Selenium Treatment Enhanced Clearance of Salmonella in Chicken Macrophages (HD11)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Cell Culture
2.2. Cytotoxicity Assay
2.3. Bacterial Strain and Growth Conditions
2.4. Quantification of Intracellular Bacteria Number
2.5. Maintenance of Cell Lines and Infection
2.6. RNA Sequencing of Cell Transcriptome Libraries
2.7. Quality Control and Differential Expression Analysis
2.8. Gene Ontology (GO) and Kyoto Encyclopedia of Genes (KEGG) Pathway Enrichment Analyses
2.9. Statistical Analyses
3. Results
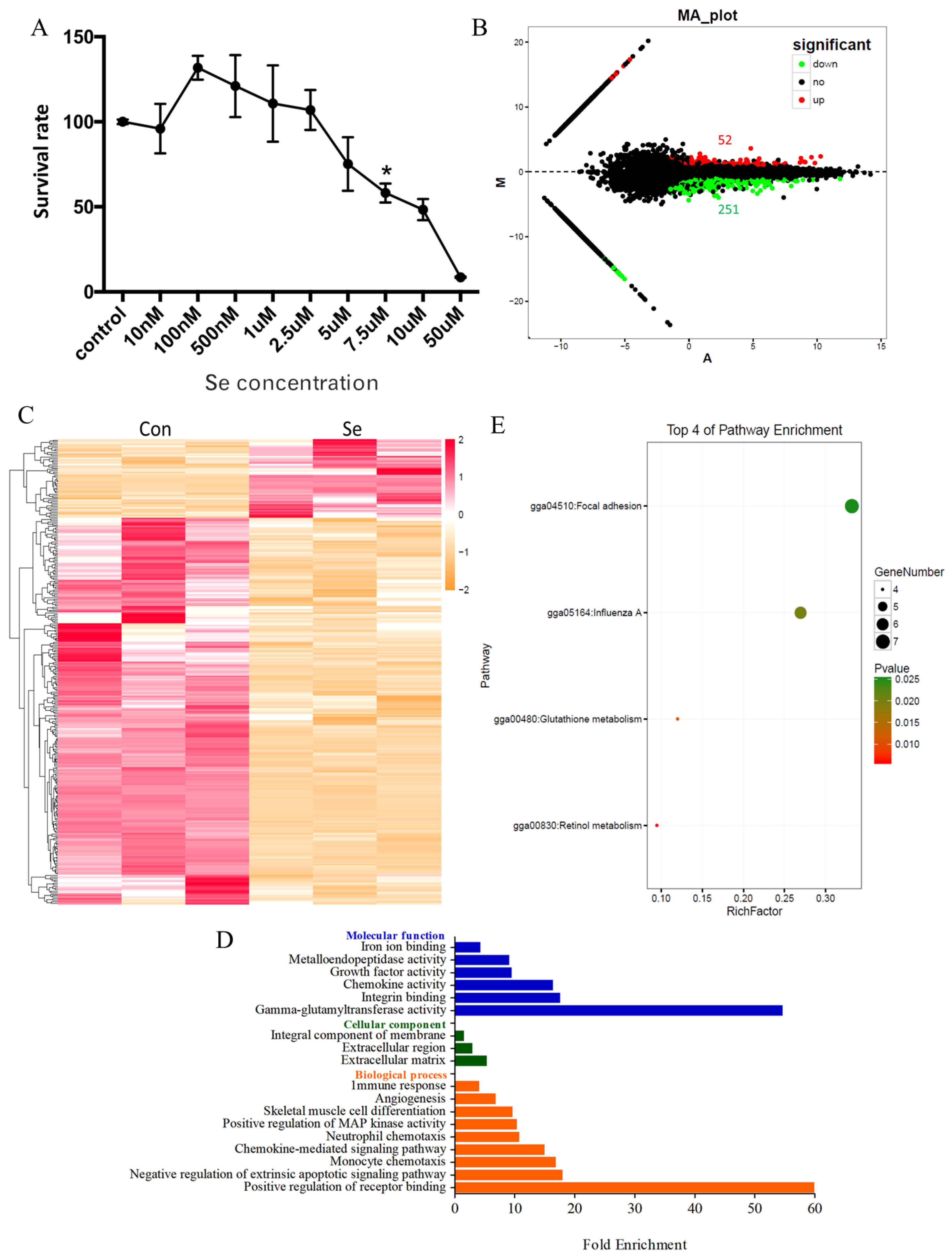
3.1. Global Transcriptome of HD11 Cells Treated with Selenium
3.2. The Global Transcriptome of HD11 Cells Infected with Salmonella
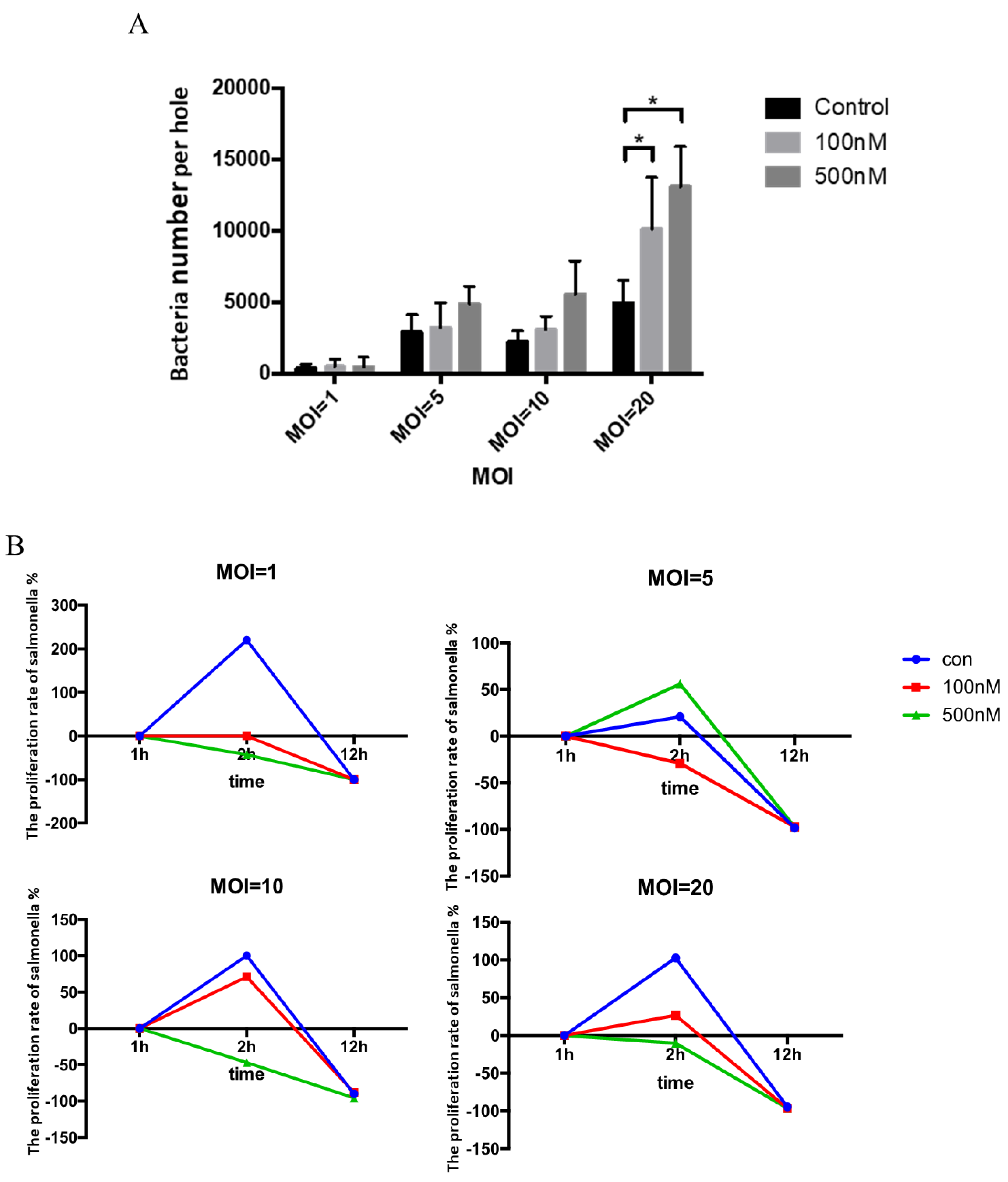
3.3. Effect of Se on Clearance of Salmonella within HD11 Cells
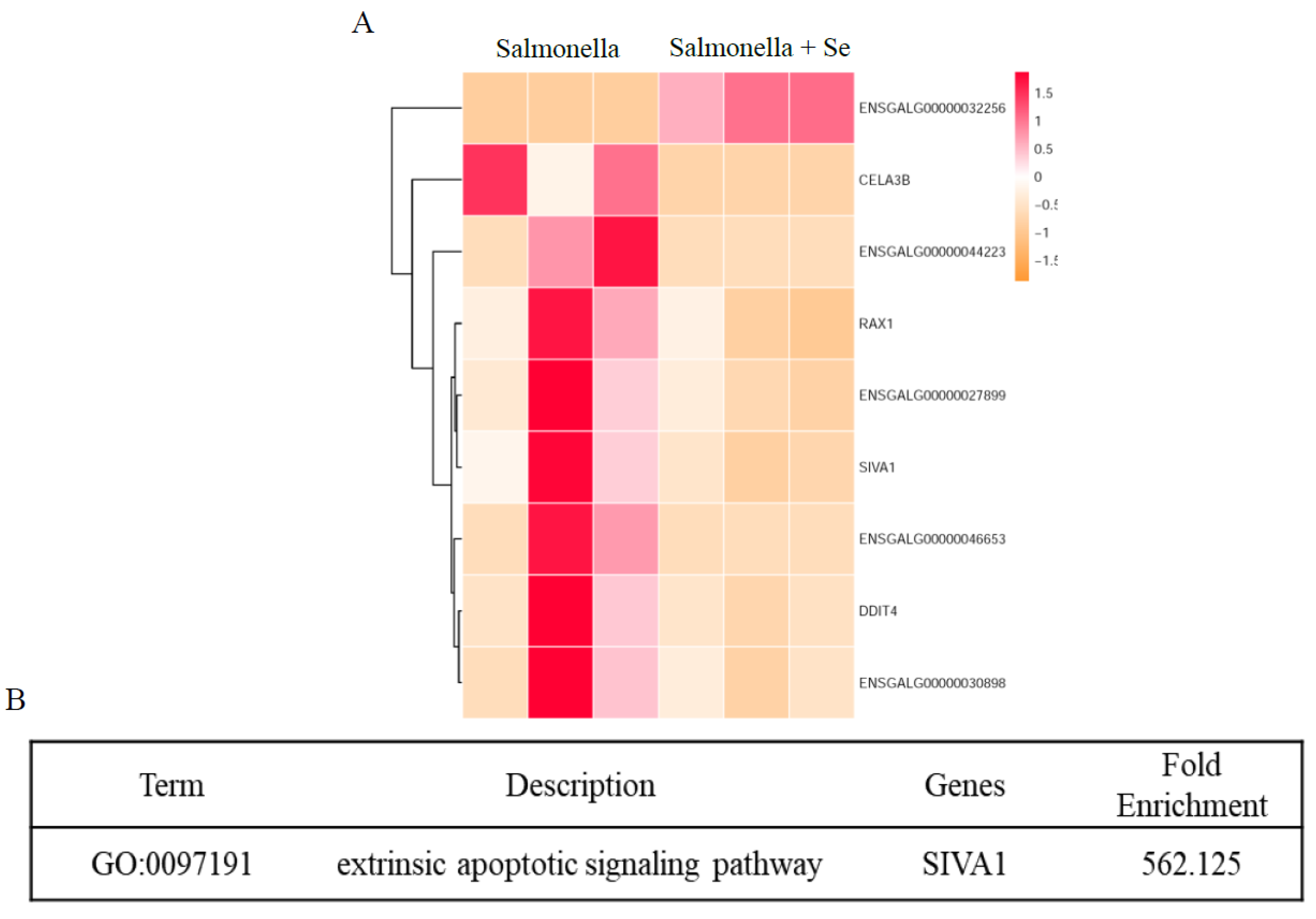
3.4. Transcriptome of HD11 Cells Infected with Salmonella Following Se Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rayman, M.P. Food-chain selenium and human health: Emphasis on intake. Br. J. Nutr. 2008, 100, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in Human Health and Disease. Antioxid. Redox Signal. 2011, 14, 1337–1383. [Google Scholar] [CrossRef] [PubMed]
- Qazi, I.H.; Angel, C.; Yang, H.; Pan, B.; Zoidis, E.; Zeng, C.J.; Han, H.; Zhou, G.B. Selenium, Selenoproteins, and Female Reproduction: A Review. Molecules 2018, 23, 3053. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Tan, H.; Mo, H.Y.; Lau, A.; Xu, Y.M. Selenium species: Current status and potentials in cancer prevention and therapy. Int. J. Mol. Sci. 2019, 20, 75. [Google Scholar] [CrossRef] [PubMed]
- Vunta, H.; Belda, B.J.; Arner, R.J.; Reddy, C.C.; Heuvel, J.P.V.; Prabhu, K.S. Selenium attenuates pro-inflammatory gene expression in macrophages. Mol. Nutr. Food Res. 2008, 52, 1316–1323. [Google Scholar] [CrossRef]
