Red-Osier Dogwood Extracts Prevent Inflammatory Responses in Caco-2 Cells and a Caco-2 BBe1/EA.hy926 Cell Co-Culture Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Maintenance of Monolayer Caco-2 Cells
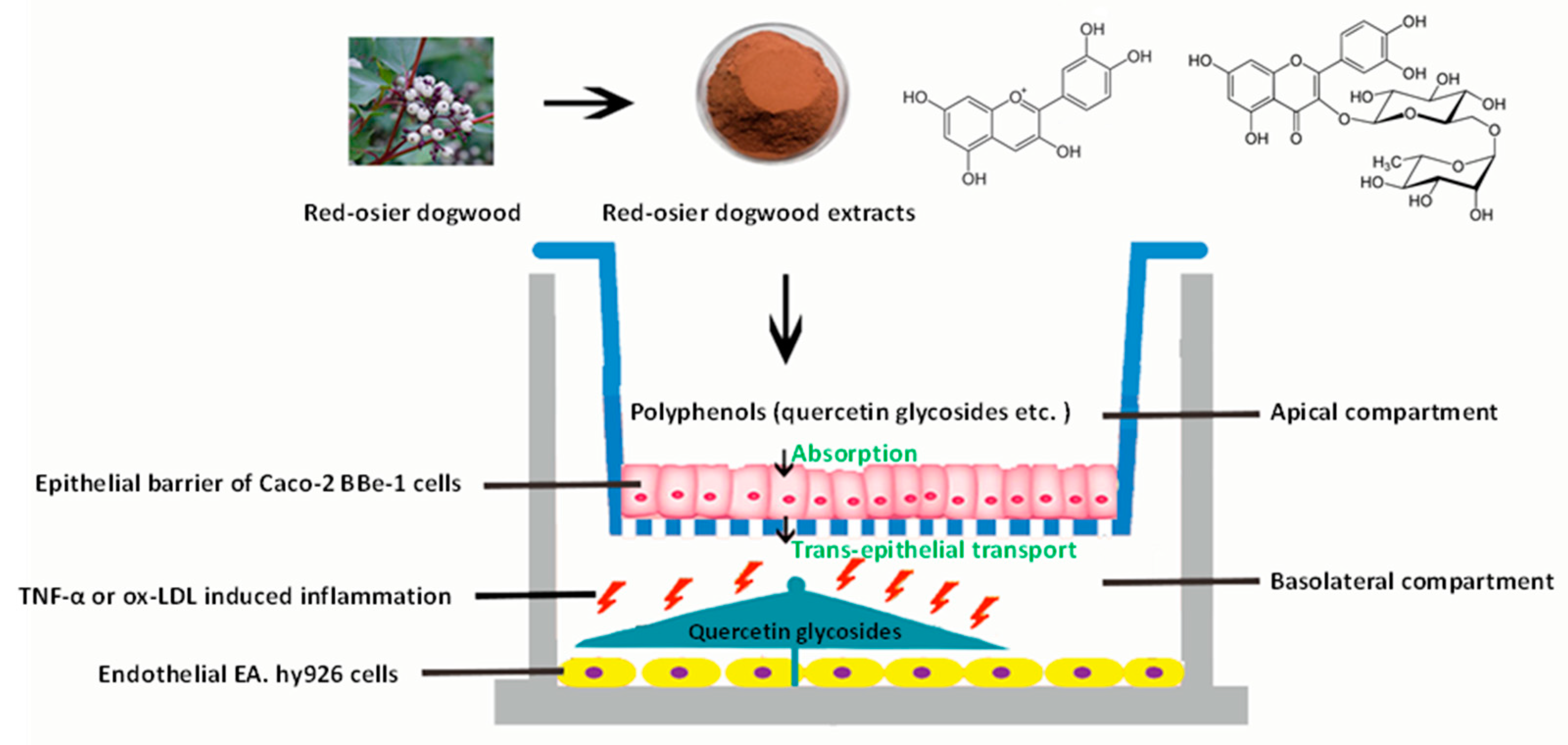
2.3. Co-Culture Model of Caco-2 BBe1/EA.hy926 Cells
2.4. Determination of RDE Cytotoxicity on Caco-2 Cells and Caco-2 BBe1 Cells
2.5. Anti-Inflammatory Experiments on Monolayer Caco-2 Cells
2.6. Anti-Inflammatory Experiments on EA.hy926 Cells in the Caco-2 BBe1/ EA.hy926 Cells Co-Culture Model
2.7. Absorption and Transepithelial Transport Experiments
2.8. Interleukin-8 (IL-8) Measurements
2.9. RNA Extraction and Real-Time PCR
2.10. Statistical Analysis
3. Results
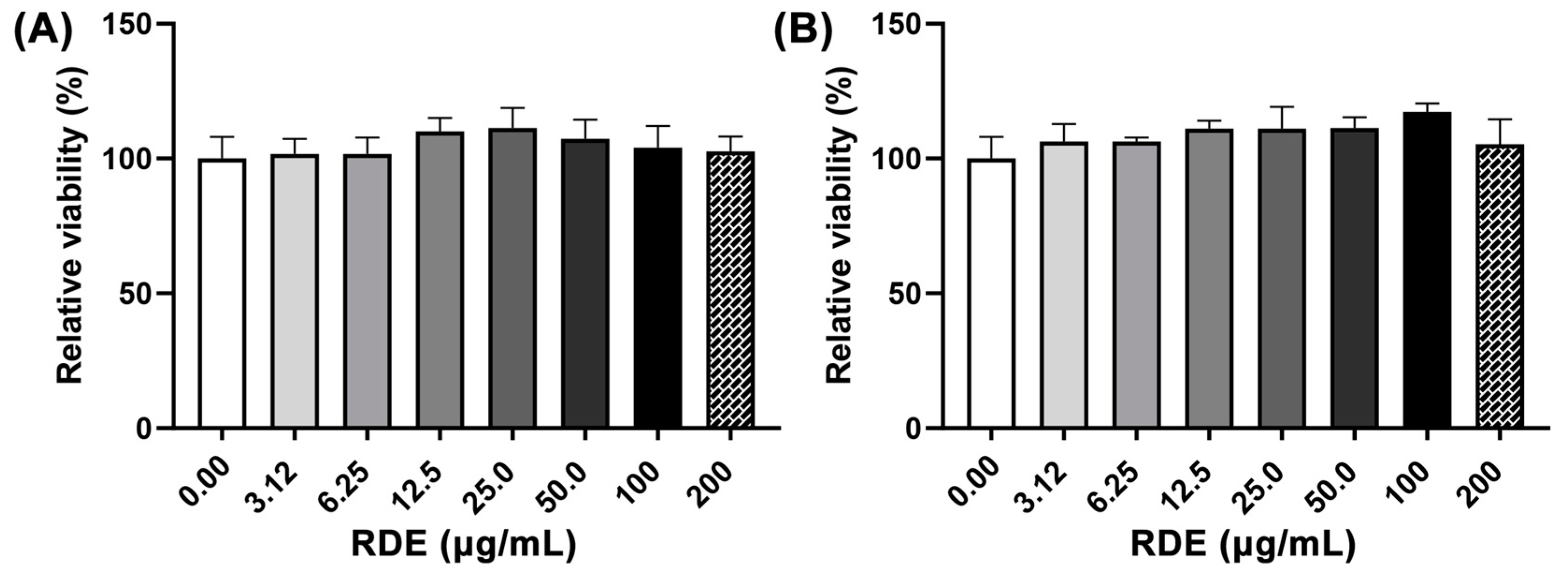
3.1. Cytotoxicity of RDE on Caco-2 and Caco-2 BBe1 Cells
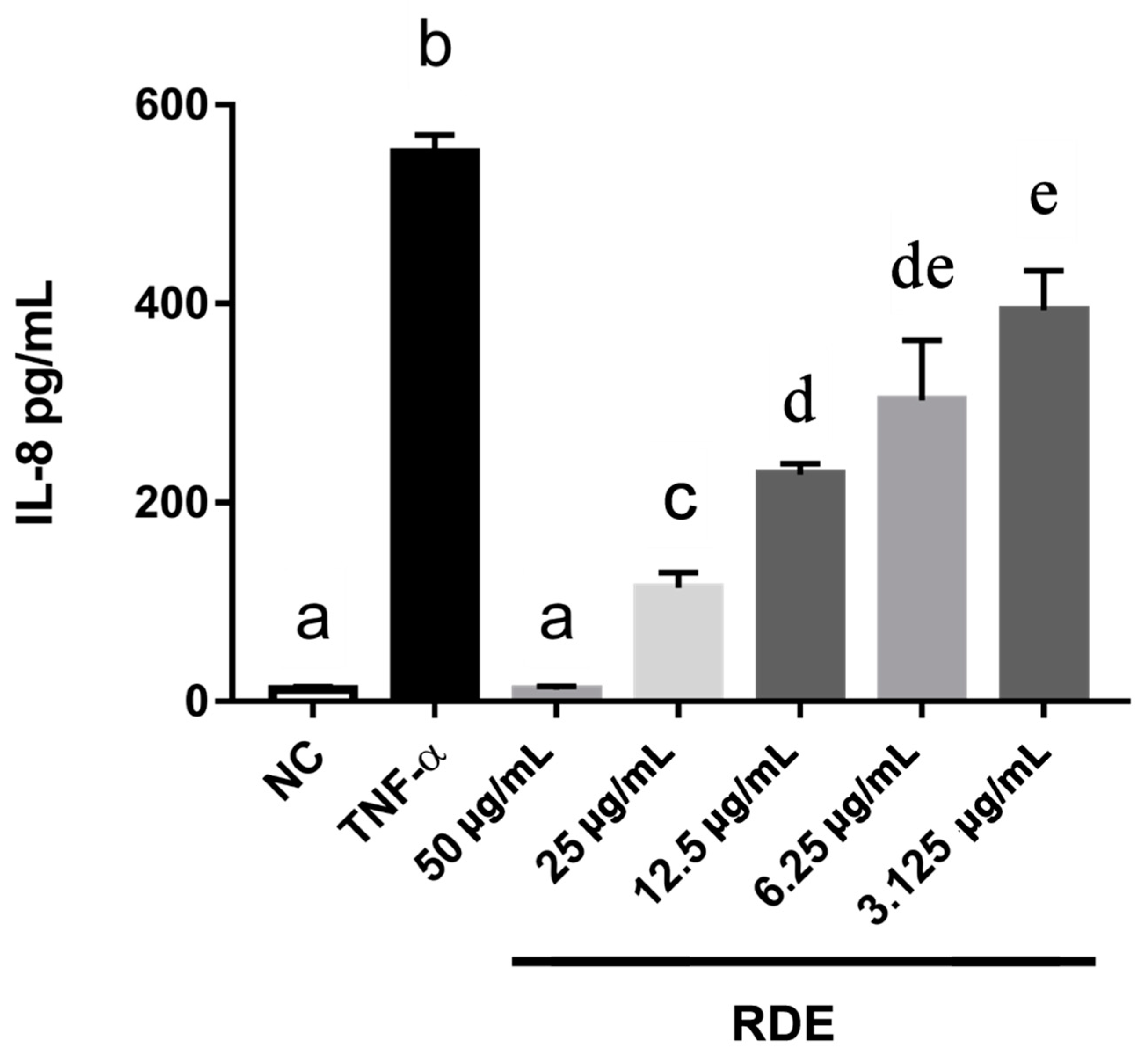
3.2. Dose-Effect of RDE on the IL-8 Production in Monolayer Caco-2 Cells
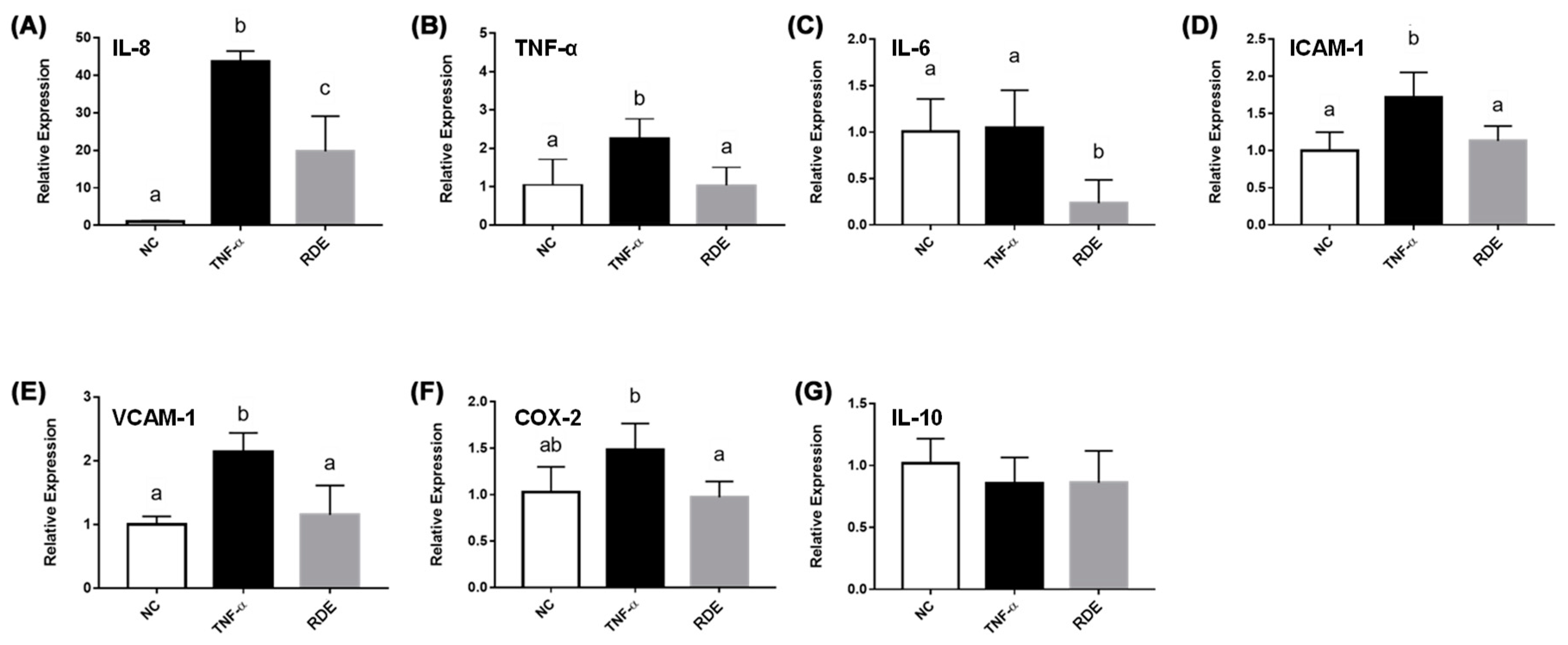
3.3. Effects of RDE on the Cytokine Gene Expression in the Monolayer Caco-2 Cells
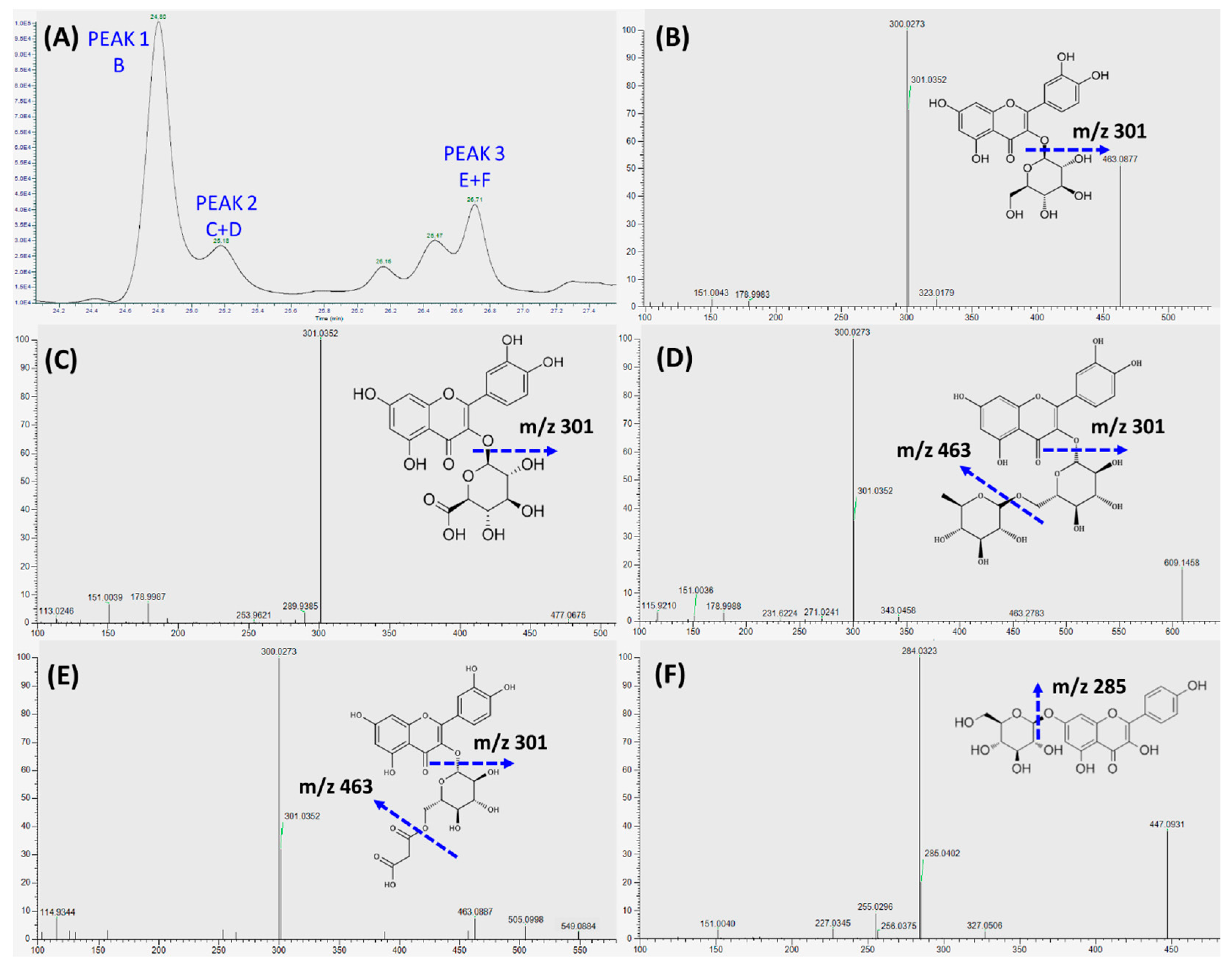
3.4. Absorption and Transport of Polyphenols by Caco-2 BBe1 Cells
3.5. Effects of Transported Polyphenols on the IL-8 Secretion and Cytokine Gene Expression in the TNF-α Inflamed EA.hy962 Cells
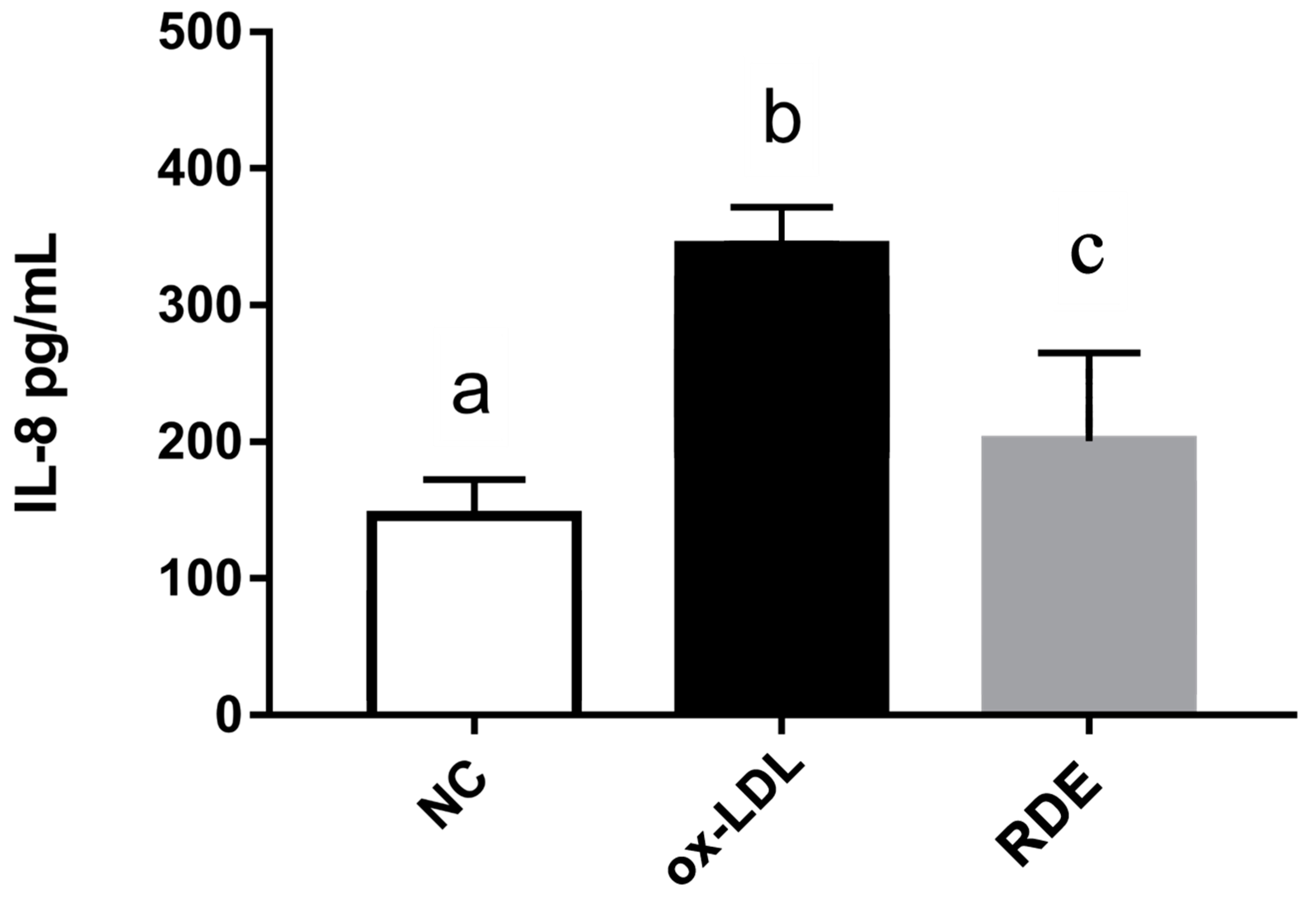
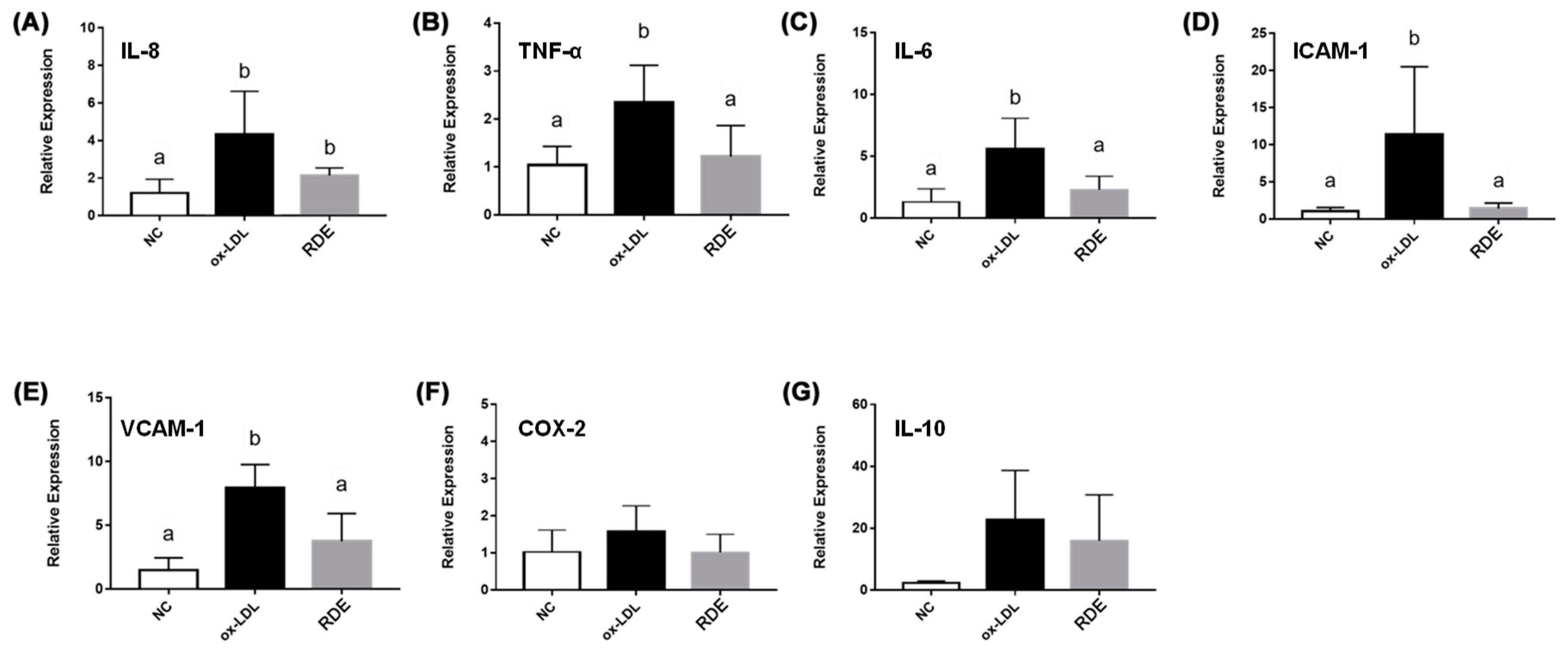
3.6. Effects of Transported Polyphenols on the IL-8 Secretion and Cytokine Gene Expression in the Ox-LDL Inflamed EA.hy962 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| RDE | red-osier dogwood extract |
| CVD | cardiovascular disease |
| IL-8 | interleukin-8 |
| TNF-α | tumor necrosis factor-alph |
| IL-6 | interleukin-6 |
| ICAM-1 | intercellular adhesion molecule-1 |
