In Vitro Antioxidant, Anti-Biofilm, and Solar Protection Activities of Melocactus zehntneri (Britton & Rose) Pulp Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Pulp Extract Preparation
2.3. Total Content of Phenolic Compounds, Sugar, and Protein
2.3.1. Phytochemistry Screening by Thin Layer Chromatography (TLC)
2.3.2. Cellulose Descendant Thin Layer Chromatography
2.4. In Vitro Antioxidant Activity
2.4.1. Total Antioxidant Capacity (TAC)
2.4.2. Hydroxyl Radical Scavenging Activity
2.4.3. Ferric Chelating
2.4.4. Copper Chelating
2.4.5. Superoxide Scavenging Activity
2.4.6. Reducing Power Test
2.4.7. DPPH Free Radical Scavenging Activity
2.5. Biofilm Assays
2.5.1. Microorganisms and Inoculum Preparation
2.5.2. Determination of Minimum Inhibitory Concentration (MIC) and Determination of Minimum Bactericidal Concentration (MBC)
2.5.3. Evaluation of Anti-Biofilm Activity of M. zehntneri Extracts against S. epidermidis 70D MRSE
2.6. Cell Viability
2.6.1. MTT Reduction Test
2.6.2. Cell Viability Test against Oxidative Stress with H2O2
2.7. In Vitro Determination of Solar Photoprotection Factor
2.8. Statistical Analysis
3. Results
3.1. Phytochemical Screening
3.2. In Vitro Antioxidant Assay
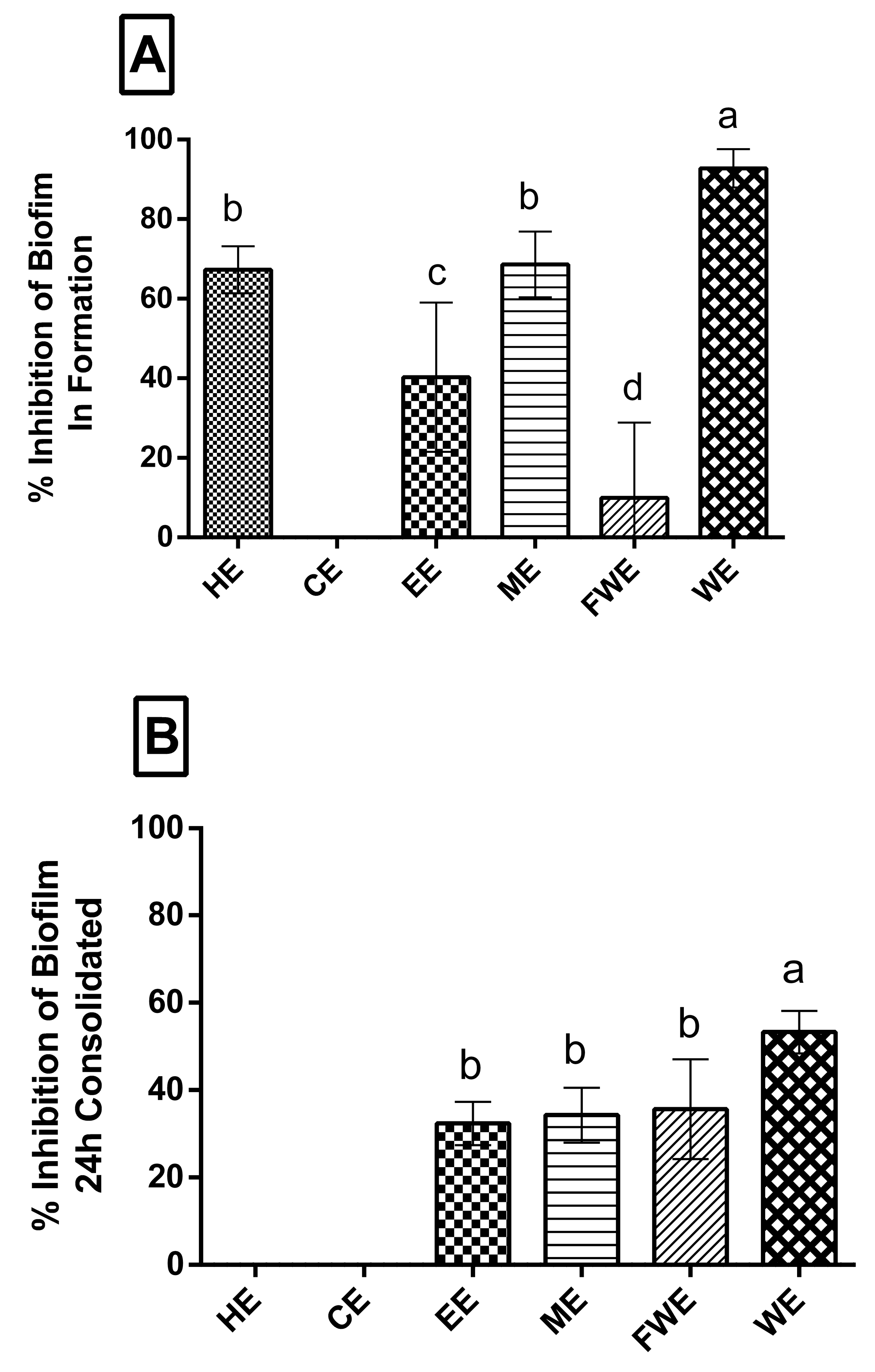
3.3. Biofilm Assay
3.4. Cell Viability
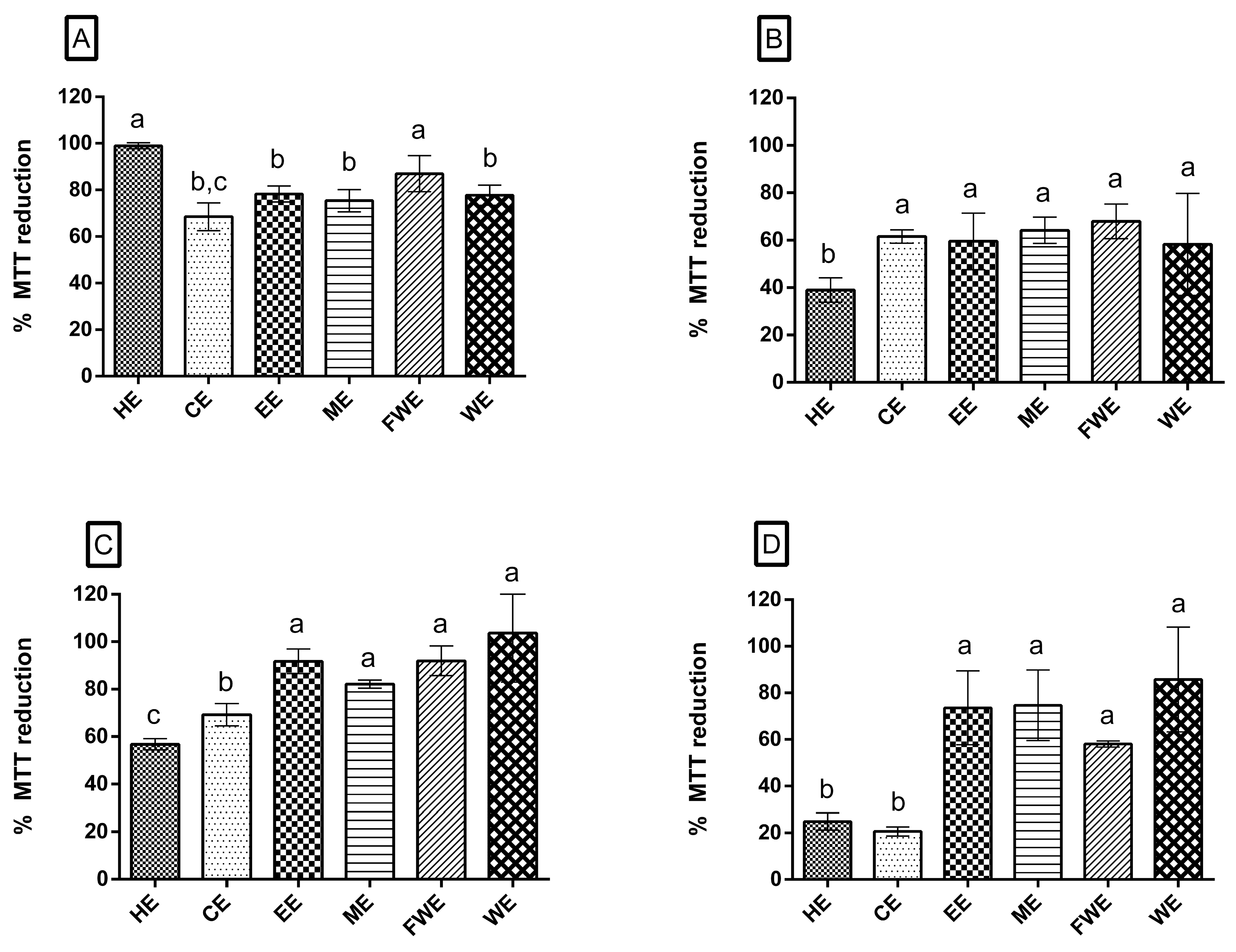
3.4.1. MTT Assay
3.4.2. Oxidative Stress
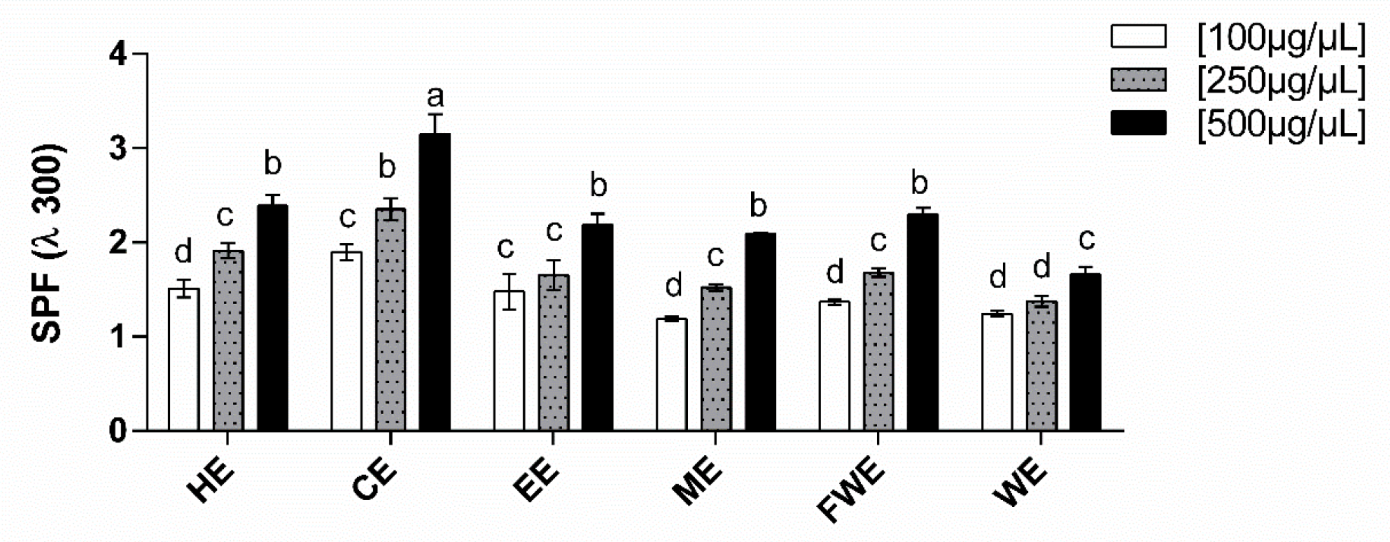
3.5. Solar Protection Factor (SPF)
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gusev, E.Y.; Zotova, N.V. Cellular stress and general pathological processes. Curr. Pharm. Des. 2019, 25, 251–297. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gong, Y.; Zhoub, B.; Yang, J.; Cheng, Y.; Zhao, J.; Qi, M. Ursolic acid ameliorates oxidative stress, inflammation and fibrosis in diabetic cardiomyopathy rats. Biomed. Pharmacother. 2018, 97, 1461–1467. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Armendáriz-Barragán, B.; Zafar, N.; Badri, W.; Galindo-Rodríguez, G.R.; Kabbaj, D.; Fessi, H.; Elaissari, A. Plant extracts: From encapsulation to application. Expert Opin. Drug Deliv. 