Ectopic or Over-Expression of Class 1 Phytoglobin Genes Confers Flooding Tolerance to the Root Nodules of Lotus japonicus by Scavenging Nitric Oxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Materials
2.2. Growth Conditions and Flooding Treatment
2.3. Nitrogenase Activity
2.4. Endogenous NO and ROS in Roots
2.5. NO Released from Nodules
2.6. Leaf Chlorophyll Content
2.7. Electrolyte Leakage from Leaves
2.8. qRT-PCR Analysis of Senescence-Related Genes
2.9. Histochemical Detection of H2O2 and O2−
2.10. Light Microscopy
3. Results
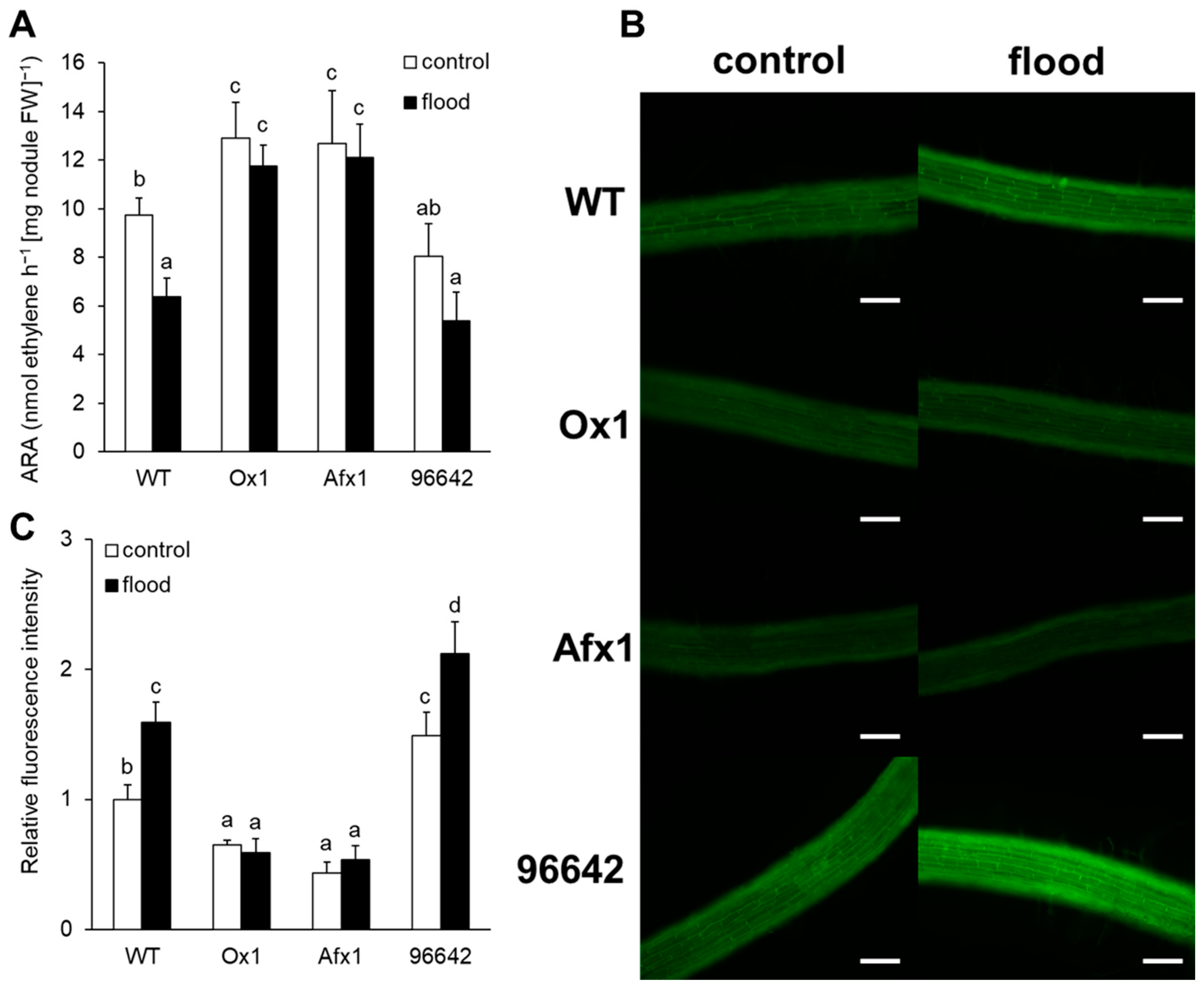
3.1. Nodules of Ox1 and Afx1 Lines have High Nitrogenase Activity and Low NO Levels
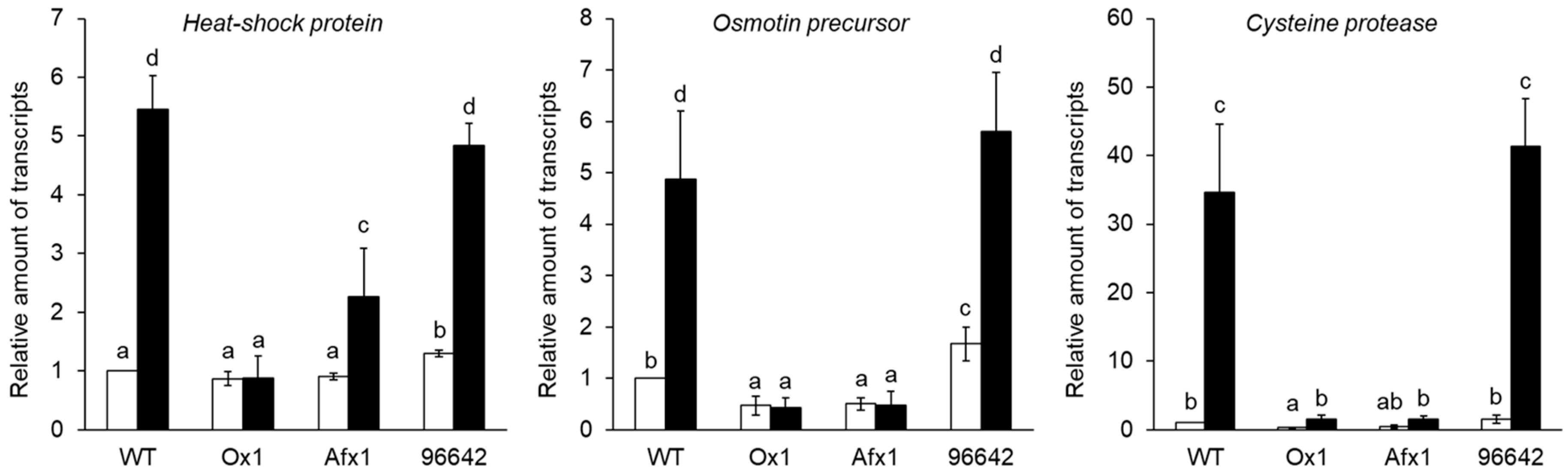
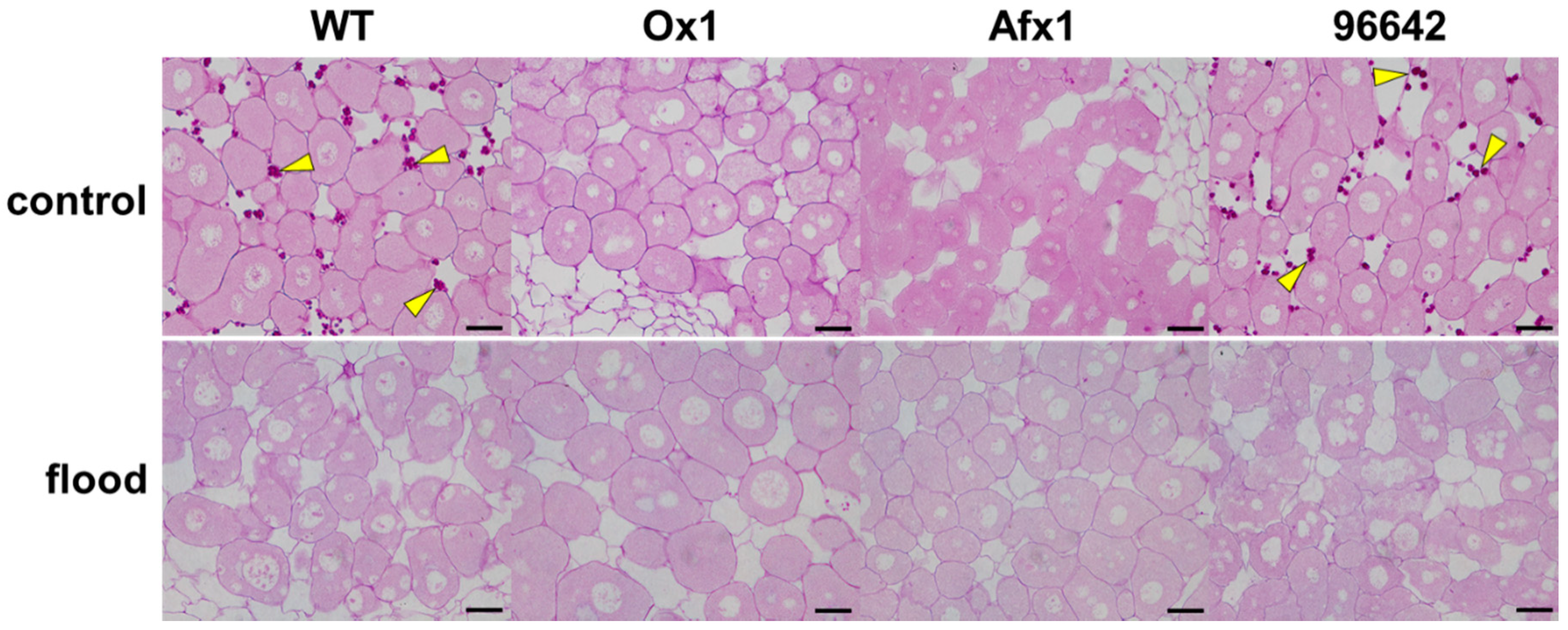
3.2. Glb1s Alleviate Nodule Senescence Caused by Flooding
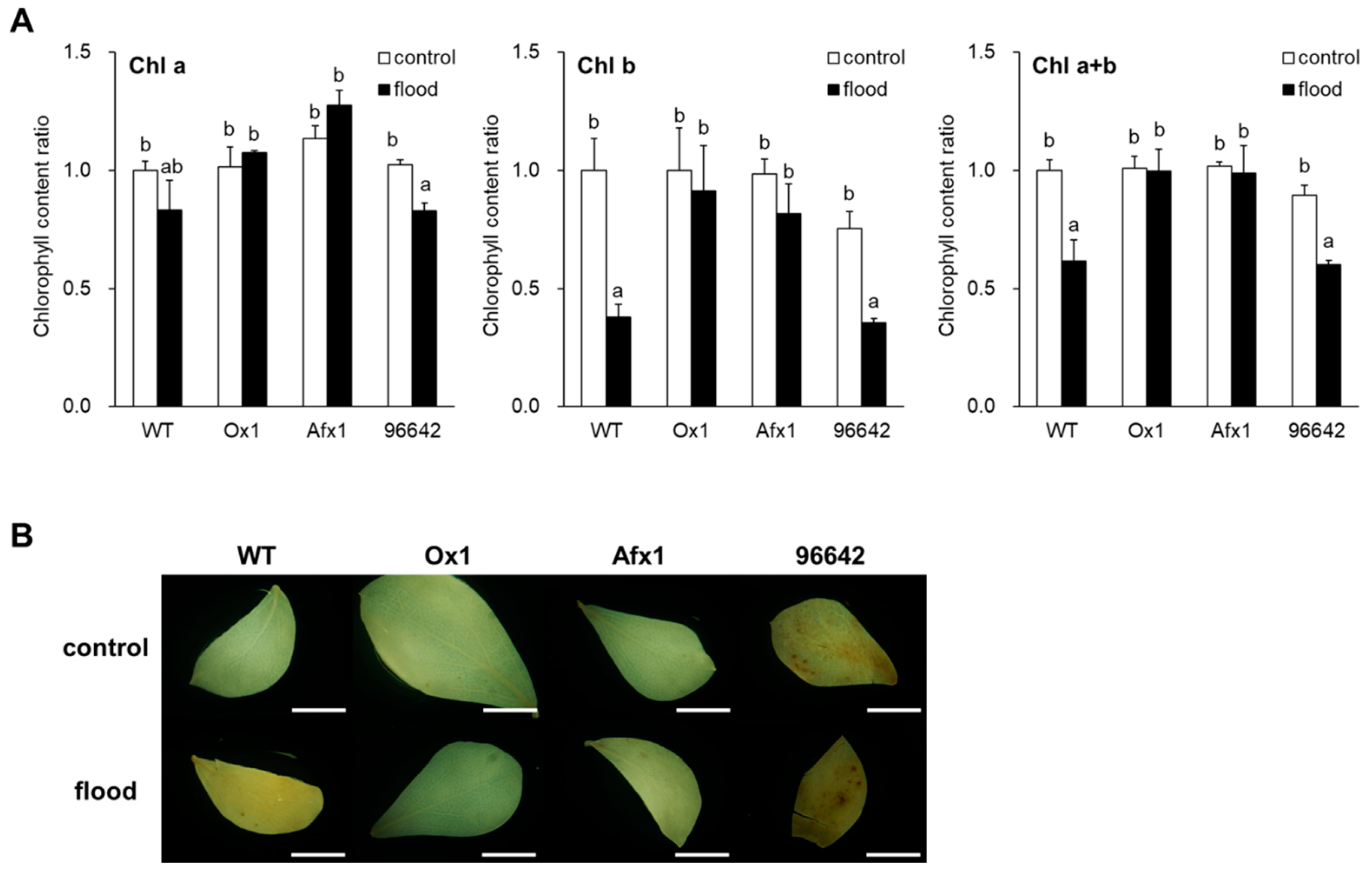
3.3. Glb1s Alleviate the Effects of Flooding in Leaves and Roots
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Chen, X.; Wang, H.; Bao, Y.; Zhang, W. Examination of the leaf proteome during flooding stress and the induction of programmed cell death in maize. Proteome Sci. 2014, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Titarenko, T. Test parameters of revealing the degree of fruit plants tolerance to the root hypoxia caused by flooding of soil. Plant Physiol. Biochem. 2000, 38, 115–117. [Google Scholar]
- Hetherington, A.M.; Hunter, S.; Crawford, R.M.M. Contrasting effects of anoxia on rhizome lipids in Iris species. Phytochemistry 1982, 21, 1275–1278. [Google Scholar] [CrossRef]
- Crawford, R.M.M.; Walton, J.C.; Wollenweber-Ratzer, W. Similarities between post-ischaemic injury to animal tissues and post-anoxic injury in plants. Proc. R. Soc. Edinb. 1994, 102, 325–332. [Google Scholar] [CrossRef]
