Effects of N-Acetyl-Cysteine Supplementation through Drinking Water on the Glutathione Redox Status during the Weaning Transition of Piglets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Feeds
2.2. Experimental Design and Measurements
2.3. Water Supplementation
2.4. Sample Collection
2.5. Laboratory Analysis
2.6. Statistics
3. Results
3.1. Animal Performance
3.2. Responses of the Glutathione Redox System in Different Tissues
3.2.1. The Erythrocyte Glutathione Redox System.
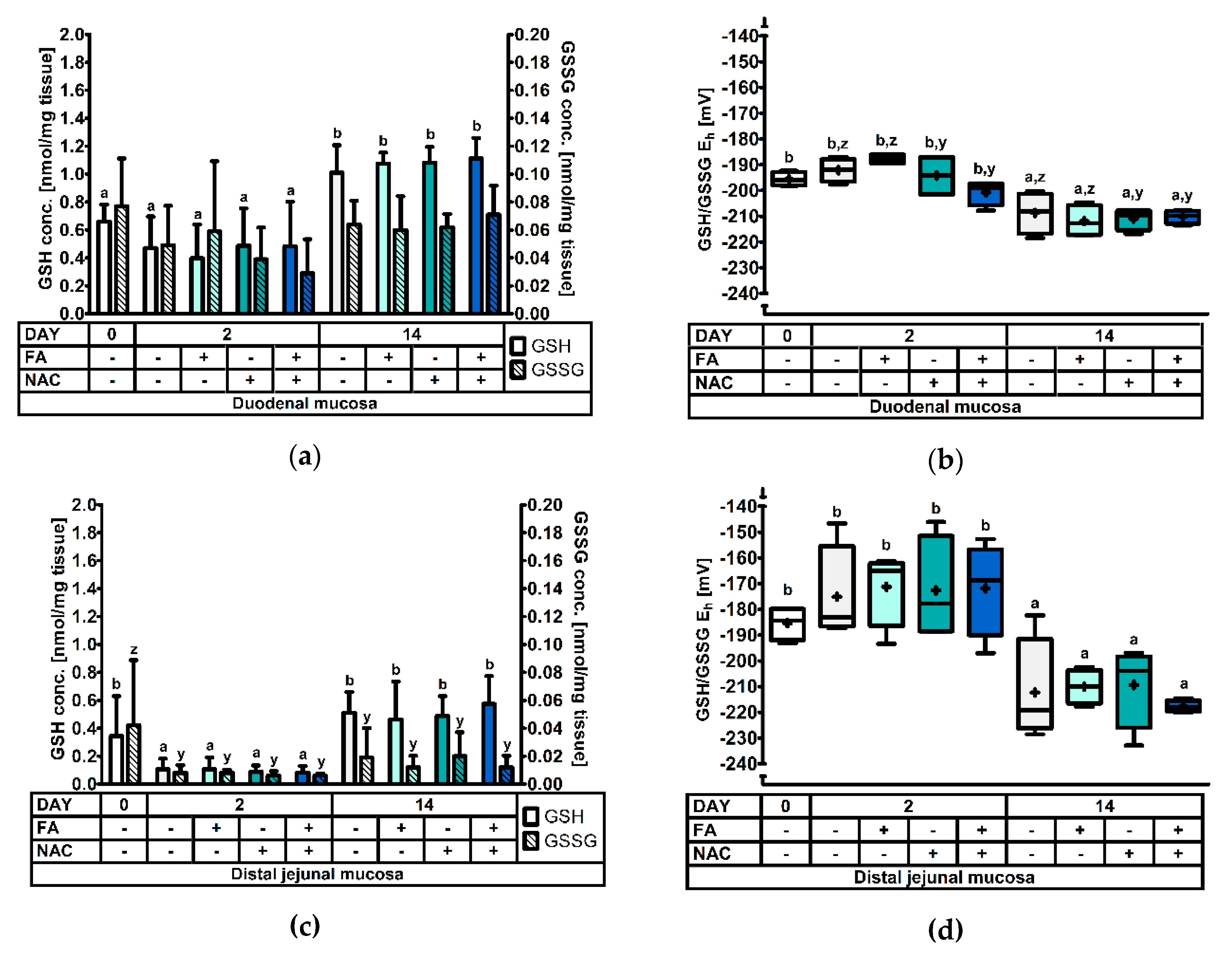
3.2.2. The Glutathione Redox System in the Duodenal and Distal Jejunal Mucosa
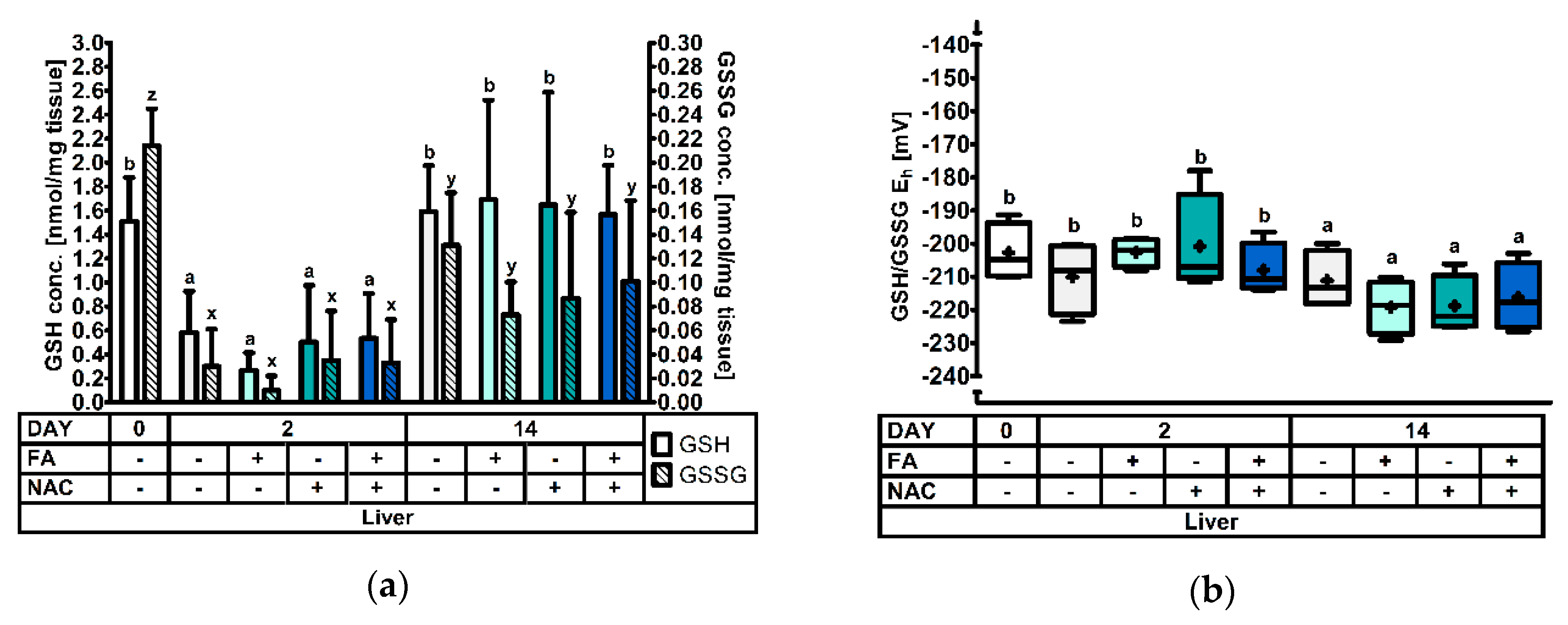
3.2.3. The Hepatic Glutathione Redox System
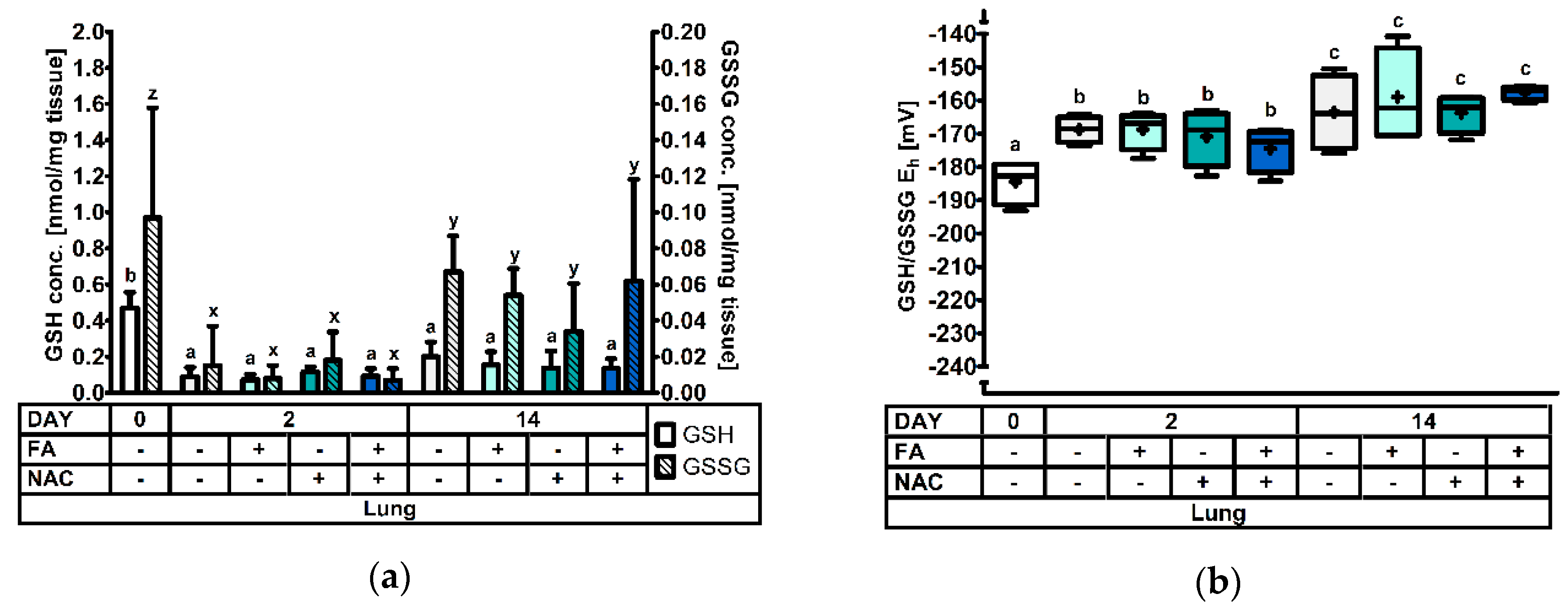
3.2.4. The Pulmonary Glutathione Redox System
3.2.5. The Renal Cortical Glutathione Redox System
3.3. Principal Component Analysis
4. Discussion
4.1. The Weaning Transition Highly Affects the Glutathione Redox System
4.2. N-Acetyl-Cysteine Did Not Improve Tissue Glutathione Levels
4.3. Fasting Did Not Amplify Weaning Induced Alterations of the Glutathione Redox System
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Degroote, J.; Michiels, J.; Claeys, E.; Ovyn, A.; De Smet, S. Changes in the pig small intestinal mucosal glutathione kinetics after weaning. J. Anim. Sci. 2012, 90, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Zhu, W.; Guo, Q.; Luo, W.; Zhang, J.; Xu, W.; Xu, J. Weaning Induced Hepatic Oxidative Stress, Apoptosis, and Aminotransferases through MAPK Signaling Pathways in Piglets. Oxidative Med. Cell. Longev. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P.; Sies, H. The Redox Code. Antioxid. Redox Signal. 2015, 23, 734–746. [Google Scholar] [CrossRef]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef]
- Circu, M.L.; Aw, T.Y. Intestinal redox biology and oxidative stress. Semin. Cell Dev. Biol. 2012, 23, 729–737. [Google Scholar] [CrossRef] [Green Version]
- Aw, T.Y. Cellular redox: A modulator of intestinal epithelial cell proliferation. News Physiol. Sci. 2003, 18, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Uchida, H.; Nakajima, Y.; Ohtake, K.; Ito, J.; Morita, M.; Kamimura, A.; Kobayashi, J. Protective effects of oral glutathione on fasting-induced intestinal atrophy through oxidative stress. World J. Gastroenterol. 2017, 23, 6650–6664. [Google Scholar] [CrossRef]