| VCAM-1 | vascular cell adhesion molecule 1 |
| COX-2 | cyclooxygenase 2 |
| ox-LDL | oxidized low-density lipoprotein |
| HBSS | Hank’s balanced salt solution |
| FBS | fetal bovine serum |
| TEER | transepithelial electrical resistance |
| ELISA | enzyme-linked immunosorbent assay |
| real-time PCR | real-time polymerase chain reaction |
| FA | formic acid |
| SPE | solid phase extraction |
| VEGF | vascular endothelial growth factor |
References
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Christian, J.W.; Stefan, K.; Stefan, D.; Peter, S.; Georg, E.; Friedrich, O.; Johann, W. Association of endotoxemia with carotid atherosclerosis and cardiovascular disease. J. Am. Coll. Cardiol. 1999, 34, 448–449. [Google Scholar]
- Wang, H.; Naghavi, M.; Allen, C.; Barber, R.M.; Bhutta, Z.A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; Coates, M.M. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Inflammation in atherosclerosis: From pathophysiology to practice. J. Am. Coll. Cardiol. 2009, 54, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Swirski, F.K.; Pittet, M.J.; Kircher, M.F.; Aikawa, E.; Jaffer, F.A.; Libby, P.; Weissleder, R. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc. Natl. Acad. Sci. USA 2006, 103, 10340–10345. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat. Rev. Immunol. 2010, 10, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.Y.; Gomaa, W.M.S.; Ametaj, B.N.; Alexander, T.W.; Yang, W.Z. Feeding red osier dogwood (Corms sericea) to beef heifers fed a high-grain diet affected feed intake and total tract digestibility. Anim. Feed Sci. Tech. 2019, 247, 83–91. [Google Scholar] [CrossRef]
