Hydroxybenzoic Acids Are Significant Contributors to the Antioxidant Effect of Borututu Bark, Cochlospermum angolensis Welw. ex Oliv
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Procedures
2.2. Preparation of Total Extract and Solvent Fractions
2.3. HPLC Fingerprinting
2.4. Chromatographic Isolation and Purification of Compounds
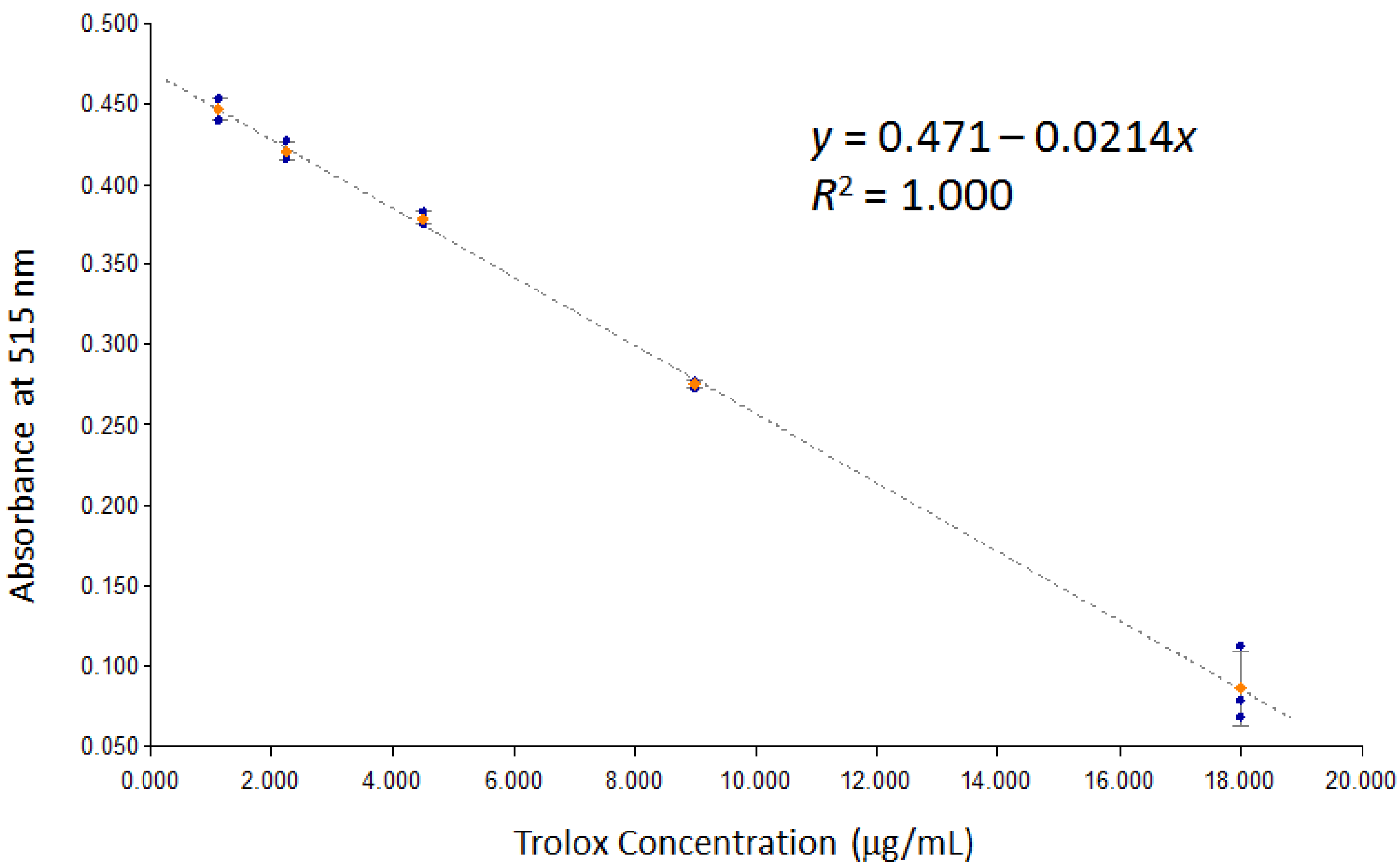
2.5. DPPH Assay [19]
3. Results & Discussion
3.1. Extraction, Fractionation, and Isolation of Active Compounds
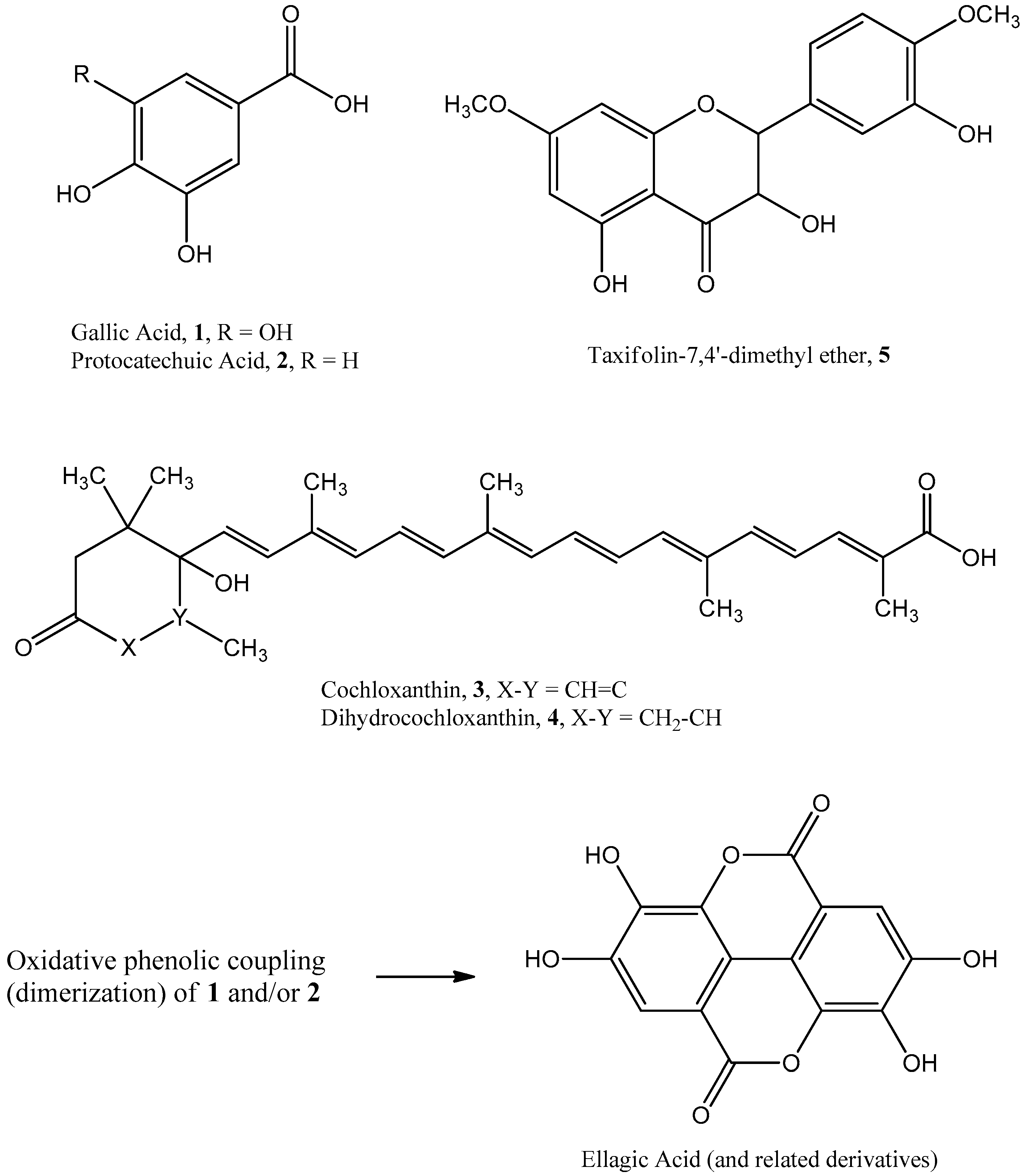
3.2. Identification of Pure Compounds
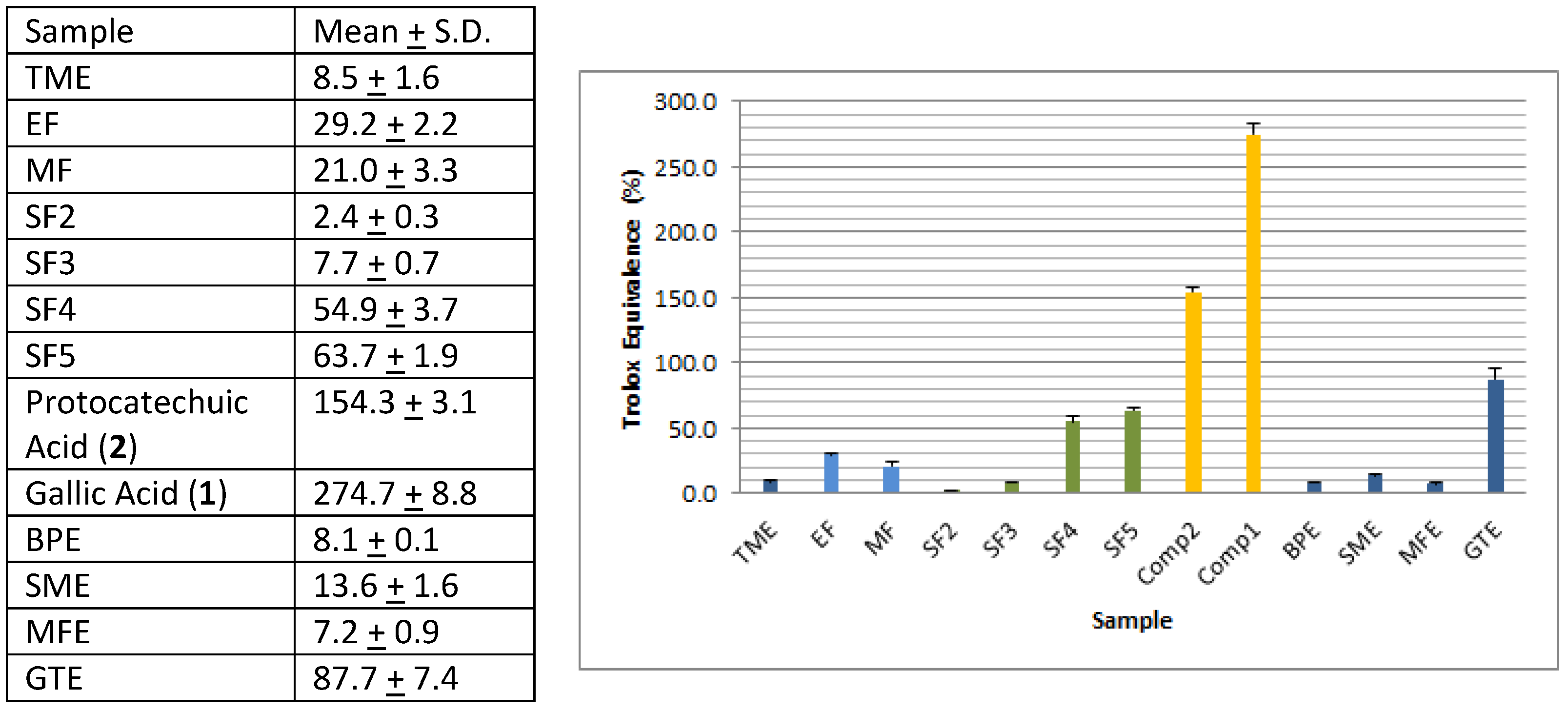
3.3. Evaluation of Antioxidant Activity
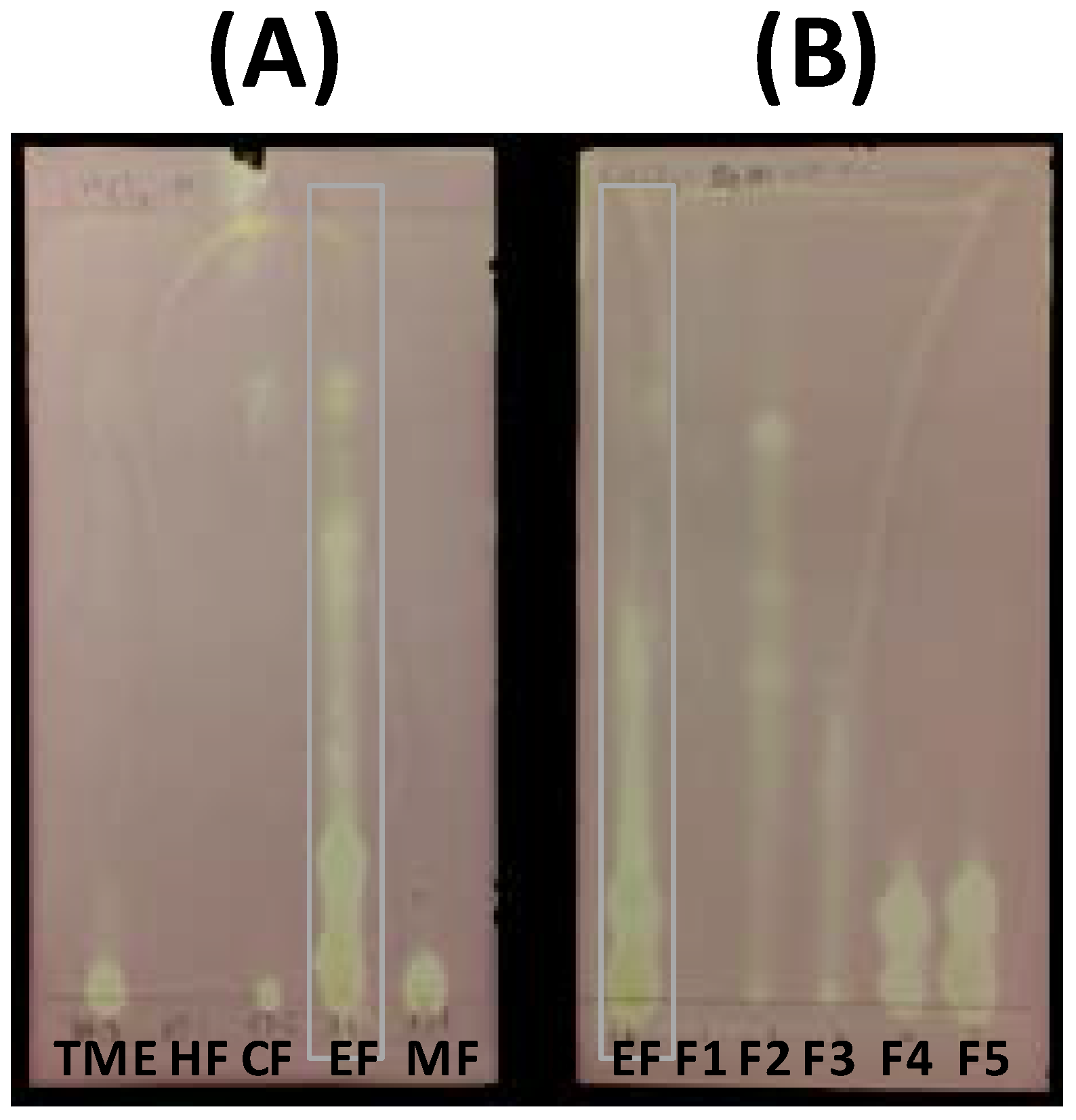
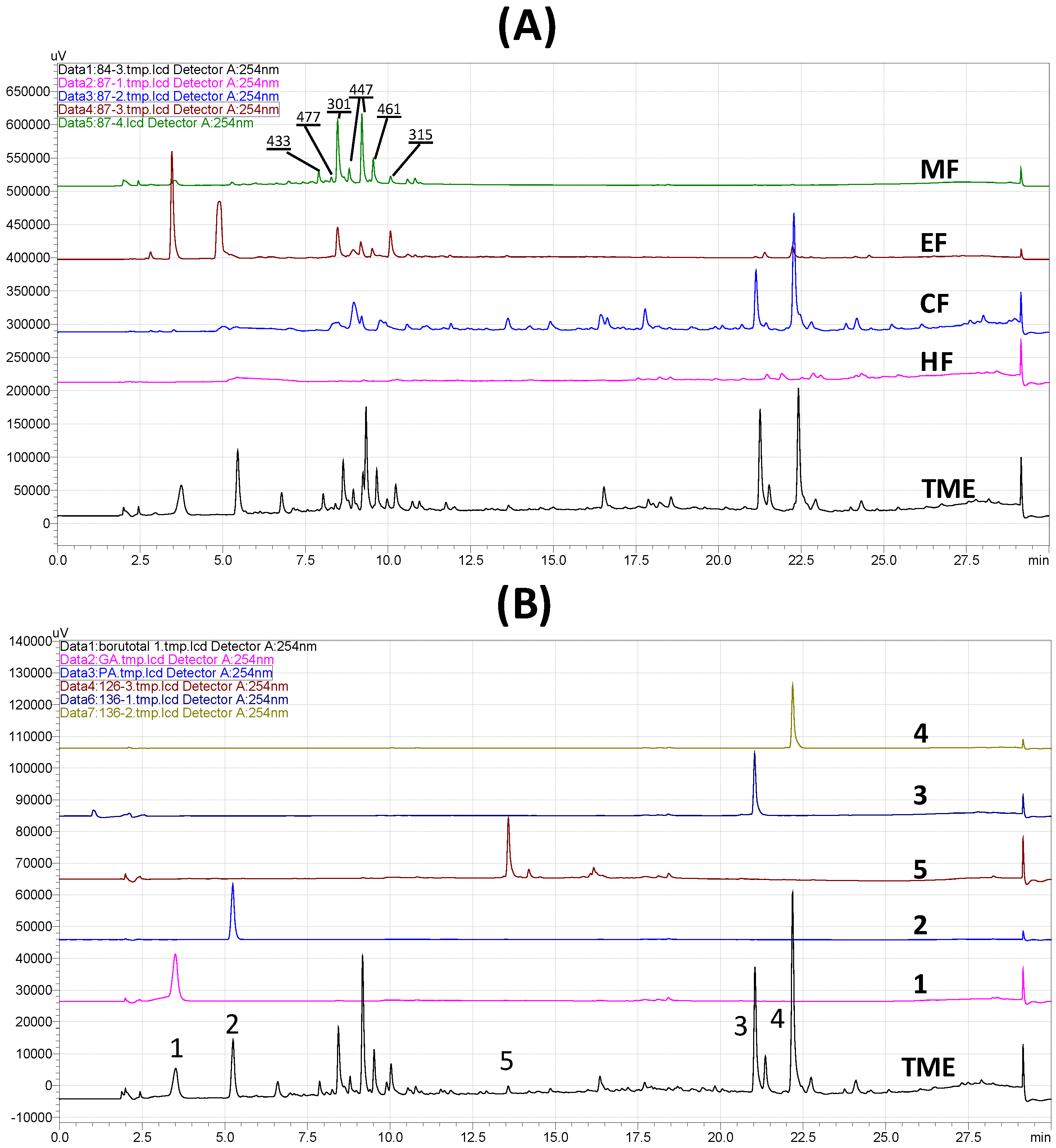
3.4. HPLC Analysis of Extract, Fractions, and Pure Compounds
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Barreira, J.C.; Morais, A.L.; Ferreira, I.C.; Oliviera, M.B. Insight on the formulation of herbal beverages with medicinal claims according with their antioxidant properties. Molecules 2013, 18, 2851–2863. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.F.R. Antioxidant properties, anti-hepatocellular carcinoma activity and hepatotoxicity of artichoke, milk thistle, and borututu. Ind. Crops Prod. 2013, 49, 61–65. [Google Scholar] [CrossRef]
- Presber, W.; Hegenscheid, B.; Hernandez-Alvarez, H.; Herrmann, D.; Brendel, C. Inhibition of the growth of Plasmodium falciparum and Plasmodium berghei in vitro by an extract of Cochlospermum angolense (Welw.). Acta Trop. 1992, 50, 331–338. [Google Scholar] [CrossRef]
- Presber, W.; Herrmann, D.K.; Hegenscheid, B. The effect of an extract from Cochlospermum angolense (“burututu”) on Plasmodium berghei in the mouse malaria suppression test. [Wirkung eines Extraktes aus Cochlospermum angolense (“Burututu”) auf Plasmodium berghei im Mausemalaria-Suppressionstest]. Angew. Parasitol. 1991, 32, 7–9. [Google Scholar] [PubMed]
- Ferreres, F.; Grosso, C.; Gil-Izquierdo, A.; Valentao, P.; Andrade, P.B. Ellagic acid and derivatives from Cochlospermum angolensis Welw. Extracts: HPLC–DAD–ESI/MSn profiling, quantification and in