Comparing Palm Oil, Tocotrienol-Rich Fraction and α-Tocopherol Supplementation on the Antioxidant Levels of Older Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Experimental Design
2.3. Sample Collection
2.4. Determination of Erythrocyte Antioxidant Enzymes (SOD, CAT, GPx)
2.5. Determination of Erythrocyte GSH and GSSG Concentration
2.6. Statistical Analysis
3. Results
3.1. Demography of Subjects
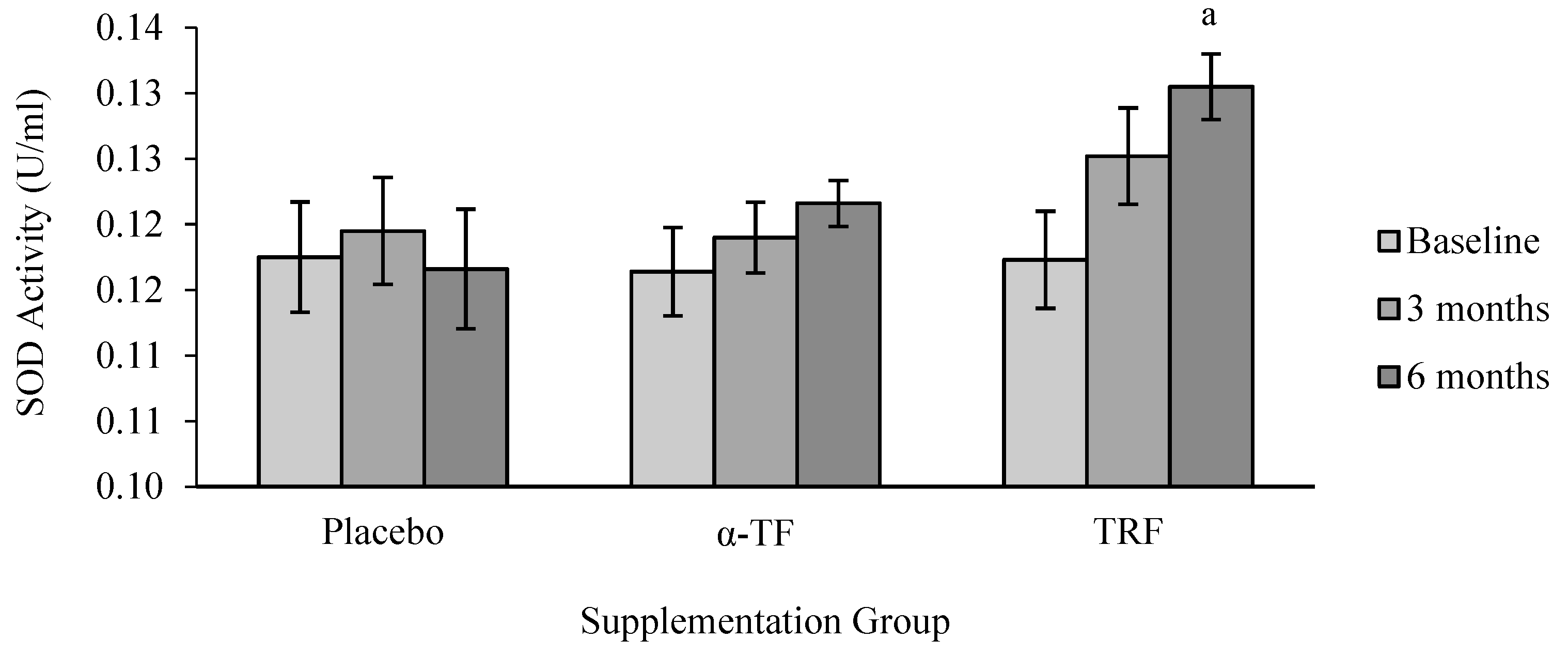
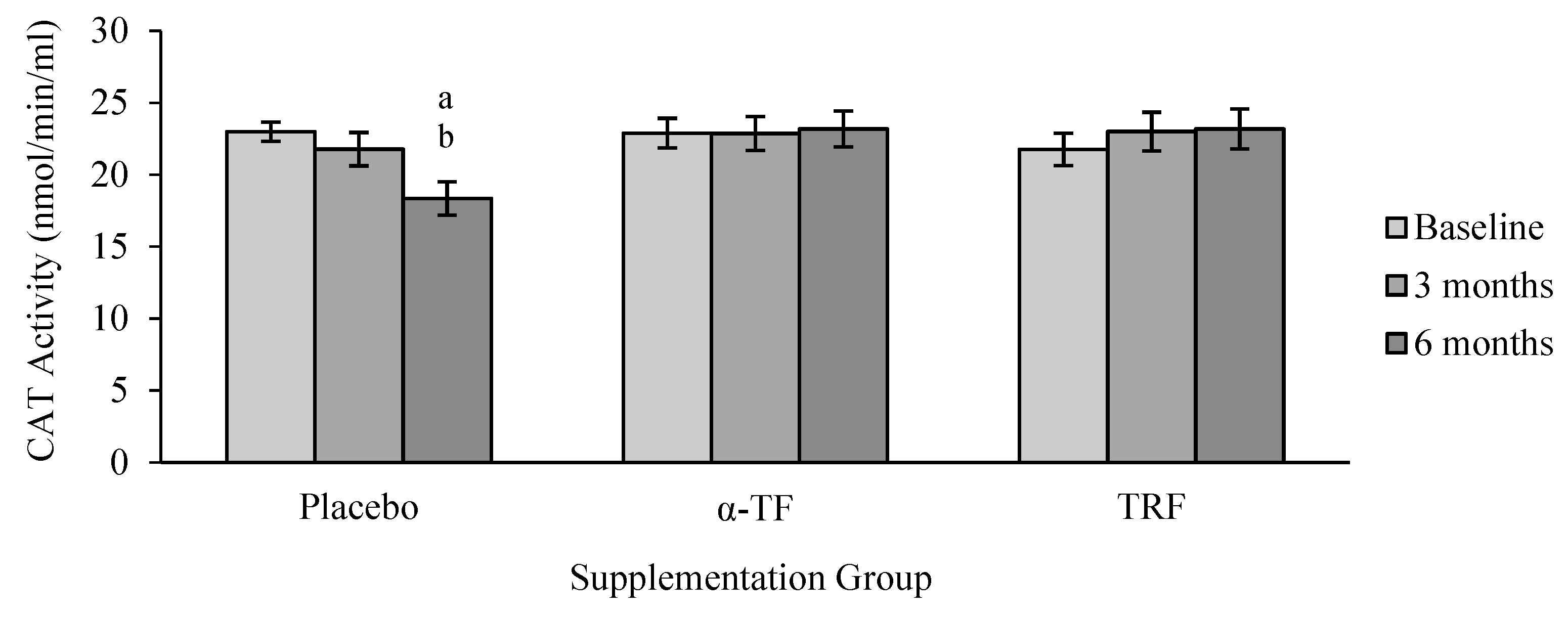
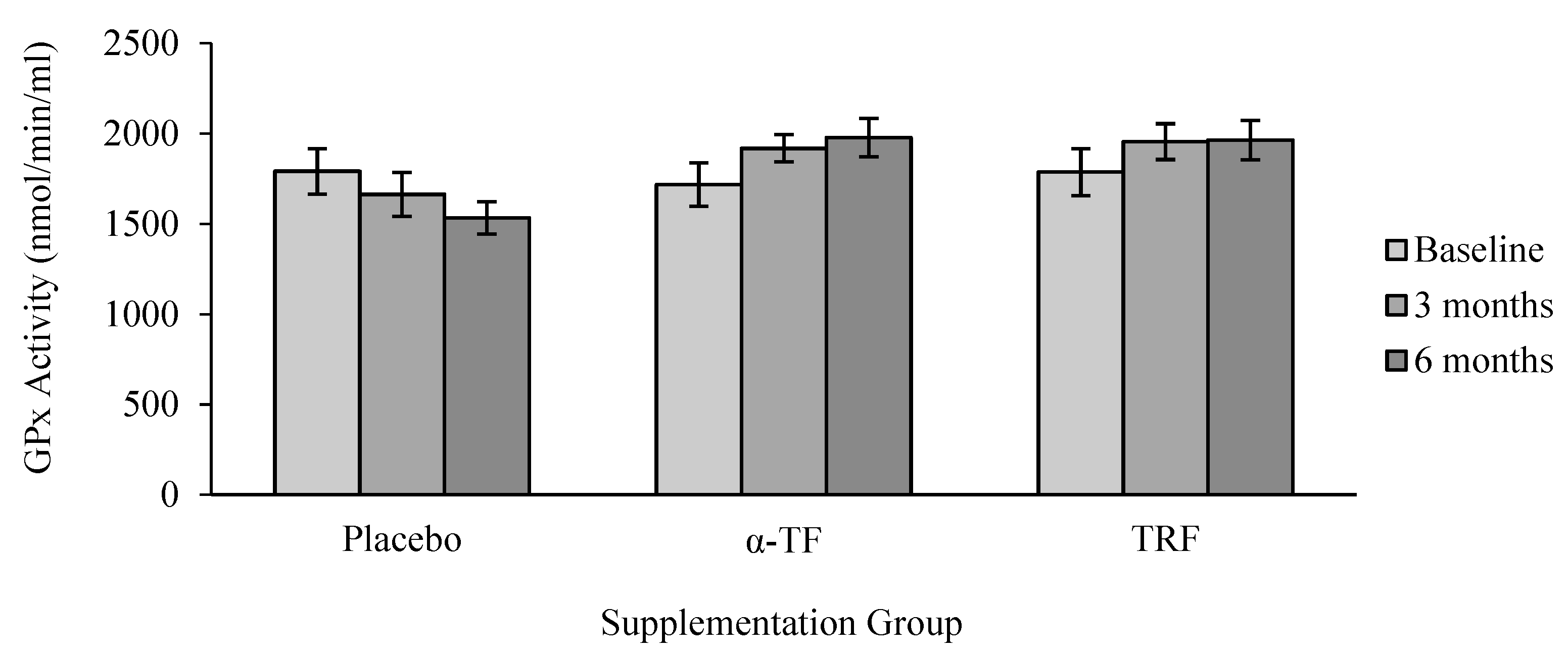
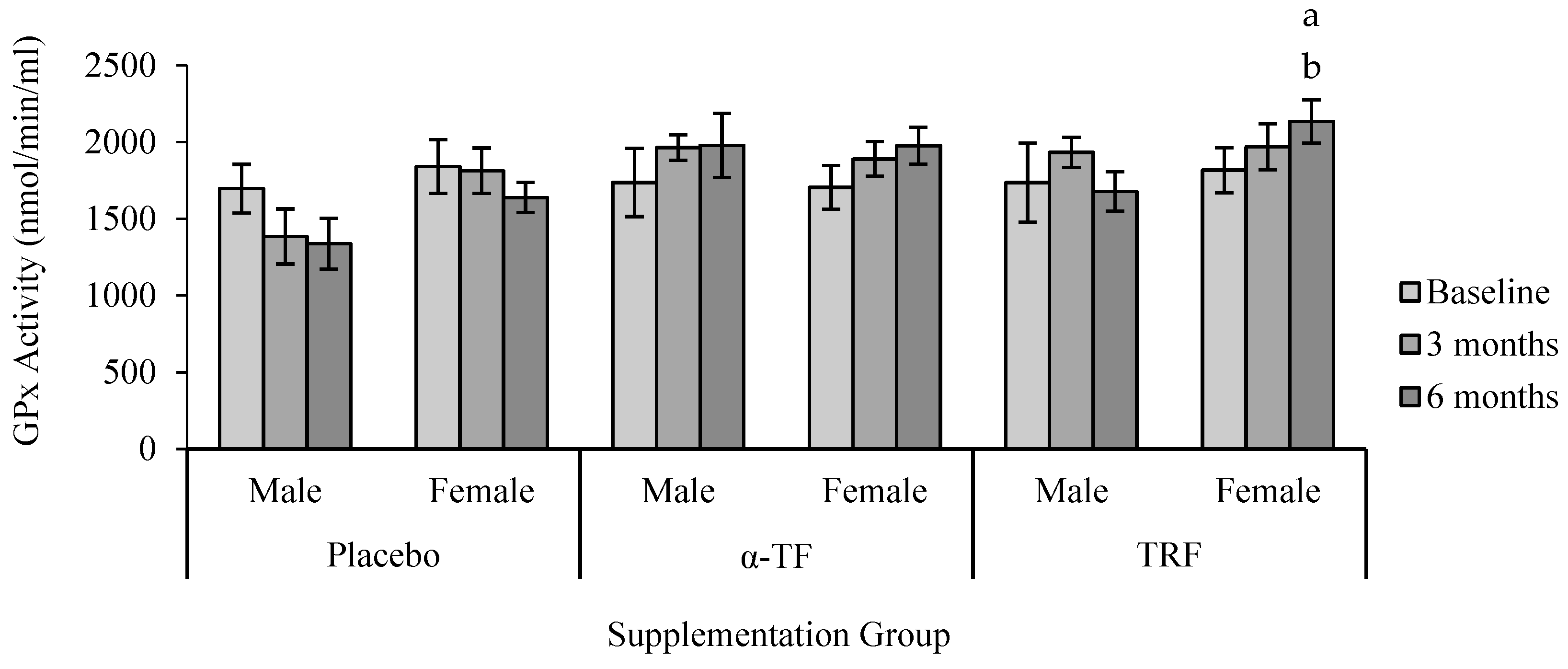
3.2. Antioxidant Enzymes Activities (SOD, CAT and GPx)
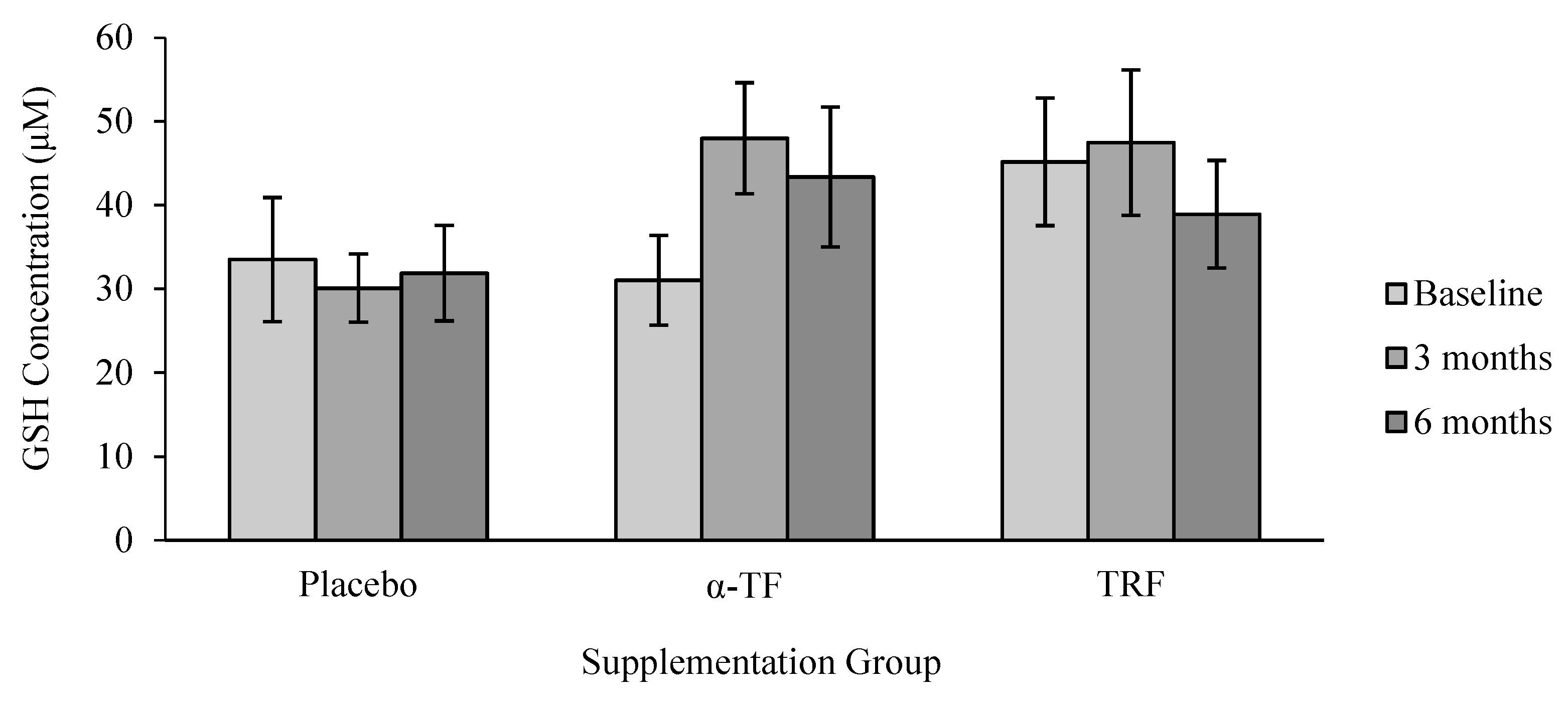
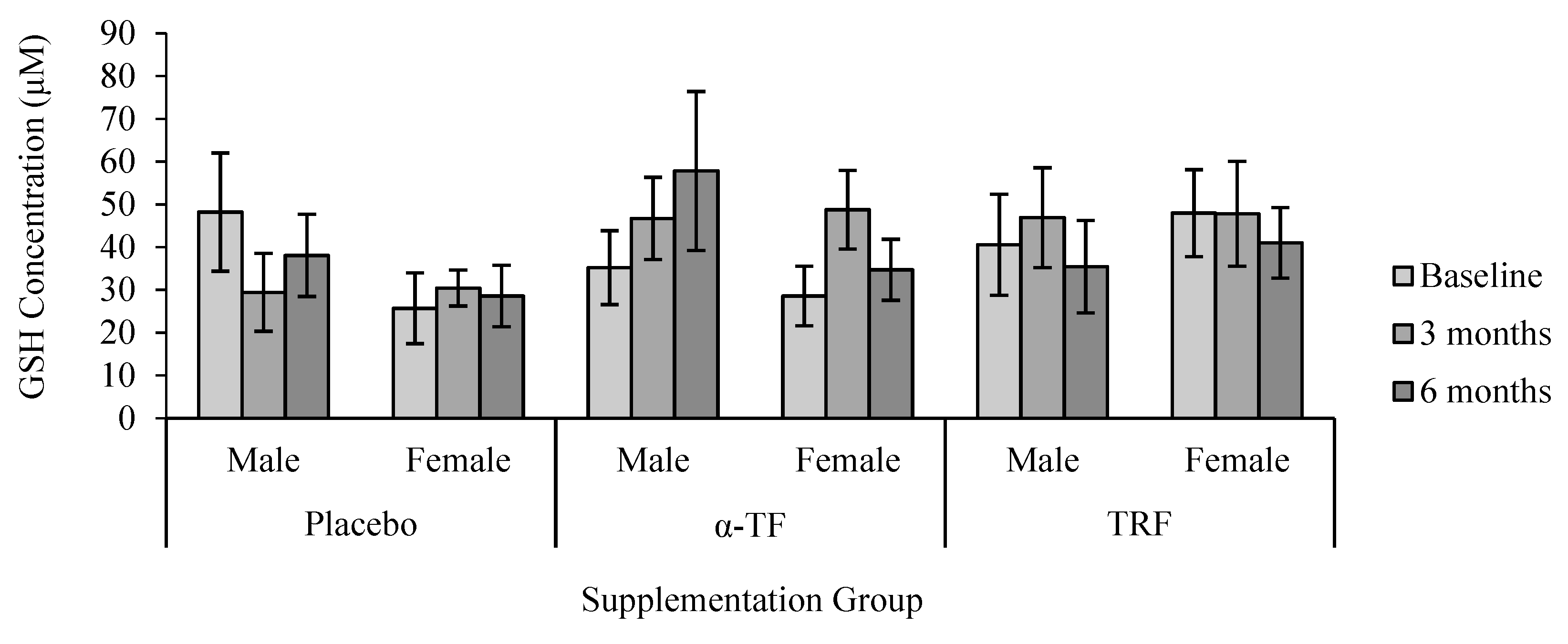
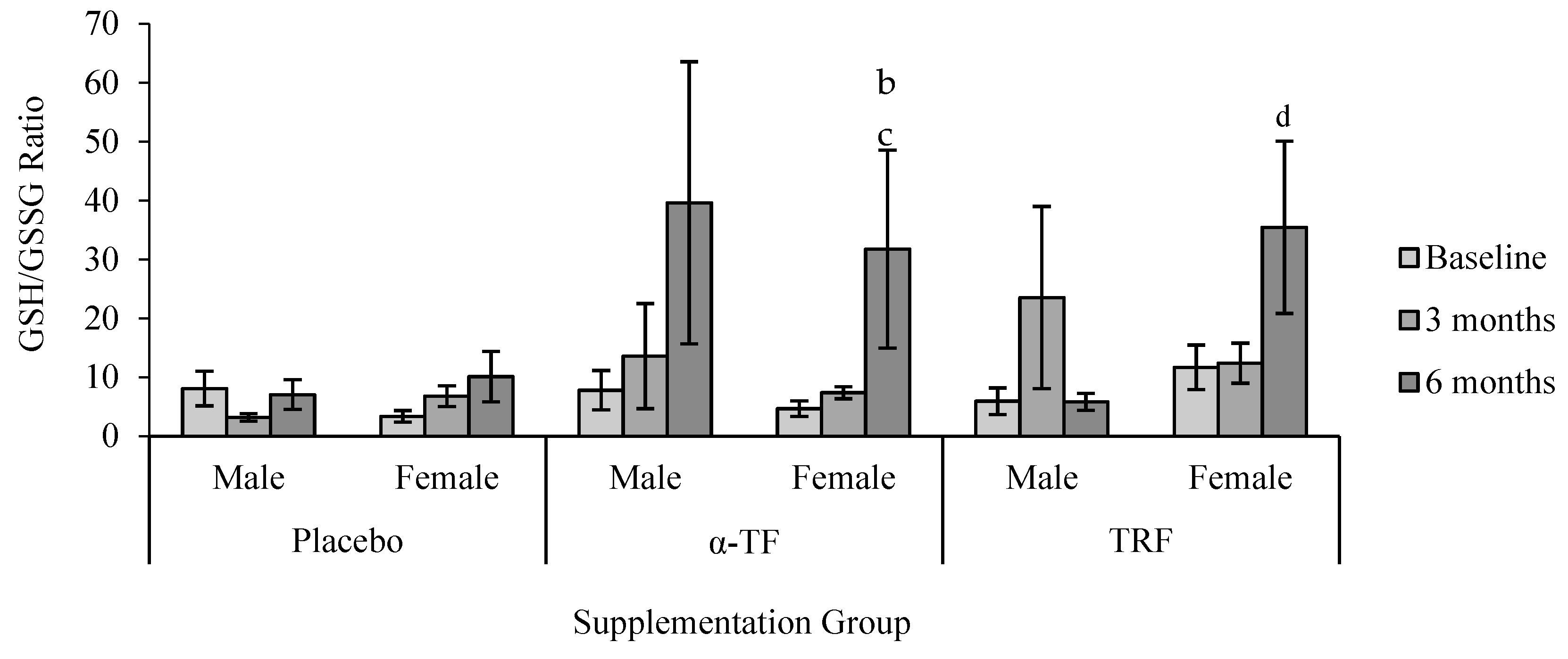
3.3. Non-Enzymatic Antioxidant (GSH and GSSG)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ulatowski, L.M.; Manor, D. Vitamin E and neurodegeneration. Neurobiol. Dis. 2015, 84, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.; Raza, S.T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The role of vitamin E in human health and some diseases. Sultan Qaboos Univ. Med. J. 2014, 14, e157–e165. [Google Scholar] [PubMed]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant and anti-inflammatory activities and the role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Comitato, R.; Ambra, R.; Virgili, F. Tocotrienols: A Family of Molecules with Specific Biological Activities. Antioxidants 2017, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Board, F.A.N.; Medicine, I.O. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; The National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- Jiang, Q.; Christen, S.; Shigenaga, M.K.; Ames, B.N. Gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am. J. Clin. Nutr. 2001, 74, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Moya-Camarena, S.; Jiang, Q. The Role of Vitamin E Forms in Cancer Prevention and Therapy—Studies in Human Intervention Trials and Animal Models. In Nutraceuticals and Cancer; Sarkar, F.H., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 323–354. [Google Scholar]
- Myung, S.-K.; Ju, W.; Cho, B.; Oh, S.-W.; Park, S.M.; Koo, B.-K.; Park, B.J.; Korean Meta-Analysis (KORMA) Study Group. Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: Systematic review and meta-analysis of randomised controlled trials. BMJ 2013, 346, f10. [Google Scholar] [CrossRef] [PubMed]
- Inokuchi, H.; Hirokane, H.; Tsuzuki, T.; Nakagawa, K.; Igarashi, M.; Miyazawa, T. Anti-angiogenic activity of tocotrienol. Biosci. Biotechnol. Biochem. 2003, 67, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Mizushina, Y.; Nakagawa, K.; Shibata, A.; Awata, Y.; Kuriyama, I.; Shimazaki, N.; Koiwai, O.; Uchiyama, Y.; Sakaguchi, K.; Miyazawa, T.; et al. Inhibitory effect of tocotrienol on eukaryotic DNA polymerase λ and angiogenesis. Biochem. Biophys. Res. Commun. 2006, 339, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Nesaretnam, K.; Dorasamy, S.; Darbre, P.D. Tocotrienols inhibit growth of ZR-75-1 breast cancer cells. Int. J. Food Sci. Nutr. 2000, 51, 95–103. [Google Scholar] [CrossRef]
- Qureshi, A.A.; Qureshi, N.; Wright, J.; Shen, Z.; Kramer, G.; Gapor, A.; Chong, Y.H.; DeWitt, G.; Ong, A.; Peterson, D.M. Lowering of serum cholesterol in hypercholesterolemic humans by tocotrienols (palmvitee). Am. J. Clin. Nutr. 1991, 53, 1021S–1026S. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.A.; Sami, S.A.; Salser, W.A.; Khan, F.A. Dose-dependent suppression of serum cholesterol by tocotrienol-rich fraction (TRF 25) of rice bran in hypercholesterolemic humans. Atherosclerosis 2002, 161, 199–207. [Google Scholar] [CrossRef]