- Chong, M.F.; Macdonald, R.; Lovegrove, J.A. Fruit polyphenols and CVD risk: A review of human intervention studies. Br. J. Nutr. 2010, 104, S28–S39. [Google Scholar] [CrossRef]
- Adefegha, S.A.; Leal, D.B.R.; de Oliveira, J.S.; Manzoni, A.G.; Bremm, J.M. Modulation of reactive oxygen species production, apoptosis and cell cycle in pleural exudate cells of carrageenan-induced acute inflammation in rats by rutin. Food Funct. 2017, 8, 4459–4468. [Google Scholar] [CrossRef]
- Nair, V.; Bang, W.Y.; Schreckinger, E.; Andarwulan, N.; Cisneros-Zevallos, L. Protective role of ternatin anthocyanins and quercetin glycosides from butterfly pea (Clitoria ternatea Leguminosae) blue flower petals against lipopolysaccharide (LPS)-induced inflammation in macrophage cells. J. Agric. Food Chem. 2015, 63, 6355–6365. [Google Scholar] [CrossRef]
- Warner, E.F.; Smith, M.J.; Zhang, Q.; Raheem, K.S.; O’Hagan, D.; O’Connell, M.A.; Kay, C.D. Signatures of anthocyanin metabolites identified in humans inhibit biomarkers of vascular inflammation in human endothelial cells. Mol. Nutr. Food Res. 2017, 61, 1700053. [Google Scholar] [CrossRef]
- Bresciani, L.; Martini, D.; Mena, P.; Tassotti, M.; Calani, L.; Brigati, G.; Brighenti, F.; Holasek, S.; Malliga, D.E.; Lamprecht, M.; et al. Absorption profile of (poly)phenolic compounds after consumption of three food supplements containing 36 different fruits, vegetables, and berries. Nutrients 2017, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Juaristi, M.; Martinez-Lopez, S.; Sarria, B.; Bravo, L.; Mateos, R. Absorption and metabolism of yerba mate phenolic compounds in humans. Food Chem. 2018, 240, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Sojka, M.; Klewicka, E.; Lipinska, L.; Klewicki, R.; Kolodziejczyk, K. Ellagitannins from rubus idaeus L. exert geno- and cytotoxic effects against human colon adenocarcinoma cell line Caco-2. J. Agric. Food Chem. 2017, 65, 2947–2955. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.; Pan, S.; Beningo, K.; Hupe, D. A permanent human cell line (EA.hy926) preserves the characteristics of endothelin converting enzyme from primary human umbilical vein endothelial cells. Life Sci. 1995, 56, 2331–2341. [Google Scholar] [CrossRef]
- Omonijo, F.A.; Liu, S.; Hui, Q.; Zhang, H.; Lahaye, L.; Bodin, J.C.; Gong, J.; Nyachoti, M.; Yang, C. Thymol improves barrier function and attenuates inflammatory responses in porcine intestinal epithelial cells during lipopolysaccharide (LPS)-induced inflammation. J. Agric. Food Chem. 2018, 67, 615–624. [Google Scholar] [CrossRef]
- Zhang, H.; Yousef, H.; Renaud, J.; Liu, R.; Yang, C.; Sun, Y.; Tsao, R. Bioaccessibility, bioavailability and anti-inflammatory effects of anthocyanins from purple root vegetables using mono-and co-culture cell models. Mol. Nutr. Food Res. 2017, 61, 1600928. [Google Scholar] [CrossRef]
- Ufnal, M.; Pham, K. The gut-blood barrier permeability—A new marker in cardiovascular and metabolic diseases? Med. Hypotheses 2017, 98, 35–37. [Google Scholar] [CrossRef]
- Lubas, A.; Kade, G.; Niemczyk, S. Renal resistive index as a marker of vascular damage in cardiovascular diseases. Int. Urol. Nephrol. 2014, 46, 395–402. [Google Scholar] [CrossRef]
- Zhong, W.; Li, Q.; Zhang, W.; Sun, Q.; Sun, X.; Zhou, Z. Modulation of intestinal barrier and bacterial endotoxin production contributes to the beneficial effect of nicotinic acid on alcohol-induced endotoxemia and hepatic inflammation in rats. Biomolecules 2015, 5, 2643–2658. [Google Scholar] [CrossRef]