2016, 13, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Davies, J.A.; Medeiros, H.G.M.; Mascio, P.D. Formation and repair of oxidative generated damage in cellular DNA. Free Radic. Biol. Med. 2017, 107, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Zhao, X.; Feng, J.; Song, F.; Pan, Y. Ursolic acid attenuates beta-amyloid-induced memory impairment in mice. Arq. Neuro-Psiquiatr. 2016, 74, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Trentin, D.S.; Giordani, R.B.; Zimmerb, K.R.; Silva, A.G.; Silva, M.V.; Correia, M.T.S.; Baumvold, I.J.R.E.; Macedo, A.J. Potential of medicinal plants from the Brazilian semi-arid region (Caatinga) against Staphylococcus epidermidis planktonic and biofilm lifestyles. J. Ethnopharmacol. 2011, 137, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Nandakumar, N.; Rengarajan, T.; Palaniswami, R.; Gnanadhas, E.N.; Lakshminarasaiah, U.; Gopas, J.; Nishigaki, I. Antioxidants and human diseases. Clin. Chim. Acta 2014, 25, 332–347. [Google Scholar] [CrossRef]
- Zappi, D.; Taylor, N.; Ribeiro, S.; Machado, M.; Moraes, E.M.; Calvente, A.B.; Correia, D.; Larocca, J.; Assis, J.G.; Aona, L.; et al. Plano De Ação Nacional Para A Conservação Das Cactáceas-Série Espécies Ameaçadas, 24th ed.; Instituto Chico Mendes de Conservação da Biodiversidade, Icmbio: Brasília, Brazil, 2011; ISBN 978-85-61842-00-01. [Google Scholar]
- Taylor, N.P.; Zappi, D.C. Cacti of Eastern Brazil; Royal Botanic Gardens, Kew: London, UK, 2004; p. 499. [Google Scholar]
- Menezes, M.O.T.; Taylor, N.P.; Loiola, M.I.B. Flora do Ceará, Brazil: Cactácea. Rodriguésia 2013, 64, 757–774. [Google Scholar] [CrossRef]
- Silva, V.A. Diversidade de uso das cactáceas no nordeste do Brasil: Uma revisão. Rev. Gaia Scie 2015, 9, 175–182. [Google Scholar]
- Kleinschmidt, S.; Huygens, F.; Faoagali, J.; Rathnayake, I.U.; Hafner, L.M. Staphylococcus epidermidis as a cause of bacteremia. Future Microbiol. 2015, 10, 1859–1879. [Google Scholar] [CrossRef] [PubMed]
- CPTEC/INPE. Available online: http://clima1.cptec.inpe.br/monitoramentobrasil/pt (accessed on 18 June 2019).