- Arthur, J.R.; McKenzie, R.C.; Beckett, G.J. Selenium in the immune system. J. Nutr. 2003, 133, 1457–1459. [Google Scholar] [CrossRef]
- Khoso, P.A.; Zhang, Y.; Yin, H.; Teng, X.; Li, S. Selenium deficiency affects immune function by influencing selenoprotein and cytokine expression in chicken spleen. Biol. Trace Elem. Res. 2019, 187, 506–516. [Google Scholar] [CrossRef]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef]
- Qazi, I.H.; Angel, C.; Yang, H.; Zoidis, E.; Pan, B.; Wu, Z.; Ming, Z.; Zeng, C.J.; Meng, Q.; Han, H. Role of Selenium and Selenoproteins in Male Reproductive Function: A Review of Past and Present Evidences. Antioxidants 2019, 8, 268. [Google Scholar] [CrossRef]
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The Role of Selenium in Inflammation and Immunity: From Molecular Mechanisms to Therapeutic Opportunities. Antioxid. Redox Signal. 2012, 16, 705–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayman, M. Selenium Intake and Status in Health & Disease. Free. Radic. Biol. Med. 2017, 112, 5. [Google Scholar]
- Avery, J.C.; Hoffmann, P.R. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Al-Quraishy, S.; Dkhil, M.A.; Wunderlich, F.; Sies, H. Dietary selenium in adjuvant therapy of viral and bacterial infections. Adv. Nutr. 2015, 6, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, F.W.; Hashimoto, A.C.; Shafer, L.A.; Dow, S.; Berry, M.J.; Hoffmann, P.R. Dietary selenium modulates activation and differentiation of CD4+ T cells in mice through a mechanism involving cellular free thiols. J. Nutr. 2010, 140, 1155–1161. [Google Scholar] [CrossRef]