- Huang, C.; Irwin, M.G.; Wong, G.T.C.; Chang, R.C.C. Evidence of the impact of systemic inflammation on neuroinflammation from a non-bacterial endotoxin animal model. J. Neuroinflamm. 2018, 15, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Kiewisz, J.; Kaczmarek, M.M.; Pawlowska, A.; Kmiec, Z.; Stompor, T. Endothelial progenitor cells participation in cardiovascular and kidney diseases: A systematic review. Acta Biochim. Pol. 2016, 63, 475–482. [Google Scholar] [CrossRef]
- Yang, R.; Hui, Q.; Jiang, Q.; Liu, S.; Zhang, H.; Wu, J.; Lin, F.; Yang, C. Effect of Manitoba-grown red-osier dogwood extracts on recovering Caco-2 cells from H2O2-induced oxidative damage. Antioxidants 2019, 8, 250. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhang, Y.; Liu, H.; Yuan, J.; Zheng, Z.; Zou, G. Transport of a cancer chemopreventive polyphenol, resveratrol: Interaction with serum albumin and hemoglobin. J. Fluoresc. 2007, 17, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Villalba, R.; Vissenaekens, H.; Pitart, J.; Romo-Vaquero, M.; Espin, J.C.; Grootaert, C.; Selma, M.V.; Raes, K.; Smagghe, G.; Possemiers, S.; et al. Gastrointestinal simulation model TWIN-SHIME shows differences between human urolithin-metabotypes in gut microbiota composition, pomegranate polyphenol metabolism, and transport along the intestinal tract. J. Agric. Food Chem. 2017, 65, 5480–5493. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Högger, P. Stability of dietary polyphenols under the cell culture conditions: Avoiding erroneous conclusions. J. Agric. Food Chem. 2015, 63, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Li, J.; Wang, K.; Hao, X.; Ge, R.; Li, Q. Isoquercitrin inhibits hydrogen peroxide-induced apoptosis of EA. hy926 cells via the PI3K/Akt/GSK3β signaling pathway. Molecules 2016, 21, 356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C. The role of inflammatory cytokines in endothelial dysfunction. Basic Res. Cardiol. 2008, 103, 398–406. [Google Scholar] [CrossRef]