- Rezaei, R.; Wang, W.; Wu, Z.; Dai, Z.; Wang, J.; Wu, G. Biochemical and physiological bases for utilization of dietary amino acids by young Pigs. J. Anim. Sci. Biotechnol. 2013, 4, 1–12. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione synthesis. Biochim. Et Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef] [Green Version]
- Griffith, O.W. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic. Biol. Med. 1999, 27, 922–935. [Google Scholar] [CrossRef]
- Grimble, R.F.; Grimble, G.K. Immunonutrition: Role of sulfur amino acids, related amino acids, and polyamines. Nutrition 1998, 14, 605–610. [Google Scholar] [CrossRef]
- Kahindi, R.; Regassa, A.; Htoo, J.; Nyachoti, M. Optimal sulfur amino acid to lysine ratio for post weaning piglets reared under clean or unclean sanitary conditions. Anim. Nutr. 2017, 3, 380–385. [Google Scholar] [CrossRef]
- Bonanomi, L.; Gazzaniga, A. Toxicological, pharmacokinetic and metabolic studies on acetylcysteine. Eur. J. Respir. Dis. Suppl. 1980, 111, 45–51. [Google Scholar]
- Cotgreave, I.A.; Berggren, M.; Jones, T.W.; Dawson, J.; Moldeus, P. Gastrointestinal metabolism of N-acetylcysteine in the rat, including an assay for sulfite in biological systems. Biopharm. Drug Dispos. 1987, 8, 377–386. [Google Scholar] [CrossRef]
- Xu, C.C.; Yang, S.F.; Zhu, L.H.; Cai, X.; Sheng, Y.S.; Zhu, S.W.; Xu, J.X. Regulation of N-acetyl cysteine on gut redox status and major microbiota in weaned piglets. J. Anim. Sci. 2014, 92, 1504–1511. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Cai, X.; Guo, Q.; Chen, X.; Zhu, S.; Xu, J. Effect of N-acetyl cysteine on enterocyte apoptosis and intracellular signalling pathways’ response to oxidative stress in weaned piglets. Br. J. Nutr. 2013, 110, 1938–1947. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.Q.; Wang, L.; Yi, D.; Ding, B.Y.; Yang, Z.G.; Li, J.; Chen, X.; Qiu, Y.S.; Wu, G.Y. N-acetylcysteine reduces inflammation in the small intestine by regulating redox, EGF and TLR4 signaling. Amino Acids 2013, 45, 513–522. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, L.; Zhang, W.; Yang, Z.; Ding, B.; Zhu, H.; Liu, Y.; Qiu, Y.; Yin, Y.; Wu, G. Protective effects of N-acetylcysteine on intestinal functions of piglets challenged with lipopolysaccharide. Amino Acids 2012, 43, 1233–1242. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, L.; Hou, Y.; Ding, B.; Zhu, H.; Liu, Y.; Yang, Z.; Zhang, W. Effects of N-acetylcysteine on growth performance and plasma biochemical indices of lipopolysaccharide challenged piglets. Chin. J. Anim. Nutr. 2010, 22, 1007–1011. [Google Scholar] [CrossRef]
- Guo, Q.; Cai, X.; Xu, C.; Luo, Z.; Sheng, Y.; Bao, J.; Chen, X.; Xu, J. Effects of dietary supplementation with N-acetyl cysteine on antioxidant capacities and the expression of inflammatory cytokines in weaned piglets. Ital. J. Anim. Sci. 2016, 15, 634–641. [Google Scholar] [CrossRef]
- Dekhuijzen, P.N.R. Antioxidant properties of N-acetylcysteine: Their relevance in relation to chronic obstructive pulmonary disease. Eur. Respir. J. 2004, 23, 629–636. [Google Scholar] [CrossRef]
- Sjodin, K.; Nilsson, E.; Hallberg, A.; Tunek, A. Metabolism of N-acetyl-l-cysteine Some structural requirements for the deacetylation and consequences for the oral bioavailability. Biochem. Pharmacol. 1989, 38, 3981–3985. [Google Scholar] [CrossRef]
- Verdonk, J.M.A.J.; Bruininx, E.M.A.M.; van der Meulen, J.; Verstegen, M.W.A. Post-weaning feed intake level modulates gut morphology but not gut permeability in weaned piglets. Livest. Sci. 2007, 108, 146–149. [Google Scholar] [CrossRef]
- Spreeuwenberg, M.A.M.; Verdonk, J.M.A.J.; Gaskins, H.R.; Verstegen, M.W.A. Small Intestine Epithelial Barrier Function Is Compromised in Pigs with Low Feed Intake at Weaning. J. Nutr. 2001, 131, 1520–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, T. Determination of reduced and oxidized glutathione in erythrocytes by high-performance liquid chromatography with ultraviolet absorbance detection. J. Chromatogr. Biomed. Sci. Appl. 1996, 678, 157–164. [Google Scholar] [CrossRef]
- Petrovic, V.; Novotny, J.; Hisira, V.; Link, R.; Leng, L.; Kovac, G. The Impact of Suckling and Post-weaning Period on Blood Chemistry of Piglets. Acta Vet. Brno 2009, 78, 365–371. [Google Scholar] [CrossRef] [Green Version]