- Sen, C.K.; Khanna, S.; Roy, S.; Packer, L. Molecular basis of vitamin E action tocotrienol potently inhibits glutamate-induced pp60(c-Src) kinase activation and death of Ht4 neuronal cells. J. Biol. Chem. 2000, 275, 13049–13055. [Google Scholar] [CrossRef] [PubMed]
- Thor, H.; Ng, T. Effects of administration of α-tocopherol and tocotrienols on serum lipids and liver HMG CoA reductase activity. Int. J. Food Sci. Nutr. 2000, 51, S3–S11. [Google Scholar] [CrossRef]
- Chin, S.F.; Ibahim, J.; Makpol, S.; Abdul Hamid, N.A.; Abdul Latiff, A.; Zakaria, Z.; Mazlan, M.; Mohd Yusof, Y.A.; Abdul Karim, A.; Wan Ngah, W.Z. Tocotrienol rich fraction supplementation improved lipid profile and oxidative status in healthy older adults: A randomized controlled study. Nutr. Metab. 2011, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Heng, E.; Karsani, S.; Abdul Rahman, M.; Abdul Hamid, N.; Hamid, Z.; Wan Ngah, W. Supplementation with tocotrienol-rich fraction alters the plasma levels of Apolipoprotein A-I precursor, Apolipoprotein E precursor, and C-reactive protein precursor from young and old individuals. Eur. J. Nutr. 2013, 52, 1811–1820. [Google Scholar] [CrossRef] [PubMed]
- Jeno, P.; Mini, T.; Moes, S.; Hintermann, E.; Horst, M. Internal sequences from proteins digested in polyacrylamide gels. Anal. Biochem. 1995, 224, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Andreescu, S.; Hepel, M. Oxidative Stress: Diagnostics, Prevention, and Therapy; ACS Symposium Series; ACS Publication: Washington, DC, USA, 2011; Volume 1083. [Google Scholar]