- Lamarca, B. The role of immune activation in contributing to vascular dysfunction and the pathophysiology of hypertension during preeclampsia. Minerva Ginecol. 2010, 62, 105–120. [Google Scholar] [PubMed]
- Kamiloglu, S.; Grootaert, C.; Capanoglu, E.; Ozkan, C.; Smagghe, G.; Raes, K.; Van Camp, J. Anti-inflammatory potential of black carrot (Daucus carota L.) polyphenols in a co-culture model of intestinal Caco-2 and endothelial EA.hy926 cells. Mol. Nutr. Food Res. 2017, 61, 1600455. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.B.; Qi, R.; Xu, L.; Chen, Y.; Yu, Z.; Guo, P.; Gong, T. The more critical murderer of atherosclerosis than lipid metabolism: Chronic stress. Lipids Health Dis. 2018, 17, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Moradi, F.; Sewell, R.D.E.; Lorigooini, Z.; Rafieian-Kopaei, M. Immunosuppression-lipid metabolism interplay and medicinal plants in atherosclerosis: A review. Curr. Pharm. Des. 2018, 24, 2789–2793. [Google Scholar] [CrossRef] [PubMed]
- Daub, K.; Seizer, P.; Stellos, K.; Kramer, B.F.; Bigalke, B.; Schaller, M.; Fateh-Moghadam, S.; Gawaz, M.; Lindemann, S. Oxidized LDL-activated platelets induce vascular inflammation. Semin. Thromb. Hemost. 2010, 36, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Kaikkonen, J.E.; Kresanov, P.; Ahotupa, M.; Jula, A.; Mikkila, V.; Viikari, J.S.; Kahonen, M.; Lehtimaki, T.; Raitakari, O.T. High serum n6 fatty acid proportion is associated with lowered LDL oxidation and inflammation: The Cardiovascular Risk in