- Jambunathan, N. Determination and detection of reactive oxygen species (ROS), lipid peroxidation, and electrolyte leakage in plants. Methods Mol. Biol. 2010, 639, 291–297. [Google Scholar] [CrossRef]
- Dordas, C.; Hasinoff, B.B.; Igamberdiev, A.U.; Manac’h, N.; Rivoal, J.; Hill, R.D. Expression of a stress-induced hemoglobin affects NO levels produced by alfalfa root cultures under hypoxic stress. Plant J. 2003, 35, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Dordas, C.; Rivoal, J.; Hill, R.D. Plant haemoglobins, nitric oxide and hypoxic stress. Ann. Bot. 2003, 91, 173–178. [Google Scholar] [CrossRef]
- Dordas, C.; Hasinoff, B.B.; Rivoal, J.; Hill, R.D. Class-1 hemoglobins, nitrate and NO levels in anoxic maize cell-suspension cultures. Planta 2004, 219, 66–72. [Google Scholar] [CrossRef]
- Fukao, T.; Serres, J.B. Plant responses to hypoxia-is survival a balancing act? Trends Plant Sci. 2004, 9, 449–456. [Google Scholar] [CrossRef]
- García-Mata, C.; Lamattina, L. Nitric oxide and abscisic acid cross talk in guard cells. Plant Physiol. 2002, 128. [Google Scholar] [CrossRef]
- Joudoi, T.; Shichiri, Y.; Kamizono, N.; Akaike, T.; Sawa, T.; Yoshitake, J.; Yamada, N.; Iwai, S. Nitrated cyclic gmp modulates guard cell signaling in Arabidopsis. Plant Cell 2013, 25, 558–571. [Google Scholar] [CrossRef] [PubMed]
- Igamberdiev, A.U.; Hill, R.D. Nitrate, NO and haemoglobin in plant adaptation to hypoxia: An alternative to classic fermentation pathways. J. Exp. Bot. 2004, 55, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- Hebelstrup, K.H.; Shah, J.K.; Igamberdiev, A.U. The role of nitric oxide and hemoglobin in plant development and morphogenesis. Physiol. Plant. 2013, 148, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Trevaskis, B.; Watts, R.A.; Andersson, C.R.; Llewellyn, D.J.; Hargrove, M.S.; Olson, J.S.; Dennis, E.S.; Peacock, W.J. Two hemoglobin genes in Arabidopsis thaliana: The evolutionary origins of leghemoglobins. Proc. Natl. Acad. Sci. USA 1997, 94, 12230–12234. [Google Scholar] [CrossRef] [PubMed]