- Robert, F.; Bebin, K.; Garrau, J.-M.; Gueriot, J.-F.; Foret, F.; Brack, B.; Garrel, C. Évaluation et correction du stress oxydatif du porcelet en post-sevrage. In Proceedings of the 41e Journées de la Recherche Porcine, Paris, France, 3–4 February 2009; pp. 173–178. [Google Scholar]
- Degroote, J.; Wang, W.; Vergauwen, H.; De Smet, S.; Van Ginneken, C.; Michiels, J. Impact of a dietary challenge with peroxidized oil on the glutathione redox status and integrity of the small intestine in weaned piglets. Animal 2018. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; Li, P.; Li, X.; Zhou, H.; Wang, F.; Li, D.; Yin, Y.; Wu, G. Gene Expression Is Altered in Piglet Small Intestine by Weaning and Dietary Glutamine Supplementation. J. Nutr. 2008, 138, 1025–1032. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wan, H.; Mercier, Y.; Zhang, X.; Wu, C.; Wu, X.; Tang, L.; Che, L.; Lin, Y.; Xu, S.; et al. Changes in plasma amino acid profiles, growth performance and intestinal antioxidant capacity of piglets following increased consumption of methionine as its hydroxy analogue. Br. J. Nutr. 2014, 112, 855–867. [Google Scholar] [CrossRef] [Green Version]
- Deneke, S.M.; Lynch, B.A.; Fanburg, B.L. Transient depletion of lung glutathione by diethylmaleate enhances oxygen toxicity. J. Appl. Physiol. 1985, 58, 571–574. [Google Scholar] [CrossRef]
- Ghezzi, P. Role of glutathione in immunity and inflammation in the lung. Int. J. Gen. Med. 2011, 4, 105–113. [Google Scholar] [CrossRef]
- Lash, L.H. Role of glutathione transport processes in kidney function. Toxicol. Appl. Pharmacol. 2005, 204, 329–342. [Google Scholar] [CrossRef]
- McCracken, B.A.; Spurlock, M.E.; Roos, M.A.; Zuckermann, F.A.; Gaskins, H.R. Weaning Anorexia May Contribute to Local Inflammation in the Piglet Small Intestine. J. Nutr. 1999, 129, 613–619. [Google Scholar] [CrossRef] [Green Version]
- Goodman, R.P.; Calvo, S.E.; Mootha, V.K. Spatiotemporal compartmentalization of hepatic NADH and NADPH metabolism. J. Biol. Chem. 2018, 293, 7508–7516. [Google Scholar] [CrossRef] [PubMed]