Young Finns Study. Free Radic. Res. 2014, 48, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhao, D.; Wang, M.; Zhao, F.; Han, X.Y.; Qi, Y.; Liu, J. Association between circulating oxidized low-density lipoprotein and atherosclerotic cardiovascular disease: A meta-analysis of prospective observational studies. J. Am. Coll. Cardiol. 2017, 70, 79–80. [Google Scholar] [CrossRef]
- Chao, P.Y.; Huang, Y.P.; Hsieh, W.B. Inhibitive effect of purple sweet potato leaf extract and its components on cell adhesion and inflammatory response in human aortic endothelial cells. Cell Adhes. Migr. 2013, 7, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Tsoy, I.; Park, J.M.; Chung, J.I.; Shin, S.C.; Chang, K.C. Anthocyanins from soybean seed coat inhibit the expression of TNF-alpha-induced genes associated with ischemia/reperfusion in endothelial cell by NF-kappaB-dependent pathway and reduce rat myocardial damages incurred by ischemia and reperfusion in vivo. FEBS Lett. 2006, 580, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, H.; Mats, L.; Liu, R.; Deng, Z.; Mine, Y.; Tsao, R. Anti-inflammatory effect and cellular uptake mechanism of peptides from common bean (Phaseolus vulga L.) milk and yogurts in Caco-2 mono- and Caco-2/EA.hy926 co-culture models. J. Agric. Food Chem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, C.; Huwel, S.; Galla, H.J. Predicting blood-brain barrier permeability of drugs: Evaluation of different in vitro assays. J. Drug Target. 2002, 10, 263–276. [Google Scholar] [CrossRef]



| Gene | Primer Sequences (5′→3′) | Length (bp) | Access No. |
|---|---|---|---|
| IL-8 | AGTCCTTGTTCCACTGTGCC | 104 | NM_000584.4 |
| GTGCTTCCACATGTCCTCAC | |||
| TNF-α | CATTGCCCTGTGAGGAGGAC | 131 | NM_000594.4 |