- Watts, R.A.; Hunt, P.W.; Hvitved, N.A.; Hargrove, M.S.; Peacock, W.J.; Dennis, E.S. A hemoglobin from plants homologous to truncated hemoglobins of microorganisms. Proc. Natl. Acad. Sci. USA 2001, 98, 10119–10124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunt, P.W.; Klok, E.J.; Trevaskis, B.; Watts, R.A.; Ellis, M.H.; Peacock, W.J.; Dennis, E.S. Increased level of hemoglobin 1 enhances survival of hypoxic stress and promotes early growth in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2002, 99, 17197–17202. [Google Scholar] [CrossRef]
- Smagghe, B.J.; Hoy, J.A.; Percifield, R.; Kundu, S.; Hargrove, M.S.; Sarath, G.; Hilbert, J.L.; Watts, R.A.; Dennis, E.S.; Peacock, W.J.; et al. Correlations between oxygen affinity and sequence classifications of plant hemoglobins. Biopolymers 2009, 91, 1083–1096. [Google Scholar] [CrossRef]
- Kubo, H. Über hämoprotein aus den wurzelknöllchen von leguminosen. Acta Phytochim. 1939, 11, 195–200. [Google Scholar]
- Ott, T.; van Dongen, J.T.; Günther, C.; Krusell, L.; Desbrosses, G.; Vigeolas, H.; Bock, V.; Czechowski, T.; Geigenberger, P.; Udvardi, M.K. Symbiotic leghemoglobins are crucial for nitrogen fixation in legume root nodules but not for general plant growth and development. Curr. Biol. 2005, 15, 531–535. [Google Scholar] [CrossRef]
- Vieweg, M.F.; Hohnjec, N.; Küster, H. Two genes encoding different truncated hemoglobins are regulated during root nodule and arbuscular mycorrhiza symbioses of Medicago truncatula. Planta 2005, 220, 757–766. [Google Scholar] [CrossRef]