vitro anti-depressant, anti-cholinesterase and anti-oxidant activities. Phytochem. Anal. 2013, 24, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.S.G.; Nunes, M.A.; Almeida, I.M.C.; Carvalho, M.R.; Barroso, M.F.; Alves, R.C.; Oliviera, M.B.P.P. Teas, dietary supplements and fruit juices: A comparative study regarding antioxidant activity and bioactive compounds. LWT-Food Sci. Technol. 2012, 49, 324–328. [Google Scholar] [CrossRef]
- Leonardi, M.; Giovanelli, S.; Cioni, P.L.; Flamini, G.; Pistelli, L. Evaluation of volatile constituents of Cochlospermum angolense. Nat. Prod. Commun. 2012, 7, 629–632. [Google Scholar] [PubMed]
- Diallo, B.; Vanhaelen, M.; Kiso, Y.; Hikino, H. Antihepatotoxic action of Cochlospermum tinctorium rhizomes. J. Ethnopharmacol. 1987, 20, 239–243. [Google Scholar] [CrossRef]
- Nergard, C.S.; Diallo, D.; Inngjerdingen, K.; Michaelsen, T.E.; Matsumoto, T.; Kiyohara, H.; Yahmada, H.; Paulsen, B.S. Medicinal use of Cochlospermum tinctorium in Mali anti-ulcer-, radical scavenging- and immunomodulating activities of polymers in the aqueous extract of the roots. J. Ethnopharmacol. 