- Athukorala, Y.; Kim, K.N.; Jeon, Y.J. Antiproliferative and antioxidant properties of an enzymatic hydrolysate from brown alga Ecklonia cava. Food Chem. Toxicol. 2006, 44. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zucolotto, S.M.; Palermo, J.A.; Schenkel, E.P. Phytochemical study of P. edulis forma flavicarpa Degener roots. Acta Farmacêutica Bonaerense 2006, 25, 5–9. [Google Scholar]
- Melo-Silveira, R.F.; Fidelis, G.P.; Pereira, C.M.S.S.; Telles, C.B.S.; Dantas, S.N.; Dantas-Santos, N.; de Oliveira-Elias, S.; Ribeiro, V.B.; Barth, A.L.; Macedo, A.J.; et al. In vitro antioxidant, anticoagulant and antimicrobial activity and in inhibition of cancer cell proliferation by xylan extracted from corncobs. Int. J. Mol. Sci. 2012, 13, 409–426. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl radical scavenging activity of compatible solute. Phytochemistry 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Zhang, Z.; Li, Z. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2008, 42, 127–132. [Google Scholar] [CrossRef]
- Anton, A. Colorimetric estimulation of aluminium with pyrocatechol violet. Anal. Chem. 1960, 32, 725–726. [Google Scholar] [CrossRef]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agri. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Breakpoints, C. CLSI Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Stepanovic, S.; Vukovic, D.; Hola, V.; Bonaventura, G.; Djukic, S.; Irkovic, I.C.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by Staphylococci. J. Compil. 2007, 115, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Mansur, J.S.; Breder, M.N.R.; Mansur, M.C.D.; Azulay, R.D. Determinaçäo do fator de proteção solar por espectrofotometria. An. Bras. Dermatol 1986, 61, 121–124. [Google Scholar]
- Nascimento, A.K.; Melo-Silveira, R.F.; Dantas-Santos, N.; Fernandes, J.M.; Zucolotto, S.M.; Rocha, H.A.O.; Scortecci, K.C. Antioxidant and antiproliferative activities of leaf extracts from Plukenetia volubilis linneo (Euphorbiaceae). Evid. Based Complement Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Somogy, A.; Rosta, K.; Pusztai, P.; Tulassay, Z.; Nagy, G. Antioxidant measurements. Physiol. Meas. 2007, 28, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, M. The success of natural products in drug discovery. Pharmacol. Pharm. 2013, 4, 17–31. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [Green Version]
