gliomas | ↓ decrease of tumor size, ↑ RSV transportation across the BBB, ↓ RSV binding to plasma protein | [96] |
| PM RSV and DTX co-loaded | in vitro cytotoxicity pharmacokinetic | MCF-7 cells, i.v. administration in rats | ↑ AUC values | [98] |
| PM RSV and PTX co-loaded | antitumor activity | PTX-resistant human lung adenocarcinoma epithelial (A549/T) cell line and mice sarcoma 180 (S180) cells, i.v. injection in S180 solid tumor bearing mice | ↑ inhibition of tumor growth | [99] |
| PM RSV co-loaded with (pHO-1) | treatment of acute lung injury. | Inhalation in Balb/c mice | inhibits the nuclear translocation of NF-kB, ↓ pro-inflammatory cytokines in lungs | [100] |
| Gelatin NP | bioavailability anti-proliferative effect in NCI-H460 lung cancer cells | i.v. injection in mice | ↑ bioavailability, ↑ anti-proliferative effect | [101] |
| chitosan-coated lipid NP | brain delivery | inhalation in rat | ↑ RSV concentration in cerebrospinal fluid | [102] |
| cyclodextrins | Bioavailability | i.v. and oral administration in rats | No modifications of bioavailability | [103] |
| Nanocarriers | Drug | Study | Outcomes | Ref. |
|---|---|---|---|---|
| SLN | RSV | in vitro penetration (pig skin) | ↑ RSV photostability, ↑ accumulation in the skin, ↑ anti-lipoperoxidative activity | [104] |
| NLC | RSV | in vitro permeation (human skin) | ↑ RSV skin permeation, ↑ RSV topical effectiveness | [105] |
| SLN | RSV | in vitro permeation (human skin) in vivo studies (ICD-induced BALB/c mice) | ↑ RSV increase of its retention in the skin layers (epidermis and dermis) ↓ tissue edema | [106] |
| SLN | RSV | skin permeation (pig skin) tyrosinase activity | ↑ percentage of tyrosinase inhibitory activity,↑ skin permeation | [107] |