2005, 96, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Ballin, N.Z.; Traore, M.; Tinto, H.; Sittie, A.; Molgaard, P.; Olsen, C.E.; Kharazmi, A.; Christensen, S.B. Antiplasmodial compounds from Cochlospermum tinctorium. J. Nat. Prod. 2002, 65, 1325–1327. [Google Scholar] [CrossRef] [PubMed]
- Musa, A.A. Cytotoxicity activity and phytochemical screening of Cochlospermum tinctorium perr ex A. rich rhizome. J. Appl. Pharm. Sci. 2012, 2, 155–159. [Google Scholar] [CrossRef]
- Lamien-Meda, A.; Kiendrebeogo, M.; Compaore, M.; Meda, R.N.T.; Bacher, M.; Koenig, K.; Pacher, T.; Fuehrer, H.P.; Noedl, H.; Willcox, M.; et al. Quality assessment and antiplasmodial activity of West African Cochlospermum species. Phytochemistry 2015, 119, 51–61. [Google Scholar] [CrossRef] [PubMed]
- E Silva, J.L.S.C.; de Paula Bicudo, B.; Rodrigues, A.B.; Mendonca, M.M.; Borges, R.R.; de Almeida, A.A.; de Oliviera, K.M.P. Evaluation of antibacterial and antifungal activity of ethanolic extract of Cochlospermum regium (Cochlospermaceae) leaf, a medicinal plant from the Cerrado of Brazil. BMC Proc. 2014, 8, 72. [Google Scholar] [CrossRef]