- Griffith, O.W.; Meister, A. Glutathione: Interorgan translocation, turnover, and metabolism. In Proceedings of the National Academy of Sciences, Toronto, ON, USA, 1 November 1979; pp. 5606–5610. [Google Scholar]
- Bauchart-Thevret, C.; Cottrell, J.; Stoll, B.; Burrin, D.G. First-pass splanchnic metabolism of dietary cysteine in weanling pigs. J. Anim. Sci. 2011, 89, 4093–4099. [Google Scholar] [CrossRef] [PubMed]
- Aw, T.Y. Biliary glutathione promotes the mucosal metabolism of luminal peroxidized lipids by rat small intestine in vivo. J. Clin. Investig. 1994, 94, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Su, W.; Ying, Z.; Chen, Y.; Zhou, L.; Li, Y.; Zhang, J.; Zhang, L.; Wang, T. N-acetylcysteine attenuates intrauterine growth retardation-induced hepatic damage in suckling piglets by improving glutathione synthesis and cellular homeostasis. Eur. J. Nutr. 2018, 57, 327–338. [Google Scholar] [CrossRef]
- Song, Z.; Tong, G.; Xiao, K.; Jiao le, F.; Ke, Y.; Hu, C. L-cysteine protects intestinal integrity, attenuates intestinal inflammation and oxidant stress, and modulates NF-kappaB and Nrf2 pathways in weaned piglets after LPS challenge. Innate Immun. 2016, 22, 152–161. [Google Scholar] [CrossRef]
- Williams, I.H. Growth of the weaned pig. In Weaning the Pig: Concepts and Consequences, 1st ed.; Pluske, J.R., Le Dividich, J., Verstegen, M.W.A., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2003; pp. 17–37. [Google Scholar]
- Burrin, D.; Stoll, B. Metabolic fate and function of dietary glutamate in the gut. Am. J. Clin. Nutr. 2009, 90, 850S–856S. [Google Scholar] [CrossRef]
- Pluske, J.R.; Williams, I.H.; Aherne, F.X. Maintenance of villous height and crypt depth in piglets by providing continuous nutrition after weaning. Anim. Sci. 1996, 62, 131–144. [Google Scholar] [CrossRef] [Green Version]
- Cho, E.S.; Sahyoun, N.; Stegink, L.D. Tissue glutathione as a cyst(e)ine reservoir during fasting and refeeding of rats. J. Nutr. 1981, 111, 914–922. [Google Scholar] [CrossRef] [PubMed]



| Nutrients (g/kg as-Fed Basis) | Weaner Diet 1 | Starter Diet 2 |
|---|---|---|
| Dry matter | 889.0 | 889.0 |
| Crude protein | 160.0 | 165.0 |
| Ether extract | 56.0 | 54.0 |
| Crude fibre | 35.0 | 40.0 |
| Crude ash | 54.5 | 57.0 |
| Calcium | 7.1 | 7.1 |