- Safir, N.; Wendel, A.; Saile, R.; Chabraoui, L. The Effect of Selenium on Immune Functions of J774.1 Cells. Clin. Chem. Lab. Med. 2003, 41, 1005–1011. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Luo, J.; Hu, Y.; Wei, L.; Duan, M.; He, H. Selenium deficiency impairs host innate immune response and induces susceptibility to Listeria monocytogenes infection. BMC Immunol. 2009, 10, 55. [Google Scholar] [CrossRef]
- Smith, K.L.; Hogan, J.S.; Weiss, W.P. Dietary vitamin E and selenium affect mastitis and milk quality. J. Anim. Sci. 1997, 75, 1659–1665. [Google Scholar] [CrossRef]
- Pagmantidis, V.; Méplan, C.; Van Schothorst, E.M.; Keijer, J.; Hesketh, J.E. Supplementation of healthy volunteers with nutritionally relevant amounts of selenium increases the expression of lymphocyte protein biosynthesis genes. Am. J. Clin. Nutr. 2008, 87, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Duntas, L.H.; Benvenga, S. Selenium: An element for life. Endocrine 2015, 48, 756–775. [Google Scholar] [CrossRef]
- Pramoolsinsap, C.; Viranuvatti, V. Salmonella hepatitis. J. Gastroenterol. Hepatol. 1998, 13, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Puertollano, M.A.; Puertollano, E.; Alvarez de Cienfuegos, G.; de Pablo, M.A. Dietary antioxidants: Immunity and host defense. Curr. Top. Med. Chem. 2011, 11, 1752–1766. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Ma, Y.; Wei, S.; Pan, B.; Qi, Y.; Hou, Y.; Meng, Q.; Zhou, G.; Han, H. Dynamic changes in the global transcriptome of bovine germinal vesicle oocytes after vitrification followed by in vitro maturation. Reprod. Fertil. Dev. 2018, 30, 1298–1313. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Babu, U.S.; Sommers, K.; Harrison, L.M.; Balan, K.V. Effects of fructooligosaccharide-inulin on Salmonella-killing and inflammatory gene expression in chicken macrophages. Vet. Immunol. Immunopathol. 2012, 149, 92–96. [Google Scholar] [CrossRef]