- Sen, S.; Chakraborty, R. The Role of Antioxidants in Human Health. In Oxidative Stress: Diagnostics, Prevention, and Therapy; ACS Publication: Washington, DC, USA, 2011; Volume 1083, pp. 1–37. [Google Scholar]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Goon, J.A.; Azman, N.H.E.N.; Ghani, S.M.A.; Hamid, Z.; Ngah, W.Z.W. Comparing palm oil tocotrienol rich fraction with a-tocopherol supplementation on oxidative stress in healthy older adults. Clin. Nutr. ESPEN 2017, 21, 1–12. [Google Scholar] [CrossRef]
- Khor, S.C.; Ngah, W.Z.W.; Yusof, Y.A.M.; Karim, N.A.; Makpol, S. Tocotrienol-Rich Fraction Ameliorates Antioxidant Defense Mechanisms and Improves Replicative Senescence-Associated Oxidative Stress in Human Myoblasts. Oxid. Med. Cell. Longev. 2017, 2017, 3868305. [Google Scholar] [CrossRef] [PubMed]
- Aliahmat, N.S.; Noor, M.R.M.; Yusof, W.J.W.; Makpol, S.; Ngah, W.Z.W.; Yusof, Y.A.M. Antioxidant enzyme activity and malondialdehyde levels can be modulated by Piper betle, tocotrienol rich fraction and Chlorella vulgaris in aging C57BL/6 mice. Clinics 2012, 67, 1447–1454. [Google Scholar] [CrossRef]
- Ford, E.S.; Ajani, U.A.; Mokdad, A.H. Brief communication: The prevalence of high intake of vitamin E from the use of supplements among US adults. Ann. Intern. Med. 2005, 143, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ma, A.; Li, Y.; Han, X.; Wang, Q.; Liang, H. Vitamin E supplementation protects erythrocyte membranes from oxidative stress in healthy Chinese middle-aged and elderly people. Nutr. Res. 2012, 32, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Werninghaus, K.; Meydani, M.; Bhawan, J.; Margolis, R.; Blumberg, J.B.; Gilchrest, B.A. Evaluation of the photoprotective effect of oral vitamin E supplementation. Arch. Dermatol. 1994, 130, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- Chin, S.