| Phosphorus | 5.5 | 5.5 |
| Potassium | 2.3 | 2.5 |
| Lysine | 11.9 | 11.7 |
| Methionine | 4.6 | 4.4 |
| Treatment | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| FA−NAC− | FA+NAC− | FA−NAC+ | FA+NAC+ | FA | NAC | FA × NAC | ||
| Body weight (BW) [kg] | ||||||||
| d0 | 7.05 | 7.05 | 7.05 | 7.06 | 0.01 | 0.880 | 0.846 | 0.746 |
| d2 | 6.92 | 6.70 | 6.91 | 6.73 | 0.01 | <0.001 | 0.768 | 0.342 |
| d14 | 9.13 | 8.87 | 8.99 | 9.03 | 0.05 | 0.301 | 0.925 | 0.165 |
| d42 | 23.04 | 22.14 | 22.64 | 22.71 | 0.21 | 0.335 | 0.845 | 0.259 |
| Average daily gain (ADG) [g/d] | ||||||||
| d0–2 | −64 | −175 | −72 | −161 | 7 | <0.001 | 0.849 | 0.465 |
| d2–14 | 185 | 183 | 176 | 193 | 4 | 0.441 | 0.962 | 0.303 |
| d14–42 | 498 | 478 | 490 | 491 | 6 | 0.457 | 0.823 | 0.410 |
| d0–42 | 383 | 362 | 372 | 375 | 5 | 0.354 | 0.882 | 0.255 |
| Average daily feed intake (ADFI) [g/d] | ||||||||
| d0–2 | 56 | 0 | 50 | 0 | 4 | 1 | 0.684 | 1 |
| d2–14 | 252 | 239 | 243 | 256 | 4 | 0.961 | 0.619 | 0.151 |
| d14–42 | 799 | 778 | 802 | 790 | 16 | 0.609 | 0.820 | 0.896 |
| d0–42 | 589 | 568 | 588 | 582 | 10 | 0.474 | 0.744 | 0.700 |
| Average daily water intake (ADWI) [mL/d] | ||||||||
| d0–2 | 329 | 322 | 311 | 308 | 17 | 0.899 | 0.646 | 0.954 |
| d2–14 | 648 | 588 | 638 | 606 | 19 | 0.251 | 0.920 | 0.723 |
| d14–42 | 2118 | 2451 | 2256 | 2314 | 115 | 0.405 | 0.997 | 0.557 |
| d0–42 | 1525 | 1716 | 1617 | 1638 | 74 | 0.484 | 0.962 | 0.572 |
| Feed conversion ratio (FCR) [ADG/ADFI] | ||||||||
| d0–2 | −1.53 | −1.18 | 0.17 | 1 | 0.612 | 1 | ||
| d2–14 | 1.36 | 1.31 | 1.38 | 1.34 | 0.02 | 0.129 | 0.325 | 0.884 |
| d14–42 | 1.61 | 1.62 | 1.65 | 1.61 | 0.03 | 0.881 | 0.791 | 0.680 |
| d0–42 | 1.54 | 1.57 | 1.59 | 1.56 | 0.03 | 0.988 | 0.715 | 0.608 |
| Water to feed ratio (WFR) [ADWI/ADFI] | ||||||||
| d0–2 | 9.03 | 7.49 | 1.62 | 1 | 0.643 | 1 | ||
| d2–14 | 2.58 | 2.46 | 2.61 | 2.37 | 0.07 | 0.199 | 0.848 | 0.688 |
| d14–42 | 2.69 | 3.20 | 2.86 | 2.93 | 0.17 | 0.393 | 0.888 | 0.525 |
| d0–42 | 2.58 | 3.04 | 2.78 | 2.82 | 0.15 | 0.404 | 0.967 | 0.483 |
| p-Value | |||||||
|---|---|---|---|---|---|---|---|
| DAY | FA | NAC | DAY × FA | DAY × NAC | FA × NAC | DAY × FA × NAC | |
| GSH | |||||||