- Hoffmann, P.R. Mechanisms by which selenium influences immune responses. Arch. Immunol. Ther. Exp. 2007, 55, 289–297. [Google Scholar] [CrossRef]
- Zheng, S.; Xing, H.; Zhang, Q.; Xue, H.; Zhu, F.; Xu, S. Pharmacokinetics of sodium selenite administered orally in blood and tissues of selenium-deficient ducklings. Biol. Trace Elem. Res. 2019, 190, 509–516. [Google Scholar] [CrossRef]
- Combs, G.F. Selenium in global food systems. Br. J. Nutr. 2001, 85, 517–547. [Google Scholar] [CrossRef] [Green Version]
- Surai, P.F.; Fisinin, V.I. Selenium in Pig Nutrition and Reproduction: Boars and Semen Quality—A Review. Asian Australas. J. Anim. Sci. 2015, 28, 730–746. [Google Scholar] [CrossRef]
- Hossain, K.F.B.; Rahman, M.M.; Sikder, M.T.; Saito, T.; Hosokawa, T.; Kurasaki, M. Inhibitory effects of selenium on cadmium-induced cytotoxicity in PC12 cells via regulating oxidative stress and apoptosis. Food Chem. Toxicol. 2018, 114, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Xiang, N.; Domann, F.E.; Zhong, W. Expression of p53 enhances selenite-induced superoxide production and apoptosis in human prostate cancer cells. Cancer Res. 2006, 66, 2296–2304. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Oberley, T.D. Redox-mediated effects of selenium on apoptosis and cell cycle in the LNCaP human prostate cancer cell line. Cancer Res. 2001, 61, 7071–7078. [Google Scholar] [PubMed]
- Aribi, M.; Meziane, W.; Habi, S.; Boulatika, Y.; Marchandin, H.; Aymeric, J.L. Macrophage Bactericidal Activities against Staphylococcus aureus Are Enhanced In Vivo by Selenium Supplementation in a Dose-Dependent Manner. PLoS ONE 2015, 10, e0135515. [Google Scholar] [CrossRef] [PubMed]