| SLN/NLC | RSV | skin hydration healthy volunteers | SLN ↑ skin hydration in comparison to NLC | [108] |
| SLN/NLC | RSV | in vitro penetration studies (rat skin) | SLN ↑ RSV accumulation in the epidermis, NLC ↑ amount of RSV in the dermis | [109] |
| Chitosan- coated Liposomes | RSV | in vitro permeation (mouse skin) | ↑ skin permeation | [110] |
| Chitosan- coated Liposomes | RSV | in vitro permeation (mouse skin) | ↓ vaginal inflammation and infections, ↑ antioxidant and anti-inflammatory activities | [111,112] |
| UDL | Psoralen and RSV | antioxidant assays B16F10 cell line | ↑ tyrosinase activity, ↑antioxidant activity | [113] |
| UDL | RSV/5-FU | in vitro permeation (human skin) anti-cancer activity on SK-MEL-28 and Colo-38 cells. | Drug accumulation in the deeper skin layers ↑ anti-cancer activity | [114] |
| Type of Prodrug | Promoiety | Linker | Chemical Stability | Stability in Blood | Solubility in Water | Ref. |
|---|---|---|---|---|---|---|
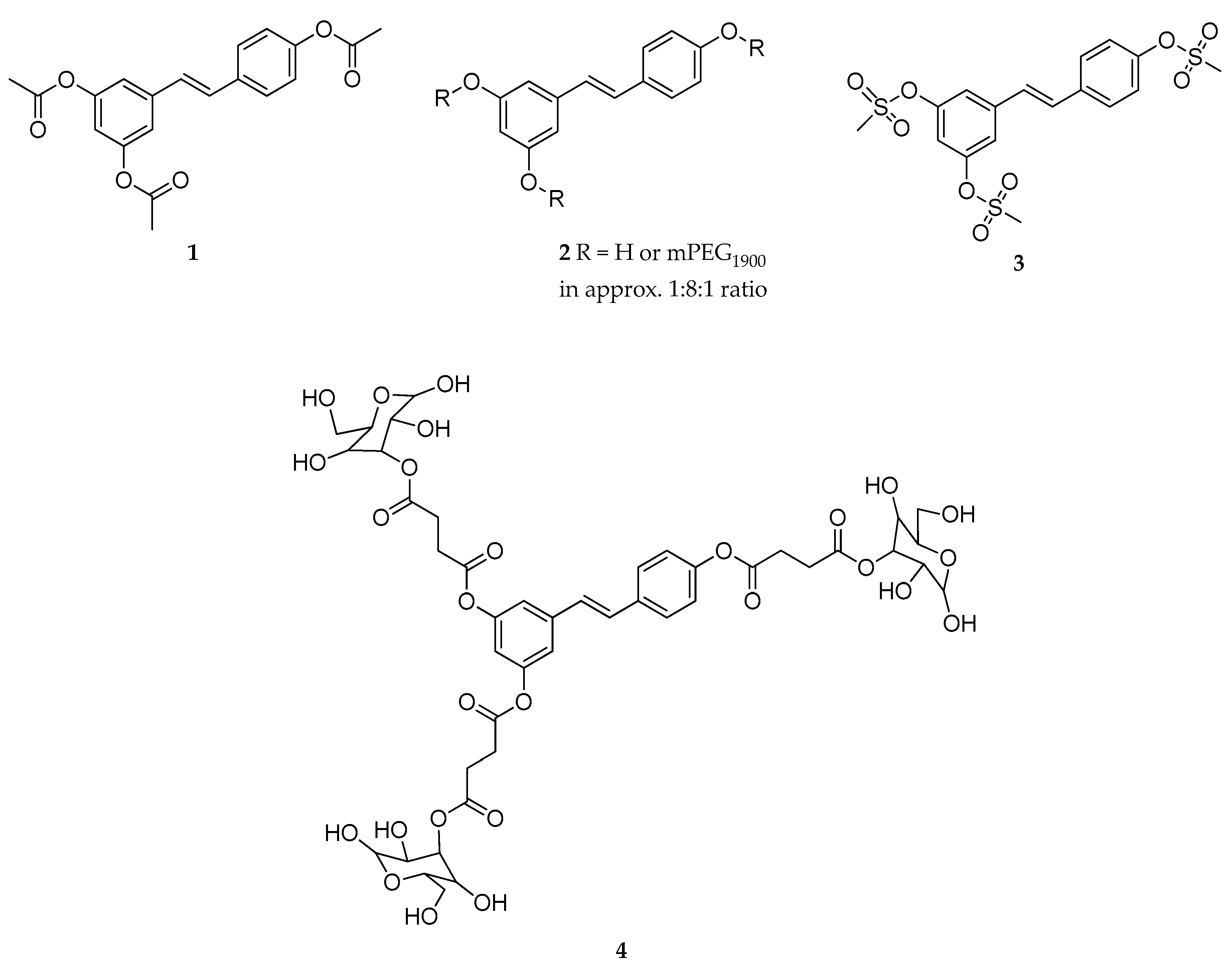
| Triester (1) | acetyl | - | slowly hydrolyzed | rapidly hydrolyzed | poorly soluble | [121,122] |
| Tri-mPEG (2) | mPEG a | - | slowly hydrolyzed | rapidly hydrolyzed | poorly soluble | [121] |
| Trimesylate (3) | mesyl | - | not hydrolyzed | not hydrolyzed | extremely soluble | [121] |
| Triester (4) | α-d-glucose | succinyl | mostly stable | rapidly hydrolyzed | highly soluble | [123] |
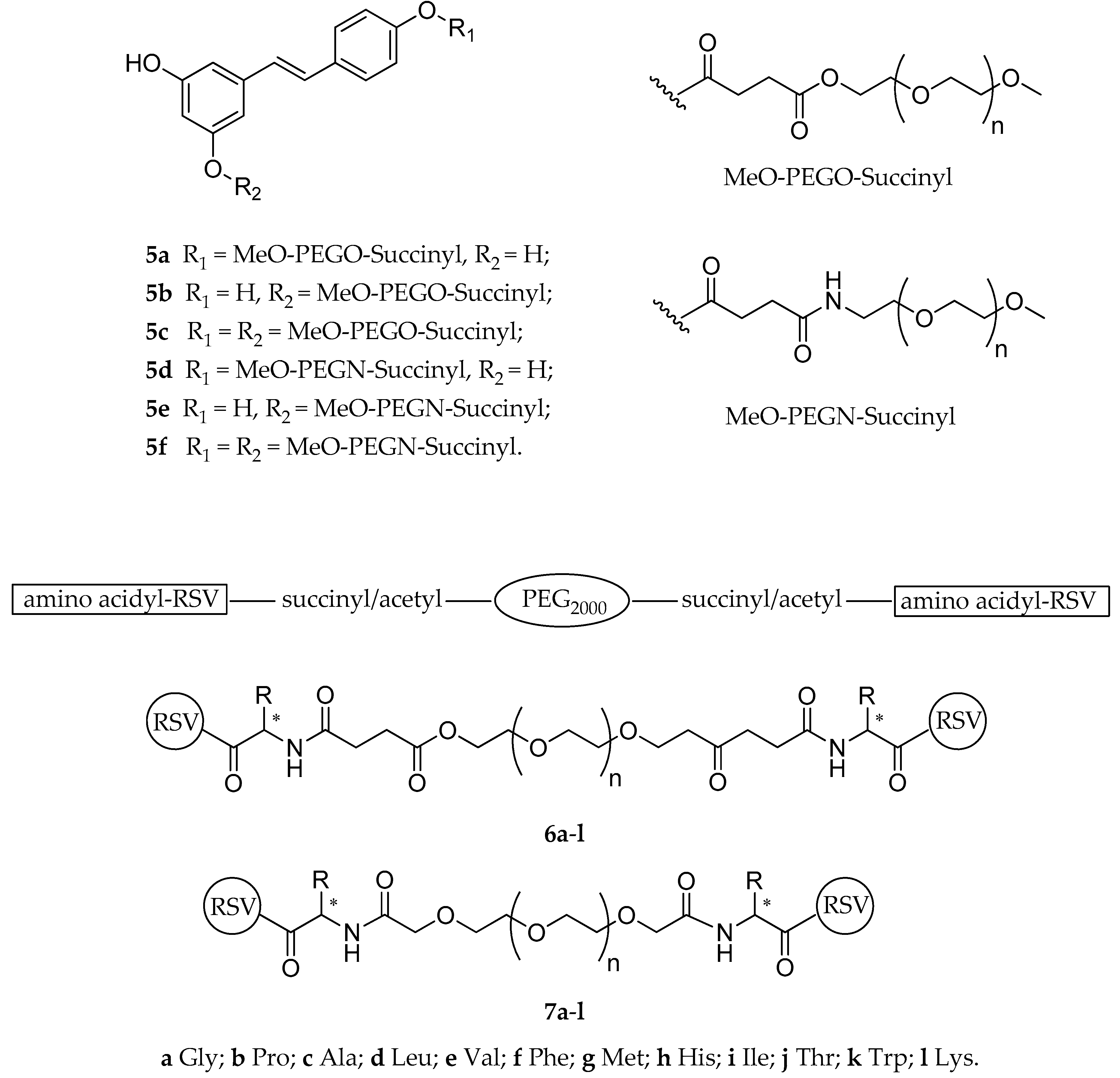
| Diesters (5a–f) | mPEG a | succinylester or succinylamide | not reported | not reported | not reported | [124] |
| Esters (6a–l, 7a–l) | PEG-succinyl or PEG-acetyl | various amino acids | not reported | hydrolyzed | highly soluble | [125] |