- Sáenz-Rivera, J.; Sarath, G.; Arredondo-Peter, R. Modeling the tertiary structure of a maize (Zea mays ssp. mays) non-symbiotic hemoglobin. Plant Physiol. Biochem. 2004, 42, 891–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perazzolli, M.; Dominici, P.; Romero-Puertas, M.C.; Zago, E.; Zeier, J.; Sonoda, M.; Lamb, C.; Delledonne, M. Arabidopsis nonsymbiotic hemoglobin AHb1 modulates nitric oxide bioactivity. Plant Cell 2004, 16, 2785–2794. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.R.; Hwang, S. Over-expression of NtHb1 encoding a non-symbiotic class 1 hemoglobin of tobacco enhances a tolerance to cadmium by decreasing NO (nitric oxide) and Cd levels in Nicotiana tabacum. Environ. Exp. Bot. 2015, 113, 18–27. [Google Scholar] [CrossRef]
- Bahmani, R.; Kim, D.; Na, J.; Hwang, S. Expression of the tobacco non-symbiotic class 1 hemoglobin gene Hb1 reduces cadmium levels by modulating Cd transporter expression through decreasing nitric oxide and ROS level in Arabidopsis. Front. Plant Sci. 2019, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Uchiumi, T.; Shimoda, Y.; Tsuruta, T.; Mukoyoshi, Y.; Suzuki, A.; Senoo, K.; Sato, S.; Kato, T.; Tabata, S.; Higashi, S.; et al. Expression of symbiotic and nonsymbiotic globin genes responding to microsymbionts on Lotus japonicus. Plant Cell Physiol. 2002, 43, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Bustos-Sanmamed, P.; Tovar-Méndez, A.; Crespi, M.; Sato, S.; Tabata, S.; Becana, M. Regulation of nonsymbiotic and truncated hemoglobin genes of Lotus japonicus in plant organs and in response to nitric oxide and hormones. New Phytol. 2011, 189, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Fukudome, M.; Calvo-Begueria, L.; Kado, T.; Osuki, K.; Rubio, M.C.; Murakami, E.; Nagata, M.; Kucho, K.; Sandal, N.; Stougaard, J.; et al. Hemoglobin LjGlb1-1 is involved in nodulation and regulates the level of nitric oxide in the Lotus japonicus-Mesorhizobium loti symbiosis. J. Exp. Bot. 2016, 67, 5275–5283. [Google Scholar] [CrossRef]