- Addae-Mensah, I.; Waibel, R.; Achenbach, H. Constituents of West African Medicinal Plants, XVI. Novel long-chain triacylbenzenes from Cochlospermum planchonii. Liebigs Ann. Chem. 1985, 6, 1284–1287. [Google Scholar] [CrossRef]
- Diallo, B.; Vanhaelen, M. Large scale purification of apocarotenoids from Cochlospermum tinctorium by countercurrent chromatography. J. Liq. Chromatogr. 1988, 1, 227–231. [Google Scholar] [CrossRef]
- Diallo, B.; Vanhaelen-Fastre, R.; Vanhaelen, M. Triacylbenzenes and long-chain volatile ketones from Cochlospermum tinctorium rhizome. Phytochemistry 1991, 30, 4153–4156. [Google Scholar] [CrossRef]
- De Almeida, S.C.X.; de Lemos, T.L.G.; Silveira, E.R.; Pessoa, O.D.L. Volatile and non-volatile chemical constituents of Cochlospermum vitifolium (Willdenow) Sprengel. Quim. Nova 2005, 28, 57–60. [Google Scholar] [CrossRef]
- Pereira, C.; Barros, L.; Ferreira, I.C.F.R. Analytical tools used to distinguish chemical profiles of plants widely consumed as infusions and dietary supplements: Artichoke, milk thistle, and borututu. Food Anal. Methods 2014, 7, 1604–1611. [Google Scholar] [CrossRef]
- Arocena-Roberson, C.L.; Abourashed, E.A.; Elsharkawy, N. Evaluation of cost versus antioxidant determinants in green tea dietary supplements. J. Am. Pharm. Assoc. 2015, 55, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Al-Mahdy, D.A.; El Dine, R.S.; Fahmy, S.; Yassin, A.; Porzel, A.; Brandt, W. Structure-activity relationships of antimicrobial gallic acid derivatives from pomegranate and acacia fruit extracts against potato bacterial wilt pathogen. Chem. Biodivers. 2015, 12, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Ayinde, B.A.; Onwukaeme, D.N.; Omogbai, E.K. Isolation and characterization of two phenolic compounds from the stem bark of Musanga Cecropioides R. Brown (Moraceae). Acta Pol. Pharm. 2007, 64, 183–185. [Google Scholar] [PubMed]