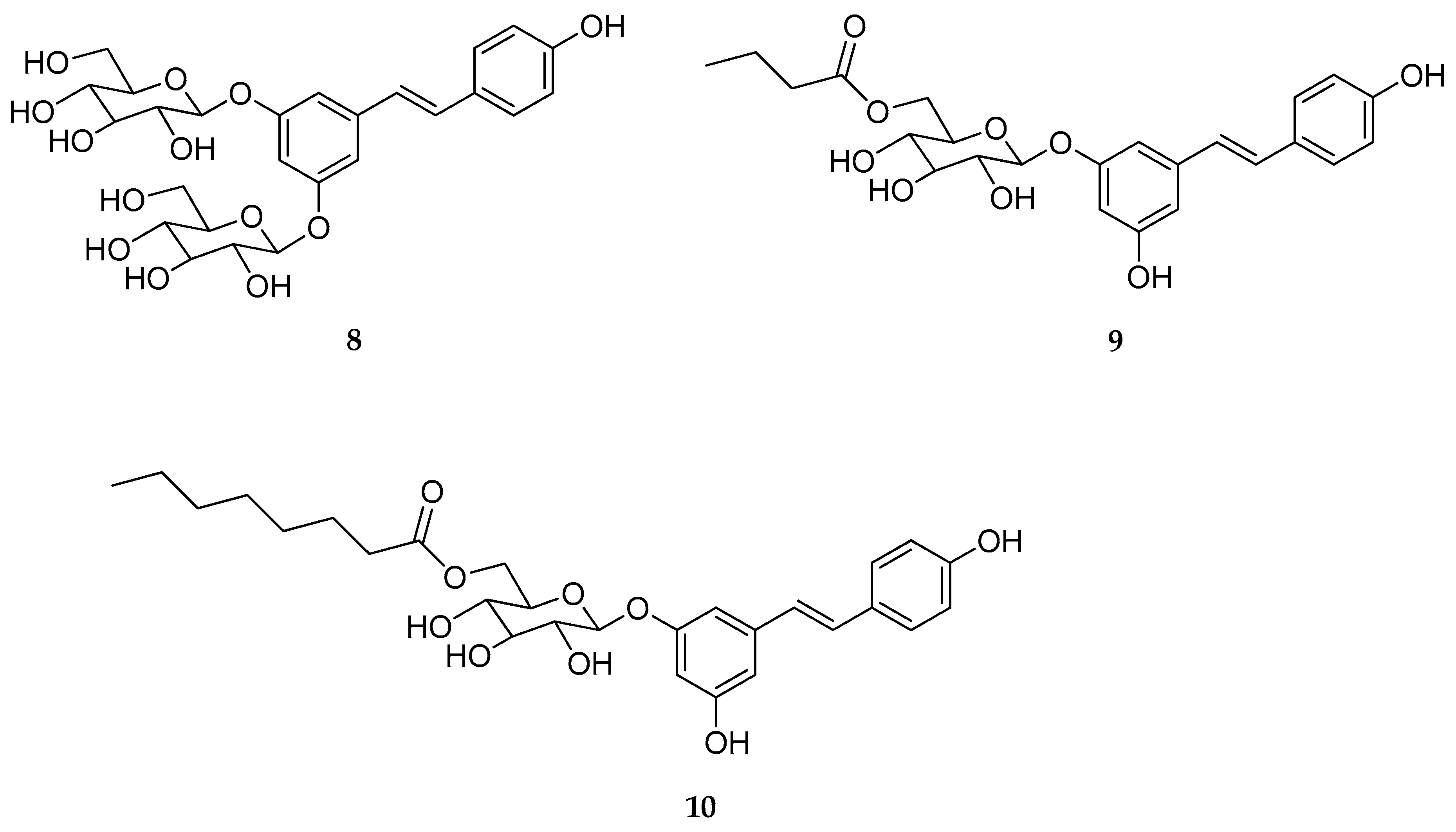
| Mono-O- glucoside and di-O-glucoside (8–10) | β-d-glucose | - | stable | stable | not reported | [126] |
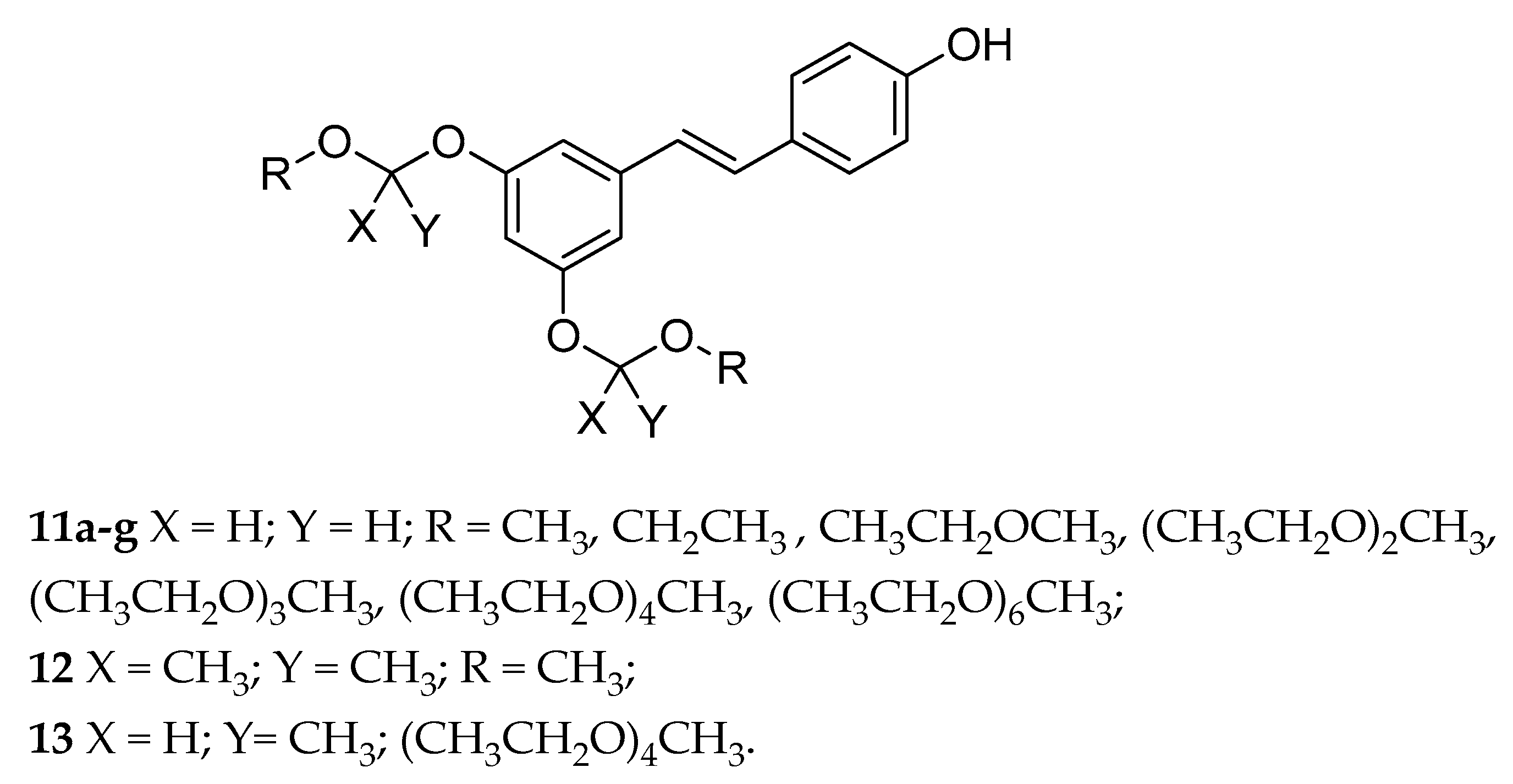
| Acetals (11a–g, 12, 13) | OEG b | - | variable | variable | from poor to highly soluble | [128] |
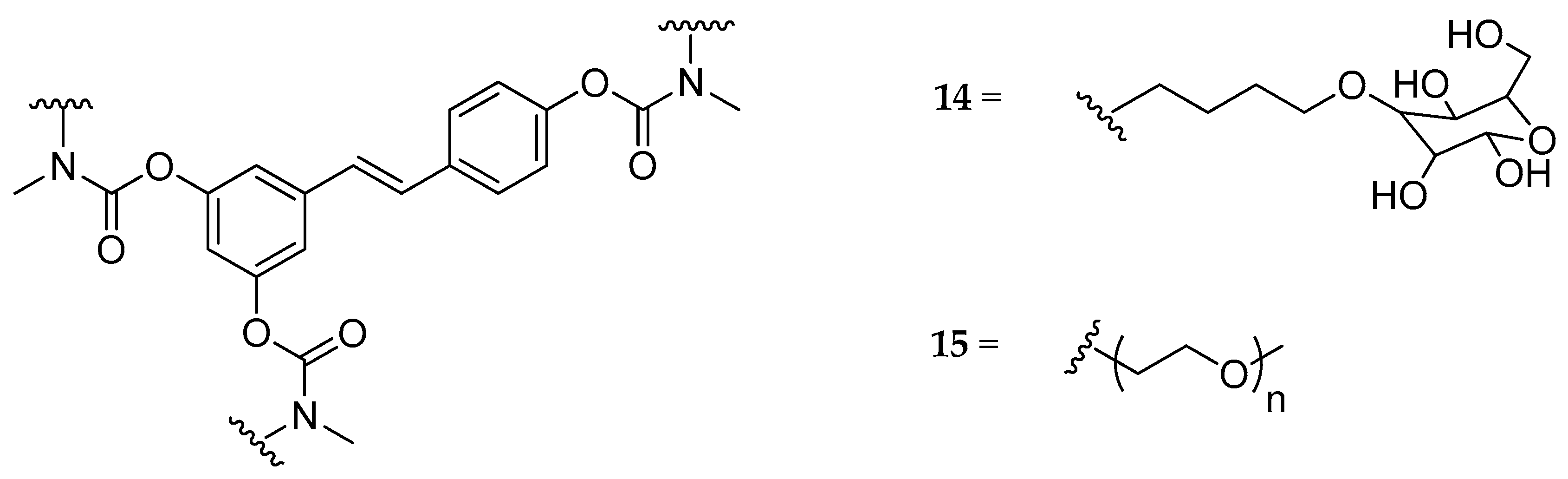
| Tri-N,N-di- substituted carbamate, (14, 15) | butyl-glucosyl or mPEG a | - | - | not hydrolyzed | highly soluble | [129] |
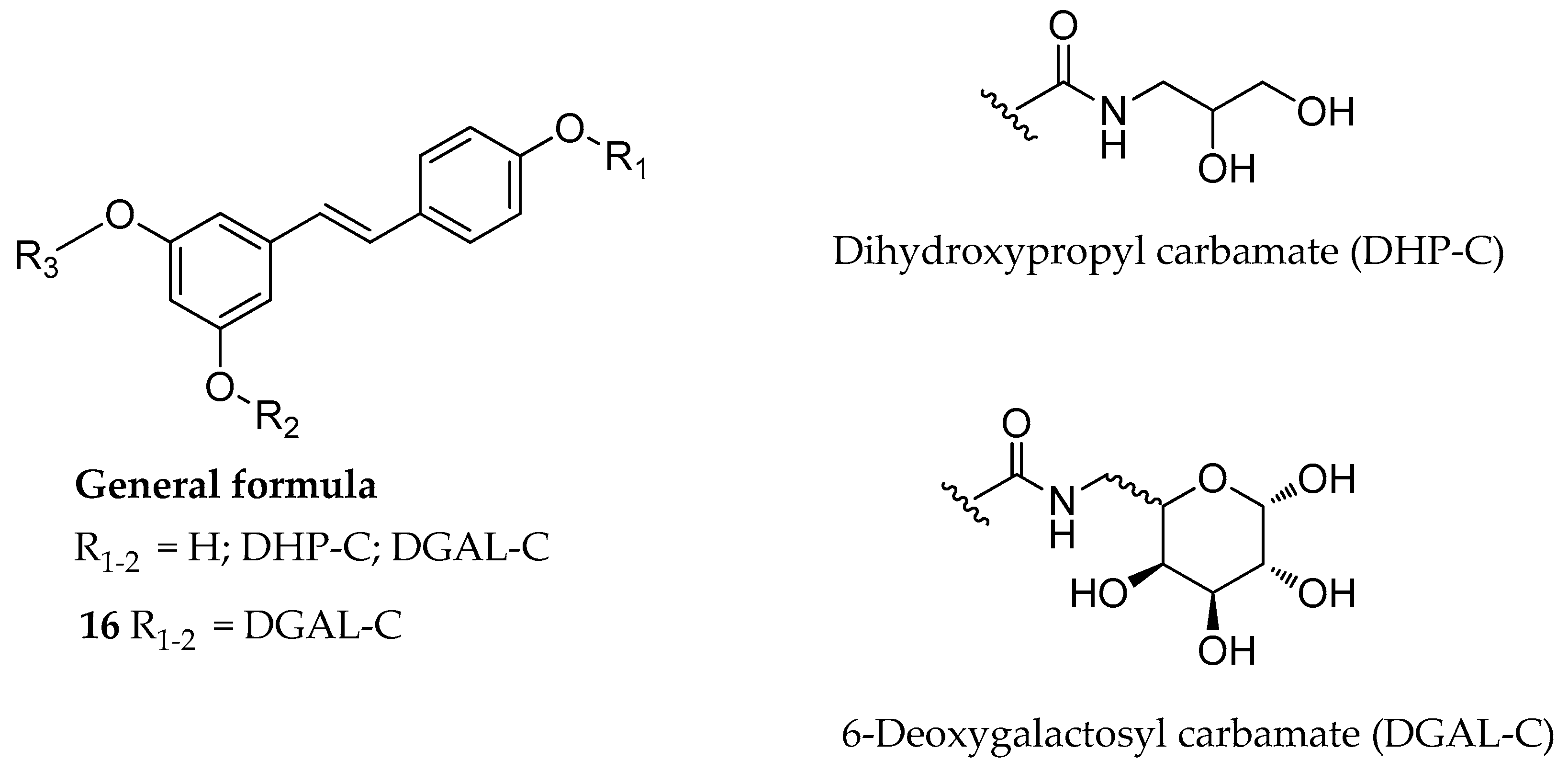
| Mono-, di- or tri-N-mono- substituted carbamate, (e.g., 16) | glycerol or galactose | - | slowly hydrolyzed | slowly hydrolyzed | highly soluble | [130] |
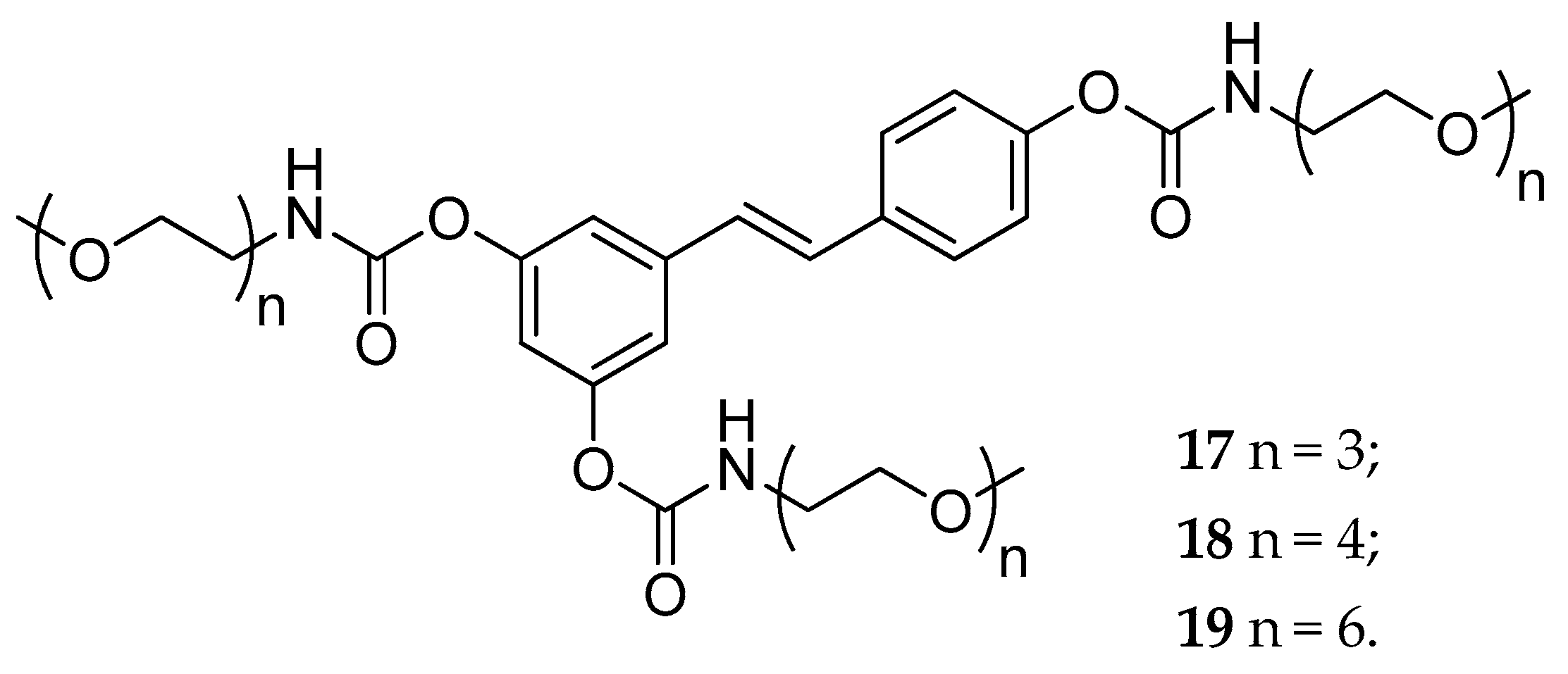
| tri-N-mono- substituted carbamate, (17–19) | OEG b | slowly hydrolyzed | fast hydrolyzed | not reported | [131] | |