- Nagata, M.; Murakami, E.; Shimoda, Y.; Shimoda-Sasakura, F.; Kucho, K.; Suzuki, A.; Abe, M.; Higashi, S.; Uchiumi, T. Expression of a class 1 hemoglobin gene and production of nitric oxide in response to symbiotic and pathogenic bacteria in Lotus japonicus. Mol. Plant-Microbe Interact. 2008, 21, 1175–1183. [Google Scholar] [CrossRef]
- Trinchant, J.C.; Rigaud, J. Nitrite and nitric oxide as inhibitors of nitrogenase from soybean bacteroids. Appl. Environ. Microbiol. 1982, 44, 1385–1388. [Google Scholar]
- Kato, K.; Kanahama, K.; Kanayama, Y. Involvement of nitric oxide in the inhibition of nitrogenase activity by nitrate in Lotus root nodules. J. Plant Physiol. 2010, 167, 238–241. [Google Scholar] [CrossRef]
- Cam, Y.; Pierre, O.; Boncompagni, E.; Hérouart, D.; Meilhoc, E.; Bruand, C. Nitric oxide (NO): A key player in the senescence of Medicago truncatula root nodules. New Phytol. 2012, 196, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, Y.; Shimoda-Sasakura, F.; Kucho, K.; Kanamori, N.; Nagata, M.; Suzuki, A.; Abe, M.; Higashi, S.; Uchiumi, T. Overexpression of class 1 plant hemoglobin genes enhances symbiotic nitrogen fixation activity between Mesorhizobium loti and Lotus japonicus. Plant J. 2009, 57, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Fukudome, M.; Watanabe, E.; Osuki, K.; Imaizumi, R.; Aoki, T.; Becana, M.; Uchiumi, T. Stably-transformed Lotus japonicus plants overexpressing phytoglobin Ljglb1-1 show decreased nitric oxide levels in roots and nodules as well as delayed nodule senescence. Plant Cell Physiol. 2019, 60, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Sasakura, F.; Uchiumi, T.; Shimoda, Y.; Suzuki, A.; Takenouchi, K.; Higashi, S.; Abe, M. A class 1 hemoglobin gene from Alnus firma functions in symbiotic and nonsymbiotic tissues to detoxify nitric oxide. Mol. Plant-Microbe Interact. 2006, 19, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Schwintzer, C.R. Effect of spring flooding on endophyte differentiation, nitrogenase activity, root growth and shoot growth in Myrica gale. Plant Soil. 1985, 87, 109–124. [Google Scholar] [CrossRef]