- Andreu, L.; Nuncio-Jáuregui, N.; Carbonell-Barrachina, Á.A.; Legua, P.; Hernández, F. Antioxidant properties and chemical characterization of Spanish Opuntia ficus-indica Mill. cladodes and fruits. J. Sci. Food Agric. 2018, 98, 1566–1573. [Google Scholar] [CrossRef]
- Nunes, E.N.; Lemos, D.M.; Silva, S.F.; Rocha, A.P.T.; Lucena, C.M.; Meiado, M.V.; Lucena, R.F.P. Quantification physicochemical in Melon Cactus [Melocactus zehntneri (Britton & Rose) Luetzelburg-Cactaceae]. Rev. Bras. Plantas Med. 2016, 18, 81–88. [Google Scholar] [CrossRef]
- Ramos-Hryb, A.B.; Pazini, F.L.; Kaster, M.P.; Rodrigues, A.L.S. Therapeutic potential of ursolic acid to manage neurodegenerative and psychiatric diseases. CNS Drugs 2017, 31, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Li, T.; Tian, J.X.; Xi, P.; Liu, R.H. Ursolic acid, a potential anticancer compound for breast cancer therapy. Crit. Rev. Food Sci. Nutr. 2017, 58, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, K.; Koike, S.; Ogasawara, Y.; Takahashi, K.; Koyama, K.; Kinoshita, K. Inhibition of amyloid β aggregation and protective effect on SH-SY5Y cells by triterpenoid saponins from the cactus Polaskia chichipe. Bioorg. Med. Chem. 2017, 25, 3377–3383. [Google Scholar] [CrossRef] [PubMed]
- Sáenz, C.; Sepúlveda, E.; Matsuhiro, B. Opuntia spp. mucilage’s: A functional component with industrial perspectives. J. Arid Environ. 2004, 57, 275–290. [Google Scholar] [CrossRef]
- Du, X.; Mu, H.; Zhou, S.; Zhang, Y.; Zhu, X. Chemical analysis and antioxidant activity of polysaccharides extracted from Inonotus obliquus sclerotia. Int. J Biol. Macromol. 2013, 62, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Vela, D. Keeping heart homeostasis in check through the balance of iron metabolism. Acta Physiol. 2019, 4, e13324. [Google Scholar] [CrossRef]
- Tesoriere, L.; Butera, D.; Pintaudi, A.M.; Allegra, M.; Livrea, M.A. Supplementation with cactus pear (Opuntia ficus-indica) fruit decreases oxidative stress in healthy humans: A comparative study with vitamin C. Am. J. Clin. Nutr. 2004, 80, 391–395. [Google Scholar] [CrossRef]