- Nessa, F.; Ismail, Z.; Mohamed, N.; Haris, M.R.H.M. Free-radical scavenging activity of organic extracts and of pure flavonoids of Blumea balsamifera DC leaves. Food Chem. 2004, 88, 243–252. [Google Scholar] [CrossRef]
- Gupta, A.D.; Bansal, V.K.; Babu, V.; Maithil, N. Chemistry, antioxidant and antimicrobial potential of nutmeg (Myristica fragrans Houtt). J. Gen. Eng. Biotech. 2013, 11, 25–31. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abourashed, E.A.; Fu, H.W. Hydroxybenzoic Acids Are Significant Contributors to the Antioxidant Effect of Borututu Bark, Cochlospermum angolensis Welw. ex Oliv. Antioxidants 2017, 6, 9. https://doi.org/10.3390/antiox6010009
Abourashed EA, Fu HW. Hydroxybenzoic Acids Are Significant Contributors to the Antioxidant Effect of Borututu Bark, Cochlospermum angolensis Welw. ex Oliv. Antioxidants. 2017; 6(1):9. https://doi.org/10.3390/antiox6010009
Chicago/Turabian StyleAbourashed, Ehab A., and Hao Wen Fu. 2017. "Hydroxybenzoic Acids Are Significant Contributors to the Antioxidant Effect of Borututu Bark, Cochlospermum angolensis Welw. ex Oliv" Antioxidants 6, no. 1: 9. https://doi.org/10.3390/antiox6010009