- Shimamura, S.; Mochizuki, T.; Nada, Y.; Fukuyama, M. Secondary paerenchyma formation and its relation to nitrogen fixation in root nodules of soybean plants (Glycine max) grown under flooded conditions. Plant Prod. Sci. 2002, 5, 294–300. [Google Scholar] [CrossRef]
- Sánchez, C.; Gates, A.J.; Meakin, G.E.; Uchiumi, T.; Girard, L.; Richardson, D.J.; Bedmar, E.J.; Delgado, M.J. Production of nitric oxide and nitrosylleghemoglobin complexes in soybean nodules in response to flooding. Mol. Plant-Microbe Interact. 2010, 23, 702–711. [Google Scholar] [CrossRef]
- Fukai, E.; Soyano, T.; Umehara, Y.; Nakayama, S.; Hirakawa, H.; Tabata, S.; Sato, S.; Hayashi, M. Establishment of a Lotus japonicus gene tagging population using the exon-targeting endogenous retrotransposon LORE1. Plant J. 2012, 69, 720–730. [Google Scholar] [CrossRef]
- Urbański, D.F.; Małolepszy, A.; Stougaard, J.; Andersen, S.U. Genome-wide LORE1 retrotransposon mutagenesis and high-throughput insertion detection in Lotus japonicus. Plant J. 2012, 69, 731–741. [Google Scholar] [CrossRef]
- Małolepszy, A.; Mun, T.; Sandal, N.; Gupta, V.; Dubin, M.; Urbański, D.; Shan, N.; Bachmann, A.; Fukai, E.; Hirakawa, H.; et al. The LORE1 insertion mutant resource. Plant J. 2016, 88, 306–317. [Google Scholar] [CrossRef]
- Aoki, T.; Kamizawa, A.; Ayabe, S. Efficient Agrobacterium-mediated transformation of Lotus japonicus with reliable antibiotic selection. Plant Cell Rep. 2002, 21, 238–243. [Google Scholar] [CrossRef]