- Cifelli, C.J.; Ross, A.C. Chronic Vitamin a Status and Acute Repletion with Retinyl Palmitate Are Determinants of the Distribution and Catabolism of all-trans-Retinoic Acid in Rats. J. Nutr. 2007, 137, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Zolfaghari, R. Cytochrome P450s in the regulation of cellular retinoic acid metabolism. Annu. Rev. Nutr. 2011, 31, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Balakrishna, S.; Prabhune, A.A. Gamma-glutamyl transferases: A structural, mechanistic and physiological perspective. Front. Biol. 2014, 9, 51–65. [Google Scholar] [CrossRef]
- Bui, T.T.; Nitta, R.T.; Kahn, S.A.; Razavi, S.M.; Agarwal, M.; Aujla, P.; Gholamin, S.; Recht, L.; Li, G. γ-Glutamyl transferase 7 is a novel regulator of glioblastoma growth. BMC Cancer 2015, 15, 225. [Google Scholar] [CrossRef]
- Dominici, S.; Paolicchi, A.; Corti, A.; Maellaro, E.; Pompella, A. Prooxidant Reactions Promoted by Soluble and Cell-Bound γ-Glutamyltransferase Activity. Methods Enzymol. 2005, 401, 484–501. [Google Scholar]
- Szulzewsky, F.; Pelz, A.; Feng, X.; Synowitz, M.; Markovic, D.; Langmann, T.; Holtman, I.R.; Wang, X.; Eggen, B.J.L.; Boddeke, H.W.G.M.; et al. Glioma-Associated Microglia/Macrophages Display an Expression Profile Different from M1 and M2 Polarization and Highly Express Gpnmb and Spp1. PLoS ONE 2015, 10, e0116644. [Google Scholar] [CrossRef]
- Catrysse, L.; Vereecke, L.; Beyaert, R.; Van Loo, G. A20 in inflammation and autoimmunity. Trends Immunol. 2014, 35, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Kattah, M.G.; Malynn, B.A.; Ma, A. Ubiquitin-Modifying Enzymes and Regulation of the Inflammasome. J. Mol. Biol. 2017, 429, 3471–3485. [Google Scholar] [CrossRef] [PubMed]