- Bayar, N.; Kriaa, M.; Kammoun, R. Extraction and characterization of three polysaccharides extracted from Opuntia ficus indica cladodes. Int. J. Biol. Macromol. 2016, 92, 441–450. [Google Scholar] [CrossRef]
- Hwang, T.L.; Shen, H.I.; Liu, F.C.; Tsai, H.I.; Wu, Y.C.; Chang, F.R. Ursolic acid inhibits superoxide production in activated neutrophils and attenuates trauma-hemorrhage shock induced organ injury in rats. PLoS ONE 2014, 9, e111365. [Google Scholar] [CrossRef]
- Araújo, I.D.R.; Aquino, N.C.; Guerra, A.C.V.A.; Almeida, R.F., Jr.; Araújo, R.M.; Araújo, R.F., Jr.; Farias, K.J.S.; Fernandes, J.V.; Andrade, V.S. Chemical composition and evaluation of the antibacterial and cytotoxic activities of the essential oil from the leaves of Myracrodruon urundeuva. BMC Complement. Altern. Med. 2017, 17, 419. [Google Scholar] [CrossRef]
- Barbosa, P.B.B.M.; de Oliveira, J.M.; Chagas, J.M.; Rabelo, L.M.A.; de Medeiros, G.F.; Giodani, R.B.; Silva, E.A.; Uchoa, A.F.; Ximenes, F.F.M. Evaluation of seed extracts from plants found in the Caatinga biome for the control of Aedes aegypti. Parasitol. Res. 2014, 113, 3565–3580. [Google Scholar] [CrossRef] [PubMed]
- Nunes, P.H.M.; Martins, M.C.C.; Oliveira, R.C.M.; Chaves, M.H.; Sousa, E.A.; Leite, J.R.S.A.; Véras, L.M.; Almeida, F.R.C. Gastric antiulcerogenic and hypokinetic activities of Terminalia fagifolia Mart. & Zucc. (Combretaceae). Biomed. Res. Int. 2014, 1–15. [Google Scholar] [CrossRef]
- Araujo, A.R.; Quelemes, P.V.; Perfeito, M.L.G.; Lima, L.I.; Sá, M.C.; Nunes, P.H.M.; Joanittis, G.A.; Eaton, P.; Soares, M.J.S.; Leite, J.R.S.A. Antibacterial, antibiofilm and cytotoxic activities of Terminalia fagifolia Mart. extract and fractions. Ann. Clin. Microbiol. Antimicrob. 2015, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Blando, F.; Russo, R.; Negro, C.; Bellis, L.; Frassinetti, S. Antimicrobial and antibiofilm activity against Staphylococcus aureus of Opuntia ficus-indica (L.) Mill. cladode polyphenolic extracts. Antioxidants 2019, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Lefsih, A.K.; Delattre, B.C.; Pierre, B.G.; Michaud, B.P.; Aminabhavic, T.M.; Dahmounea, F.; Madania, K. Extraction, characterization and gelling behavior enhancement of pectins from the cladodes of Opuntia ficus indica. Int. J. Biol. Macromol. 