- Kaneko, T.; Nakamura, Y.; Sato, S.; Asamizu, E.; Kato, T.; Sasamoto, S.; Watanabe, A.; Idesawa, K.; Ishikawa, A.; Kawashima, K.; et al. Complete genome structure of the nitrogen-fixing symbiotic bacterium Mesorhizobium loti. DNA Res. 2000, 7, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Fåhraues, G. The infection of clover root hair by nodule bacteria studied by a single glass slide technique. Microbiology 1957, 16, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Rolny, N.; Costa, L.; Carrión, C.; Guiamet, J.J. Is the electrolyte leakage assay an unequivocal test of membrane deterioration during leaf senescence? Plant Physiol. Biochem. 2011, 49, 1220–1227. [Google Scholar] [CrossRef]
- Thordal-Christensen, H.; Zhang, Z.; Wei, Y.; Collinge, D.B. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997, 11, 1187–1194. [Google Scholar] [CrossRef]
- Signorelli, S.; Corpas, F.J.; Borsani, O.; Barroso, J.B.; Monza, J. Water stress induces a differential and spatially distributed nitro-oxidative stress response in roots and leaves of Lotus japonicus. Plant Sci. 2013, 137–146. [Google Scholar] [CrossRef]
- Jabs, T.; Dietrich, R.A.; Dangl, J.L. Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science 1996, 273, 1853–1856. [Google Scholar] [CrossRef]
- Fujie, M.; Shintaku, H.; Maeno, H.; Kajihara, R.; Usami, S.; Yamada, T. Molecular cytological analysis of cysteine proteinases from nodules of Lotus japonicus. Cytologia 2009, 74, 343–354. [Google Scholar] [CrossRef]
- Chungopast, S.; Hirakawa, H.; Sato, S.; Hanada, Y.; Saito, K.; Kawaguchi, M.; Tajima, S.; Nomura, M. Transcriptomic profiles of nodule senescence in Lotus japonicus and Mesorhizobium loti symbiosis. Plant Biotechnol. 2014, 31, 345–349. [Google Scholar] [CrossRef]
- Hossain, M.S.; Umehara, Y.; Kouchi, H. A novel fix-symbiotic mutant of Lotus japonicus, Ljsym105, shows impaired development and premature deterioration of nodule infected cells and symbiosomes. Mol. Plant Microbe Interact. 2006, 19, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.; Licausi, F.; Araújo, W.L.; Nunes-Nesi, A.; Sodek, L.; Fernie, A.R.; Van Dongen, J.T. Glycolysis and the tricarboxylic acid cycle are linked by alanine aminotransferase during hypoxia induced by waterlogging of Lotus japonicus. Plant Physiol. 2010, 152, 1501–1513. [Google Scholar] [CrossRef] [PubMed]