-F.; Hamid, N.A.A.; Latiff, A.A.; Zakaria, Z.; Mazlan, M.; Yusof, Y.A.; Karim, A.A.; Ibahim, J.; Hamid, Z.; Ngah, W.Z.; et al. Reduction of DNA damage in older healthy adults by Tri E® Tocotrienol supplementation. Nutrition 2008, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mastaloudis, A.; Leonard, S.W.; Traber, M.G. Oxidative stress in athletes during extreme endurance exercise. Free Radic. Biol. Med. 2001, 31, 911–922. [Google Scholar] [CrossRef]
- O’Byrne, D.; Grundy, S.; Packer, L.; Devaraj, S.; Baldenius, K.; Hoppe, P.P.; Kraemer, K.; Jialal, I.; Traber, M.G. Studies of LDL oxidation following α-, γ-, or δ-tocotrienyl acetate supplementation of hypercholesterolemic humans. Free Radic. Biol. Med. 2000, 29, 834–845. [Google Scholar] [CrossRef]
- Andriollo-Sanchez, M.; Hininger-Favier, I.; Meunier, N.; Venneria, E.; O’Connor, J.M.; Maiani, G.; Coudray, C.; Roussel, A.M. Age-related oxidative stress and antioxidant parameters in middle-aged and older European subjects: The ZENITH study. Eur. J. Clin. Nutr. 2005, 59, S58–S62. [Google Scholar] [CrossRef] [PubMed]
- Guemouri, L.; Artur, Y.; Herbeth, B.; Jeandel, C.; Cuny, G.; Siest, G. Biological variability of superoxide dismutase, glutathione peroxidase, and catalase in blood. Clin. Chem. 1991, 37, 1932–1937. [Google Scholar] [PubMed]
- Žitňanová, I.; Rakovan, M.; Paduchová, Z.; Dvořáková, M.; Andrezálová, L.; Muchová, J.; Šimko, M.; Waczulíková, I.; Ďuračková, Z. Oxidative stress in women with perimenopausal symptoms. Menopause 2011, 18, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Prokai, L.; Prokai-Tatrai, K.; Perjési, P.; Simpkins, J.W. Mechanistic insights into the direct antioxidant effects of estrogens. Drug Dev. Res. 2005, 66, 118–125. [Google Scholar] [CrossRef]