| CGACCCTAAGCCCCCAATTC | |||
| IL-6 | CCAGCTATGAACTCCTTCTC | 234 | NM_001318095.1 |
| GCTTGTTCCTCACATCTCTC | |||
| ICAM-1 | TCATCACTGTGGTAGCAGCC | 159 | NM_000201.3 |
| GATAGGTTCAGGGAGGCGTG | |||
| VCAM-1 | AATTCCACGCTGACCCTGAG | 151 | NM_001078.4 |
| GGCCACCACTCATCTCGATT | |||
| COX-2 | GAATGGGGTGATGAGCAGTT | 561 | NM_000963.4 |
| CAGAAGGGCAGGATACAGC | |||
| IL-10 | TGGAATGCGAGCAATCCTGA | 148 | NM_000572.3 |
| TTACCTGGAGGAGGTGATGC | |||
| GGCCTTGCTCTTGTTTTCAC | |||
| GAPDH | GCACCGTCAAGGCTGAGAAC | 142 | NM_001289745.2 |
| ATGGTGGTGAAGACGCCAGT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Q.; Zhang, H.; Yang, R.; Hui, Q.; Chen, Y.; Mats, L.; Tsao, R.; Yang, C. Red-Osier Dogwood Extracts Prevent Inflammatory Responses in Caco-2 Cells and a Caco-2 BBe1/EA.hy926 Cell Co-Culture Model. Antioxidants 2019, 8, 428. https://doi.org/10.3390/antiox8100428
Jiang Q, Zhang H, Yang R, Hui Q, Chen Y, Mats L, Tsao R, Yang C. Red-Osier Dogwood Extracts Prevent Inflammatory Responses in Caco-2 Cells and a Caco-2 BBe1/EA.hy926 Cell Co-Culture Model. Antioxidants. 2019; 8(10):428. https://doi.org/10.3390/antiox8100428
Chicago/Turabian StyleJiang, Qian, Hua Zhang, Runqiang Yang, Qianru Hui, Yuhuan Chen, Lili Mats, Rong Tsao, and Chengbo Yang. 2019. "Red-Osier Dogwood Extracts Prevent Inflammatory Responses in Caco-2 Cells and a Caco-2 BBe1/EA.hy926 Cell Co-Culture Model" Antioxidants 8, no. 10: 428. https://doi.org/10.3390/antiox8100428
APA StyleJiang, Q., Zhang, H., Yang, R., Hui, Q., Chen, Y., Mats, L., Tsao, R., & Yang, C. (2019). Red-Osier Dogwood Extracts Prevent Inflammatory Responses in Caco-2 Cells and a Caco-2 BBe1/EA.hy926 Cell Co-Culture Model. Antioxidants, 8(10), 428. https://doi.org/10.3390/antiox8100428