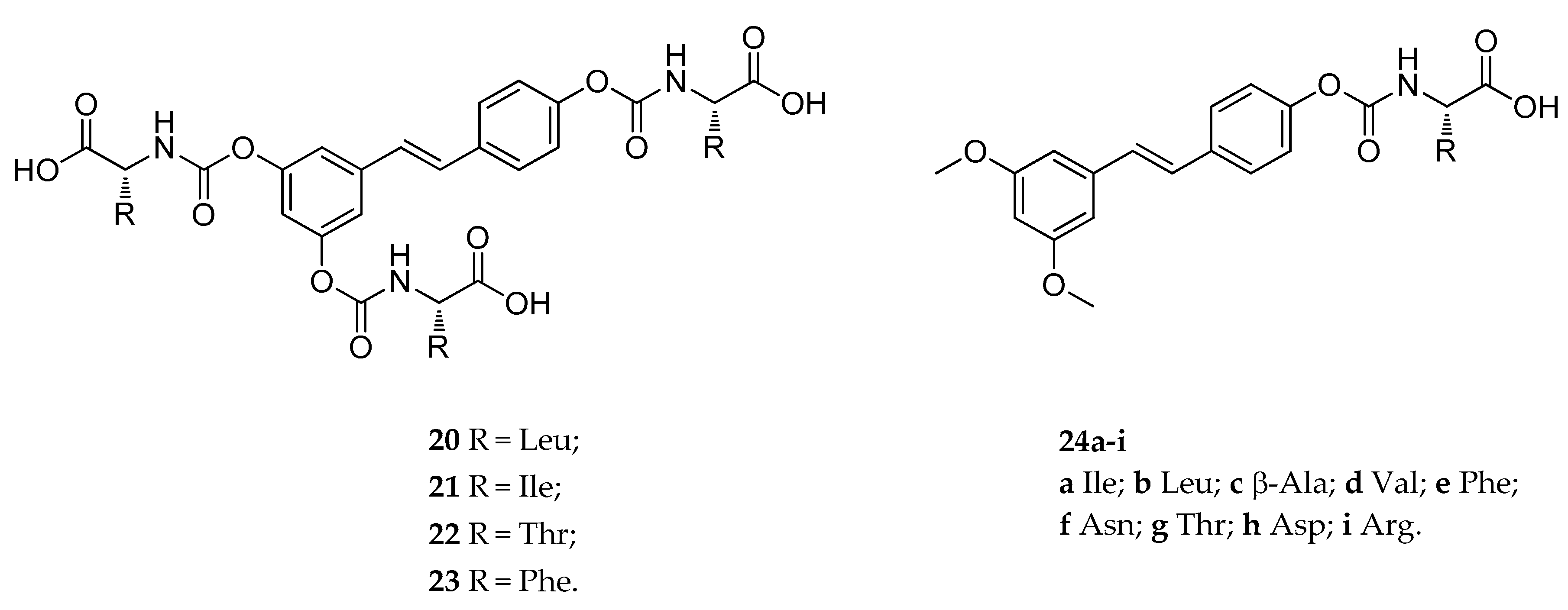
| tri-N-mono- substituted carbamate (20–23) | various amino acids | carbamoyl | slowly hydrolyzed | suitable hydrolyzed | not reported | [133] |
| mono-N-mono- substituted carbamate (24a–i) | various amino acids and metyl | carbamoyl | slowly hydrolyzed | suitable hydrolyzed | not reported | [133] |
| Type of Prodrug | Compounds | Effects | Ref. |
|---|---|---|---|
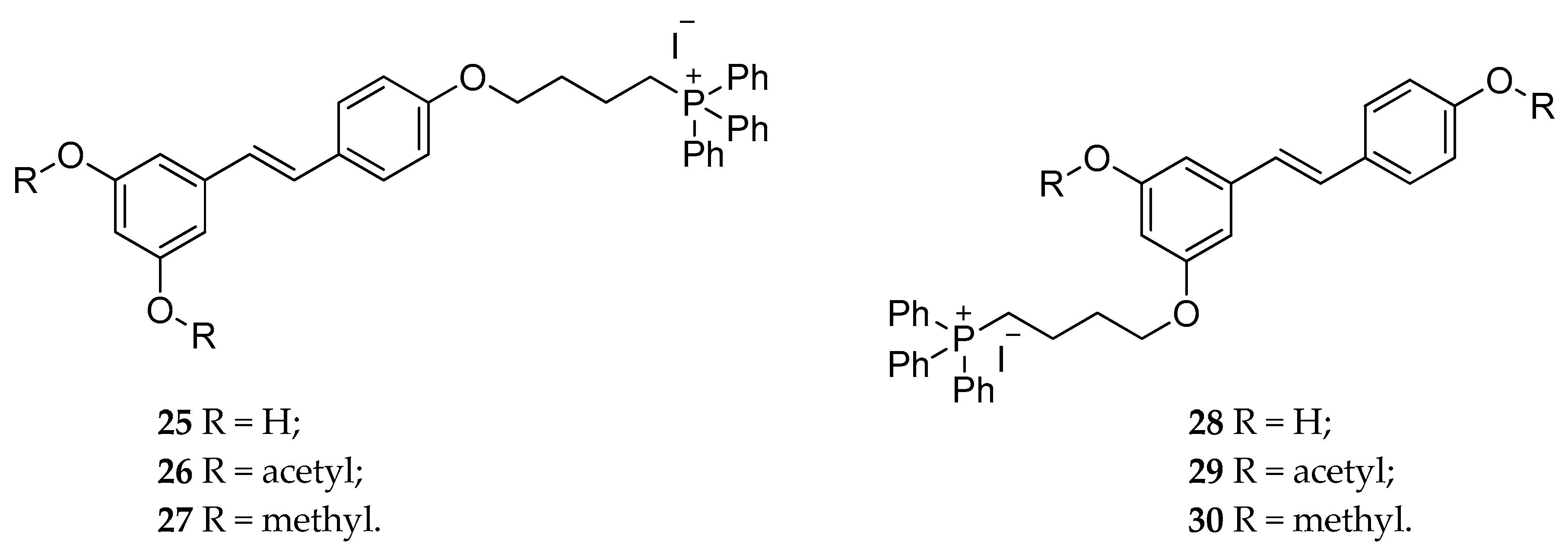
| RSV-triphenylphosphonium | 25–30 | cytotoxicity effect in C-26 murine colon cancer cell line | [134] |
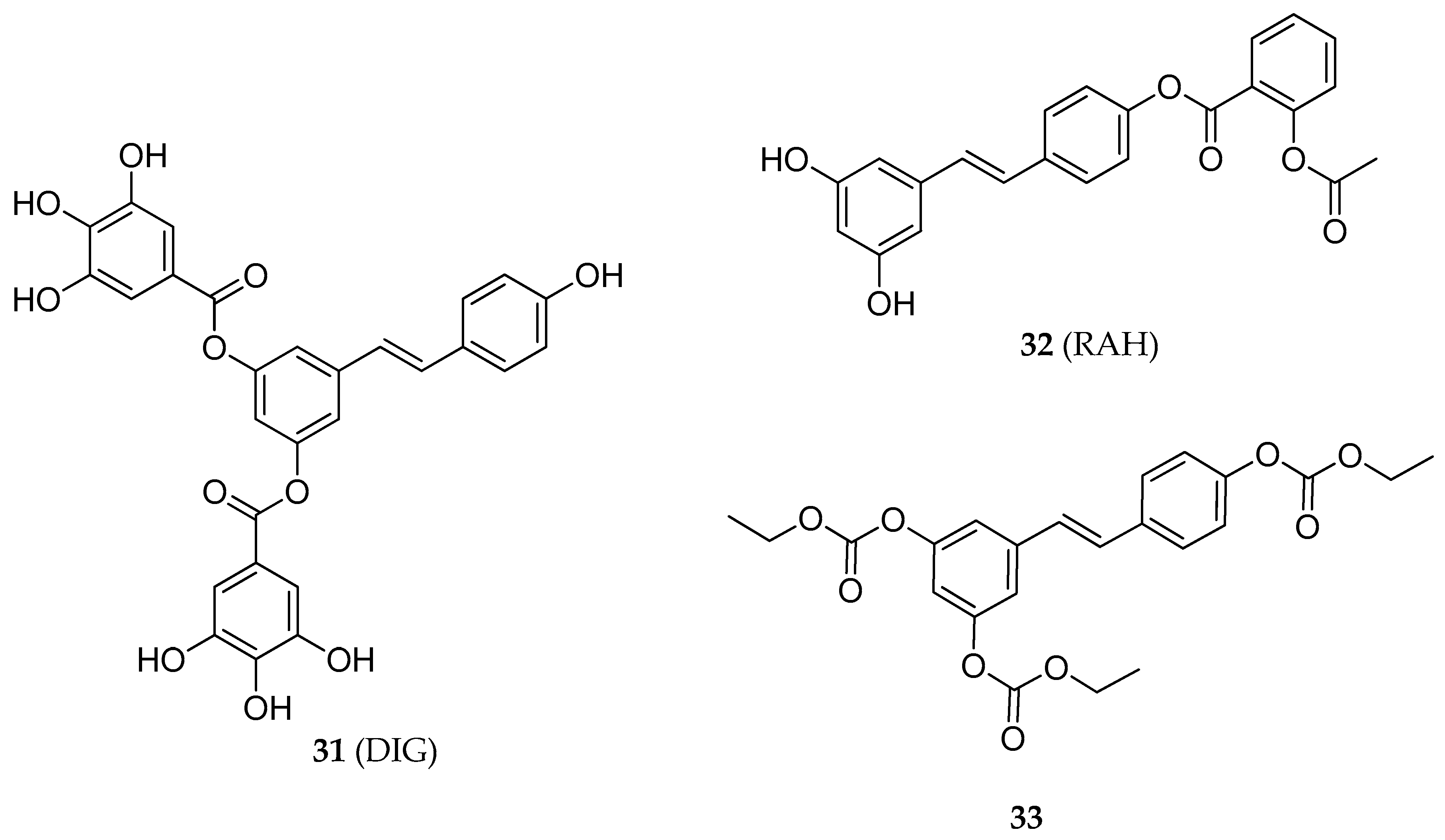
| 3,5-RSV diester | 31 | apoptotic effect in HT-29 human colon cancer cells line | [141] |
| 4′-RSV ester | 32 | growth inhibition in HCT-116 and HT-29 human colon cancer cells line | [145] |
| RSV tricarbonate | 33 | antiproliferative and pro-apoptotic activities in Jurkat T-cells. | [148] |