| Erythrocytes | 0.002 | 0.125 | 0.206 | 0.450 | 0.982 | 0.788 | 0.594 |
| Duodenum | <0.001 | 0.952 | 0.480 | 0.552 | 0.973 | 0.905 | 0.725 |
| Distal jejunum | <0.001 | 0.887 | 0.865 | 0.854 | 0.585 | 0.600 | 0.565 |
| Liver | <0.001 | 0.740 | 0.877 | 0.698 | 0.745 | 0.634 | 0.505 |
| Lung | <0.001 | 0.345 | 0.705 | 0.924 | 0.166 | 0.716 | 0.601 |
| Kidney | <0.001 | 0.257 | 0.138 | 0.415 | 0.905 | 0.055 | 0.038 |
| GSSG | |||||||
| Erythrocytes | 0.440 | 0.608 | 0.918 | 0.518 | 0.619 | 0.081 | 0.686 |
| Duodenum | 0.077 | 0.895 | 0.428 | 0.883 | 0.216 | 0.857 | 0.412 |
| Distal jejunum | 0.027 | 0.568 | 0.894 | 0.572 | 0.849 | 0.908 | 0.993 |
| Liver | <0.001 | 0.320 | 0.844 | 0.736 | 0.501 | 0.167 | 0.418 |
| Lung | <0.001 | 0.943 | 0.597 | 0.485 | 0.539 | 0.413 | 0.340 |
| Kidney | <0.001 | 0.377 | 0.121 | 0.587 | 0.082 | 0.757 | 0.874 |
| GSH/GSSG Eh | |||||||
| Erythrocytes | <0.001 | 0.222 | 0.060 | 0.866 | 0.713 | 0.041 | 0.276 |
| Duodenum | <0.001 | 0.552 | 0.044 | 0.971 | 0.062 | 0.366 | 0.058 |
| Distal jejunum | <0.001 | 0.944 | 0.985 | 0.623 | 0.763 | 0.531 | 0.731 |
| Liver | 0.007 | 0.709 | 0.945 | 0.673 | 0.537 | 0.775 | 0.076 |
| Lung | <0.001 | 0.537 | 0.571 | 0.218 | 0.435 | 0.861 | 0.659 |
| Kidney | 0.017 | 0.821 | 0.725 | 0.766 | 0.175 | 0.454 | 0.149 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Degroote, J.; Van Noten, N.; Wang, W.; De Smet, S.; Michiels, J. Effects of N-Acetyl-Cysteine Supplementation through Drinking Water on the Glutathione Redox Status during the Weaning Transition of Piglets. Antioxidants 2019, 8, 24. https://doi.org/10.3390/antiox8010024
Degroote J, Van Noten N, Wang W, De Smet S, Michiels J. Effects of N-Acetyl-Cysteine Supplementation through Drinking Water on the Glutathione Redox Status during the Weaning Transition of Piglets. Antioxidants. 2019; 8(1):24. https://doi.org/10.3390/antiox8010024
Chicago/Turabian StyleDegroote, Jeroen, Noémie Van Noten, Wei Wang, Stefaan De Smet, and Joris Michiels. 2019. "Effects of N-Acetyl-Cysteine Supplementation through Drinking Water on the Glutathione Redox Status during the Weaning Transition of Piglets" Antioxidants 8, no. 1: 24. https://doi.org/10.3390/antiox8010024