- Borras, C.; Sastre, J.; Garcia-Sala, D.; Lloret, A.; Pallardo, F.V.; Vina, J. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic. Biol. Med. 2003, 34, 546–552. [Google Scholar] [CrossRef]
- Borrás, C.; Gambini, J.; López-Grueso, R.; Pallardó, F.V.; Viña, J. Direct antioxidant and protective effect of estradiol on isolated mitochondria. Biochem. Biophys. Acta 2010, 1802, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Greenblatt, R.B.; Oettinger, M.; Bohler, C.S. Estrogen-Androgen Levels in Aging Men and Women: Therapeutic Considerations. J. Am. Geriatr. Soc. 1976, 24, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Scholz, R.W.; Graham, K.S.; Gumpricht, E.; Reddy, C.C. Mechanism of Interaction of Vitamin E and Glutathione in the Protection against Membrane Lipid Peroxidation. Ann. N. Y. Acad. Sci. 1989, 570, 514–517. [Google Scholar] [CrossRef]
- Halprin, K.M.; Ohkawara, A. The Measurement of Glutathione in Human Epidermis Using Glutathione Reductase. J. Investig. Dermatol. 1967, 48, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Codoñer-Franch, P.; Pons-Morales, S.; Boix-García, L.; Valls-Bellés, V. Oxidant/antioxidant status in obese children compared to pediatric patients with type 1 diabetes mellitus. Pediatr. Diabetes 2010, 11, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Moskaug, J.Ø.; Carlsen, H.; Myhrstad, M.C.; Blomhoff, R. Polyphenols and glutathione synthesis regulation. Am. J. Clin. Nutr. 2005, 81, 277S–283S. [Google Scholar] [CrossRef] [PubMed]
- Papp, L.V.; Holmgren, A.; Khanna, K.K. Selenium and selenoproteins in health and disease. Antioxid. Redox Signal. 2010, 12, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, M.; Karkowsky, A. Glutathione metabolism and some possible functions of glutathione in the nervous system. Int. Rev. Neurobiol. 1976, 19, 75–121. [Google Scholar] [PubMed]