- Kool, M.; Van Loo, G.; Waelput, W.; De Prijck, S.; Muskens, F.; Sze, M.; Van Praet, J.; Branco-Madeira, F.; Janssens, S.; Reizis, B.; et al. The Ubiquitin-Editing Protein A20 Prevents Dendritic Cell Activation, Recognition of Apoptotic Cells, and Systemic Autoimmunity. Immunity 2011, 35, 82–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vereecke, L.; Beyaert, R.; Van Loo, G. The ubiquitin-editing enzyme A20 (TNFAIP3) is a central regulator of immunopathology. Trends Immunol. 2009, 30, 383–391. [Google Scholar] [CrossRef]
- Onose, A.; Hashimoto, S.; Hayashi, S.; Maruoka, S.; Kumasawa, F.; Mizumura, K.; Jibiki, I.; Matsumoto, K.; Gon, Y.; Kobayashi, T.; et al. An inhibitory effect of A20 on NF-κB activation in airway epithelium upon influenza virus infection. Eur. J. Pharmacol. 2006, 541, 198–204. [Google Scholar] [CrossRef]
- Lin, R.; Yang, L.; Nakhaei, P.; Sun, Q.; Sharif-Askari, E.; Julkunen, I.; Hiscott, J. Negative regulation of the retinoic acid-inducible gene I-induced antiviral state by the ubiquitin-editing protein A20. J. Biol. Chem. 2006, 281, 2095–2103. [Google Scholar] [CrossRef]
- Saitoh, T.; Yamamoto, M.; Miyagishi, M.; Taira, K.; Nakanishi, M.; Fujita, T.; Akira, S.; Yamamoto, N.; Yamaoka, S. A20 is a negative regulator of IFN regulatory factor 3 signaling. J. Immunol. 2005, 174, 1507–1512. [Google Scholar] [CrossRef]
- Prabhu, K.S.; Zamamiri-Davis, F.; Stewart, J.B.; Thompson, J.T.; Sordillo, L.M.; Reddy, C.C. Selenium deficiency increases the expression of inducible nitric oxide synthase in RAW 264.7 macrophages: Role of nuclear factor-κB in up-regulation. Biochem. J. 2002, 366, 203–209. [Google Scholar] [CrossRef]
- Krammer, P.H. CD95′s deadly mission in the immune system. Nature 2000, 407, 789–795. [Google Scholar] [CrossRef]
- Mizuno, T.; Zhong, X.; Rothstein, T. Fas-induced apoptosis in B cells. Apoptosis 2003, 8, 451–460. [Google Scholar] [CrossRef]