- Mira, M.; Hill, R.D.; Stasolla, C. Regulation of programmed cell death by phytoglobins. J. Exp. Bot. 2016, 67, 5901–5908. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.B. Long-distance signalling from roots to shoots assessed: The flooding story. J. Exp. Bot. 2002, 53, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Igamberdiev, A.U.; Stoimenova, M.; Seregélyes, C.; Hill, R.D. Class-1 hemoglobin and antioxidant metabolism in alfalfa roots. Planta 2006, 223, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.S.; Mira, M.M.; Renault, S.; Hill, R.D.; Stasolla, C. Phytoglobin expression influences soil flooding response of corn plants. Ann. Bot. 2016, 118, 919–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukudome, M.; Watanabe, E.; Osuki, K.-i.; Uchi, N.; Uchiumi, T. Ectopic or Over-Expression of Class 1 Phytoglobin Genes Confers Flooding Tolerance to the Root Nodules of Lotus japonicus by Scavenging Nitric Oxide. Antioxidants 2019, 8, 206. https://doi.org/10.3390/antiox8070206
Fukudome M, Watanabe E, Osuki K-i, Uchi N, Uchiumi T. Ectopic or Over-Expression of Class 1 Phytoglobin Genes Confers Flooding Tolerance to the Root Nodules of Lotus japonicus by Scavenging Nitric Oxide. Antioxidants. 2019; 8(7):206. https://doi.org/10.3390/antiox8070206
Chicago/Turabian StyleFukudome, Mitsutaka, Eri Watanabe, Ken-ichi Osuki, Nahoko Uchi, and Toshiki Uchiumi. 2019. "Ectopic or Over-Expression of Class 1 Phytoglobin Genes Confers Flooding Tolerance to the Root Nodules of Lotus japonicus by Scavenging Nitric Oxide" Antioxidants 8, no. 7: 206. https://doi.org/10.3390/antiox8070206