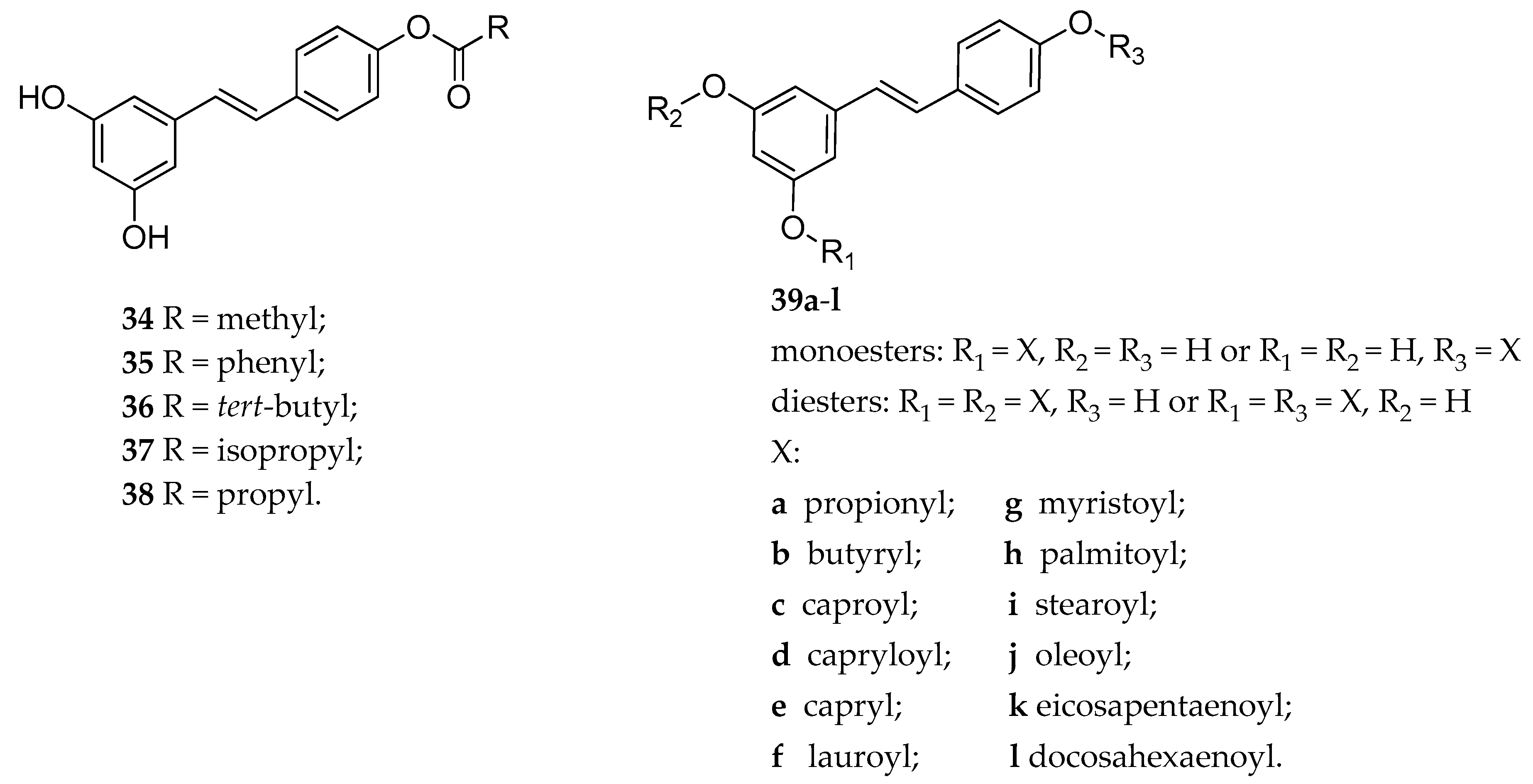
| 4′-RSV ester | 34–36 | anti-depressant activity of 33 in rats (Porsalt forced-swim test) | [149] |
| 4′-RSV ester | 36–38 | decreasing cell viability in MDA-MB-231 cancer cells line | [150] |
| RSV mono and diester | 39a–l | antioxidant activity in both DPPH and ABTS radical cation scavenging assays | [151] |
| RSV mono and diester | 39a–l | inhibition of hydroxyl radical-induced DNA scission | [152] |
| Type of Prodrug | Compounds | Cosmetic Application | Ref. |
|---|---|---|---|
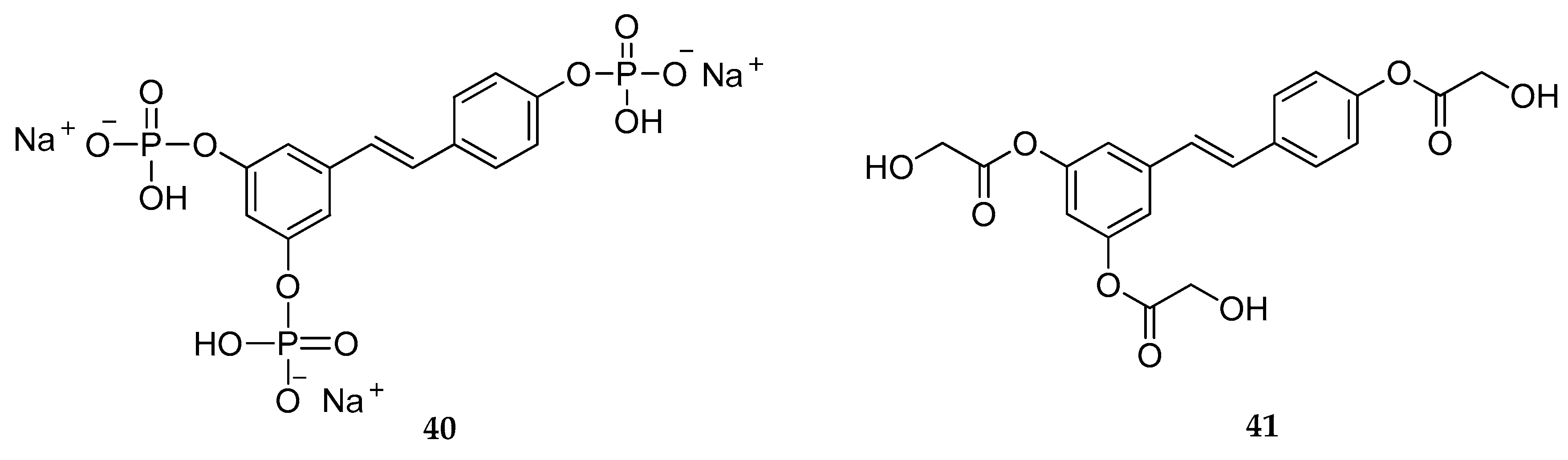
| Triphosphate | 40 | not stated | [160] |
| Triacetate | 1 | anti-melanogenic agents/whitening effect | [156,161] |
| Triglycolate | 42 | anti-melanogenic agents/whitening effect | [159,162] |
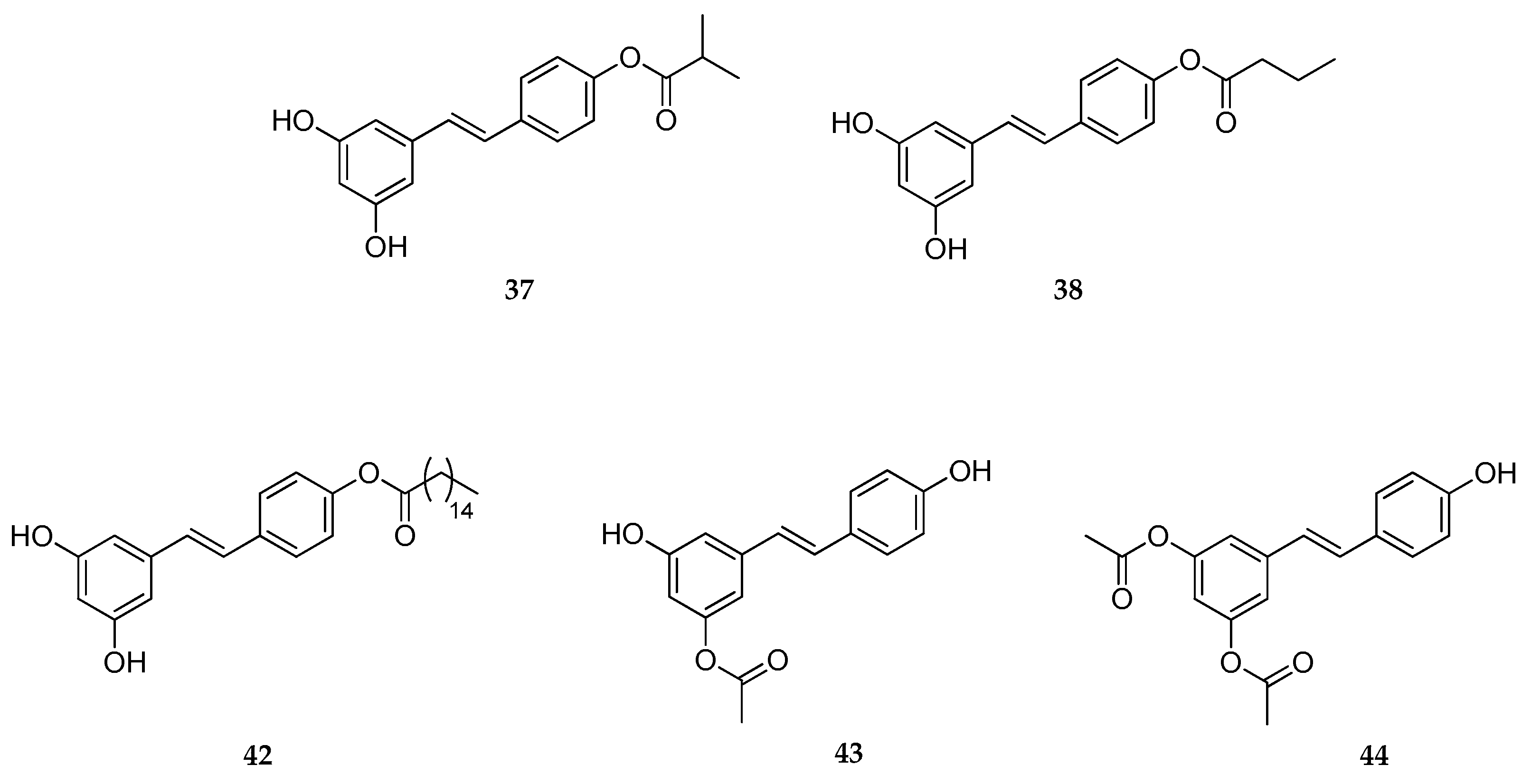
| 4′-Acetate | 43 | skin antioxidant, skin anti-aging | [157] |
| 4′-, 3-, 3,5- Esters | 37, 38, 42–44 | skin antioxidant, skin anti-aging | [158] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Intagliata, S.; Modica, M.N.; Santagati, L.M.; Montenegro, L. Strategies to Improve Resveratrol Systemic and Topical Bioavailability: An Update. Antioxidants 2019, 8, 244. https://doi.org/10.3390/antiox8080244
Intagliata S, Modica MN, Santagati LM, Montenegro L. Strategies to Improve Resveratrol Systemic and Topical Bioavailability: An Update. Antioxidants. 2019; 8(8):244. https://doi.org/10.3390/antiox8080244
Chicago/Turabian StyleIntagliata, Sebastiano, Maria N. Modica, Ludovica M. Santagati, and Lucia Montenegro. 2019. "Strategies to Improve Resveratrol Systemic and Topical Bioavailability: An Update" Antioxidants 8, no. 8: 244. https://doi.org/10.3390/antiox8080244