- Resch, U.; Schichl, Y.M.; Winsauer, G.; Gudi, R.; Prasad, K.; de Martin, R. Siva1 is a XIAP-interacting protein that balances NFκB and JNK signalling to promote apoptosis. J. Cell Sci. 2009, 122, 2651–2661. [Google Scholar] [CrossRef] [PubMed]
- Py, B.; Slomianny, C.; Auberger, P.; Petit, P.X.; Benichou, S. Siva-1 and an alternative splice form lacking the death domain, Siva-2, similarly induce apoptosis in T lymphocytes via a caspase-dependent mitochondrial pathway. J. Immunol. 2004, 172, 4008–4017. [Google Scholar] [CrossRef]
- Prasad, K.; Ao, Z.; Yoon, Y.; Wu, M.X.; Rizk, M.; Jacquot, S.; Schlossman, S.F. CD27, a member of the tumor necrosis factor receptor family, induces apoptosis and binds to Siva, a proapoptotic protein. Proc. Natl. Acad. Sci. USA 1997, 94, 6346–6351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, Y.; Ao, Z.; Cheng, Y.; Schlossman, S.F.; Prasad, K.V.S. Murine Siva-1 and Siva-2, alternate splice forms of the mouse Siva gene, both bind to CD27 but differentially transduce apoptosis. Oncogene 1999, 18, 7174–7179. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Huang, J.; Nie, Y.; Qazi, I.H.; Cao, Y.; Wang, L.; Ai, Y.; Zhou, G.; Wu, K.; Han, H. Selenium Treatment Enhanced Clearance of Salmonella in Chicken Macrophages (HD11). Antioxidants 2019, 8, 532. https://doi.org/10.3390/antiox8110532
Liu Z, Huang J, Nie Y, Qazi IH, Cao Y, Wang L, Ai Y, Zhou G, Wu K, Han H. Selenium Treatment Enhanced Clearance of Salmonella in Chicken Macrophages (HD11). Antioxidants. 2019; 8(11):532. https://doi.org/10.3390/antiox8110532
Chicago/Turabian StyleLiu, Zhexi, Jianwei Huang, Yijuan Nie, Izhar Hyder Qazi, Yutao Cao, Linli Wang, Yue Ai, Guangbin Zhou, Keliang Wu, and Hongbing Han. 2019. "Selenium Treatment Enhanced Clearance of Salmonella in Chicken Macrophages (HD11)" Antioxidants 8, no. 11: 532. https://doi.org/10.3390/antiox8110532