- Sies, H. Hydroperoxides and Thiol Oxidants in the Study of Oxidative Stress in Intact Cells and Organs. In Oxidative Stress; Academic Press: London, UK, 1985; pp. 73–90. [Google Scholar]
- White, C.W.; Mimmack, R.E.; Repine, J.E. Accumulation of lung tissue oxidized glutathione (GSSG) as a marker of oxidant induced lung injury. Chest 1986, 89, 111S–113S. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Rossi, R.; Colombo, R.; Giustarini, D.; Milzani, A. Biomarkers of oxidative damage in human disease. Clin. Chem. 2006, 52, 601–623. [Google Scholar] [CrossRef] [PubMed]
- Owen, J.B.; Butterfield, D.A. Measurement of oxidized/reduced glutathione ratio. Methods Mol. Biol. 2010, 648, 269–277. [Google Scholar] [PubMed]
- Bonnefont-Rousselot, D.; Lacomblez, L.; Jaudon, M.-C.; Lepage, S.; Salachas, F.; Bensimon, G.; Bizard, C.; Doppler, V.; Delattre, J.; Meininger, V. Blood oxidative stress in amyotrophic lateral sclerosis. J. Neurol. Sci. 2000, 178, 57–62. [Google Scholar] [CrossRef]
- Cecchi, C.; Latorraca, S.; Sorbi, S.; Iantomasi, T.; Favilli, F.; Vincenzini, M.T.; Liguri, G. Gluthatione level is altered in lymphoblasts from patients with familial Alzheimer’s disease. Neurosci. Lett. 1999, 275, 152–154. [Google Scholar] [CrossRef]
- Lang, C.A.; Mills, B.J.; Mastropaolo, W.; Liu, M.C. Blood glutathione decreases in chronic diseases. J. Lab. Clin. Med. 2000, 135, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Mills, B.J.; Weiss, M.M.; Lang, C.A.; Liu, M.C.; Ziegler, C. Blood glutathione and cysteine changes in cardiovascular disease. J. Lab. Clin. Med. 2000, 135, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Samiec, P.S.; Drews-Botsch, C.; Flagg, E.W.; Kurtz, J.C.; Sternberg, P., Jr.; Reed, R.L.; Jones, D.P. Glutathione in human plasma: Decline in association with aging, age-related macular degeneration, and diabetes. Free Radic. Biol. Med. 1998, 24, 699–704. [Google Scholar] [CrossRef]