2016, 82, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Radad, K.; Gille, G.; Liu, L.; Rausch, W.D. Use of ginseng in medicine with emphasis in neurodegenerative disorders. J. Pharmacol. Sci. 2006, 100, 175–186. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.R.; Enayatifard, K.M.M.; Ghaffarloo, S.M.; Yazdani, C.J. Correlation between sun protection factor and antioxidant activity, phenol and flavonoid contents of some medicinal plants. Iran. J. Pharm. Res. 2014, 13, 1041–1047. [Google Scholar]
- Wagemaker, T.A.L.; Carvalho, C.R.L.; Maia, N.B. Sun protection factor, content and composition of lipid fraction of green coffee beans. Ind. Crops Prod. 2011, 33, 469–473. [Google Scholar] [CrossRef] [Green Version]
- Saewan, N.; Jimtaisong, A. Natural products as photoprotection. J. Cosmet. Dermatol. 2015, 14, 47–63. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aquino-Martins, V.G.d.Q.; Melo, L.F.M.d.; Silva, L.M.P.; Targino de Lima, T.R.; Fernandes Queiroz, M.; Viana, R.L.S.; Zucolotto, S.M.; Andrade, V.S.; Rocha, H.A.O.; Scortecci, K.C. In Vitro Antioxidant, Anti-Biofilm, and Solar Protection Activities of Melocactus zehntneri (Britton & Rose) Pulp Extract. Antioxidants 2019, 8, 439. https://doi.org/10.3390/antiox8100439
Aquino-Martins VGdQ, Melo LFMd, Silva LMP, Targino de Lima TR, Fernandes Queiroz M, Viana RLS, Zucolotto SM, Andrade VS, Rocha HAO, Scortecci KC. In Vitro Antioxidant, Anti-Biofilm, and Solar Protection Activities of Melocactus zehntneri (Britton & Rose) Pulp Extract. Antioxidants. 2019; 8(10):439. https://doi.org/10.3390/antiox8100439
Chicago/Turabian StyleAquino-Martins, Verônica Giuliani de Queiroz, Luciana Fentanes Moura de Melo, Larissa Marina Pereira Silva, Thales Rodrigo Targino de Lima, Moacir Fernandes Queiroz, Rony Lucas Silva Viana, Silvana Maria Zucolotto, Vania Sousa Andrade, Hugo Alexandre Oliveira Rocha, and Katia Castanho Scortecci. 2019. "In Vitro Antioxidant, Anti-Biofilm, and Solar Protection Activities of Melocactus zehntneri (Britton & Rose) Pulp Extract" Antioxidants 8, no. 10: 439. https://doi.org/10.3390/antiox8100439