- Van Haaften, R.I.M.; Haenen, G.R.M.M.; Evelo, C.T.A.; Bast, A. Tocotrienols Inhibit Human Glutathione S-Transferase P1-1. IUBMB Life 2002, 54, 81–84. [Google Scholar] [CrossRef] [PubMed]





| Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo | α-Tocopherol | (Tocotrienol-Rich Fraction) TRF | |||||||
| Male | Female | Total | Male | Female | Total | Male | Female | Total | |
| Subjects (n) | 8 | 15 | 23 | 9 | 15 | 24 | 9 | 15 | 24 |
| Age (years) | 52.7 ± 1.6 | 52.1 ± 1.7 | 52.2 ± 2.1 | 53.5 ± 1.5 | 52.7 ± 1.2 | 52.5 ± 2.5 | 53.1 ± 1.3 | 52.7 ± 1.9 | 53.4 ± 1.5 |
| p value | - | - | - | 0.335 | 0.49 | 0.845 | 0.609 | 0.678 | 0.935 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nor Azman, N.H.E.; Goon, J.A.; Abdul Ghani, S.M.; Hamid, Z.; Wan Ngah, W.Z. Comparing Palm Oil, Tocotrienol-Rich Fraction and α-Tocopherol Supplementation on the Antioxidant Levels of Older Adults. Antioxidants 2018, 7, 74. https://doi.org/10.3390/antiox7060074
Nor Azman NHE, Goon JA, Abdul Ghani SM, Hamid Z, Wan Ngah WZ. Comparing Palm Oil, Tocotrienol-Rich Fraction and α-Tocopherol Supplementation on the Antioxidant Levels of Older Adults. Antioxidants. 2018; 7(6):74. https://doi.org/10.3390/antiox7060074
Chicago/Turabian StyleNor Azman, Nor Helwa Ezzah, Jo Aan Goon, Siti Madiani Abdul Ghani, Zalina Hamid, and Wan Zurinah Wan Ngah. 2018. "Comparing Palm Oil, Tocotrienol-Rich Fraction and α-Tocopherol Supplementation on the Antioxidant Levels of Older Adults" Antioxidants 7, no. 6: 74. https://doi.org/10.3390/antiox7060074





