Effects of Nesting Material Provision and High-Dose Vitamin C Supplementation during the Peripartum Period on Prepartum Nest-Building Behavior, Farrowing Process, Oxidative Stress Status, Cortisol Levels, and Preovulatory Follicle Development in Hyperprolific Sows
Abstract
:1. Introduction
2. Material and Methods
2.1. Animals and Management
2.2. Experimental Design
2.3. Data Collection
2.3.1. Saliva Collection and Assays
Saliva Collection
Assay for Oxidative Stress Parameters
Cortisol Assay
2.3.2. Colostrum Collection and Assays
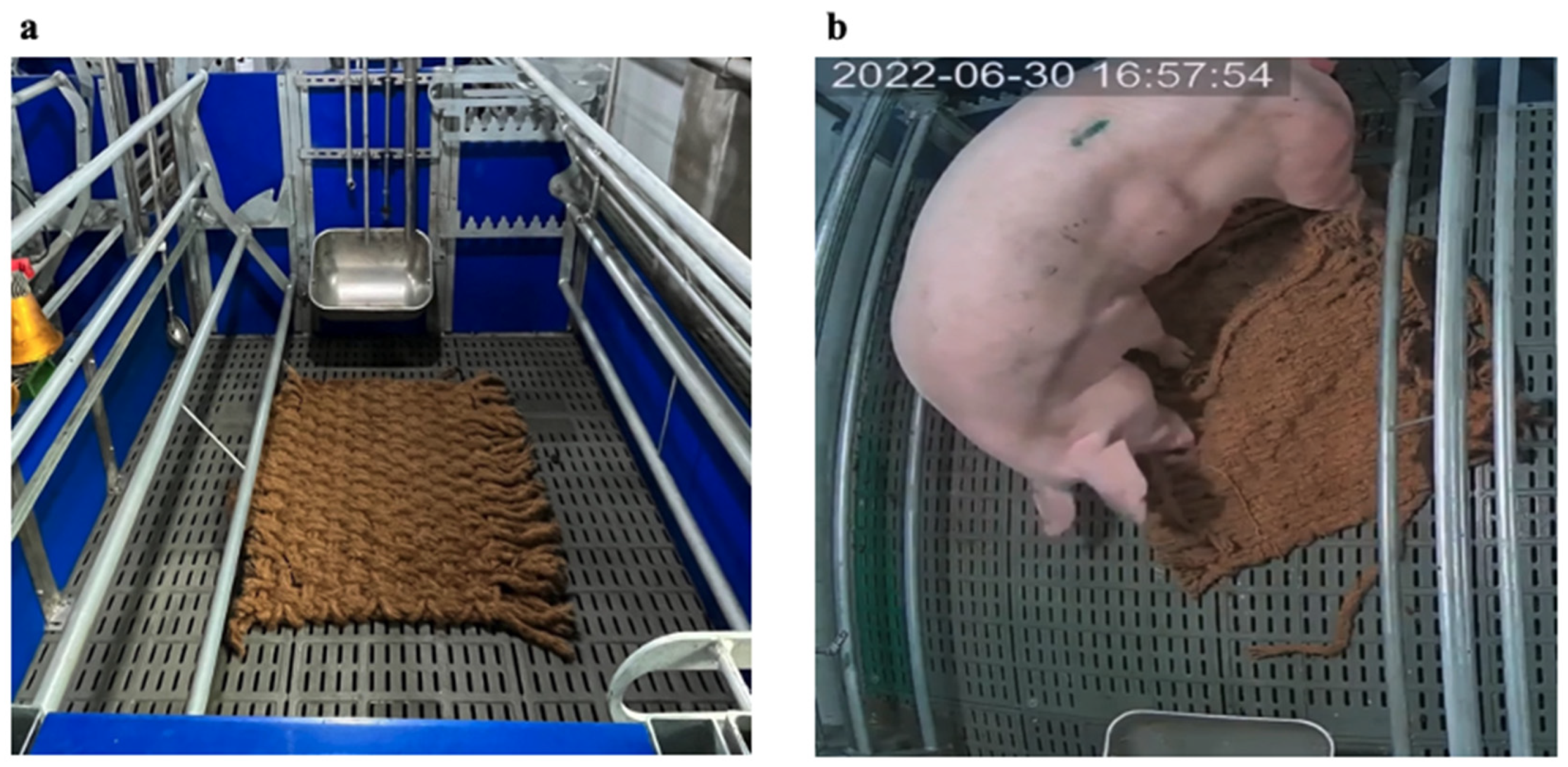
2.3.3. Behavioral Observation
2.3.4. Measurement of Follicle Size
2.4. Statistical Analysis
3. Results
3.1. Oxidative Stress Parameters
3.2. Salivary Cortisol Levels
3.3. Colostrum Oxytocin, Prolactin, and Ig Levels
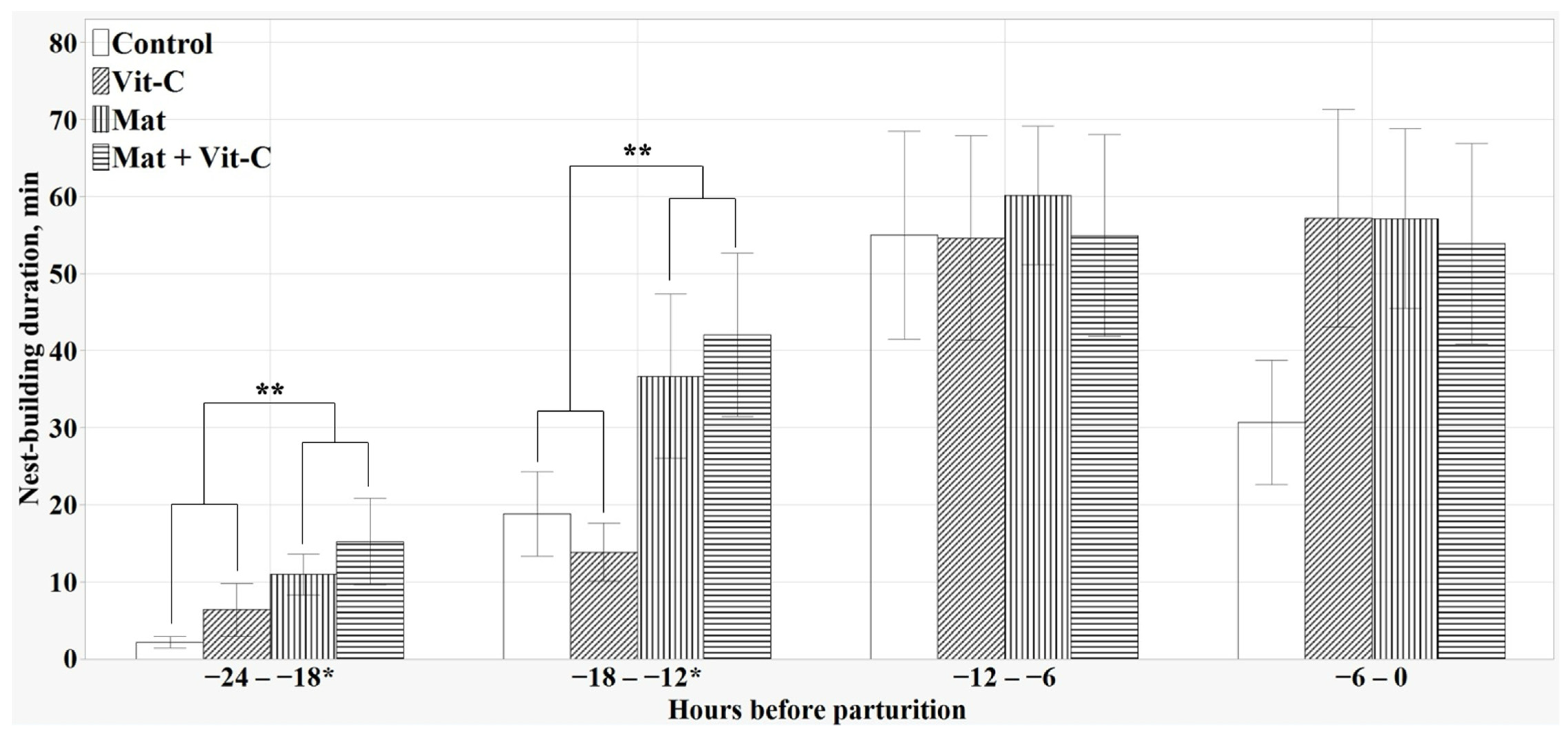
3.4. Prepartum NB
3.5. Farrowing Process and Litter Characteristics
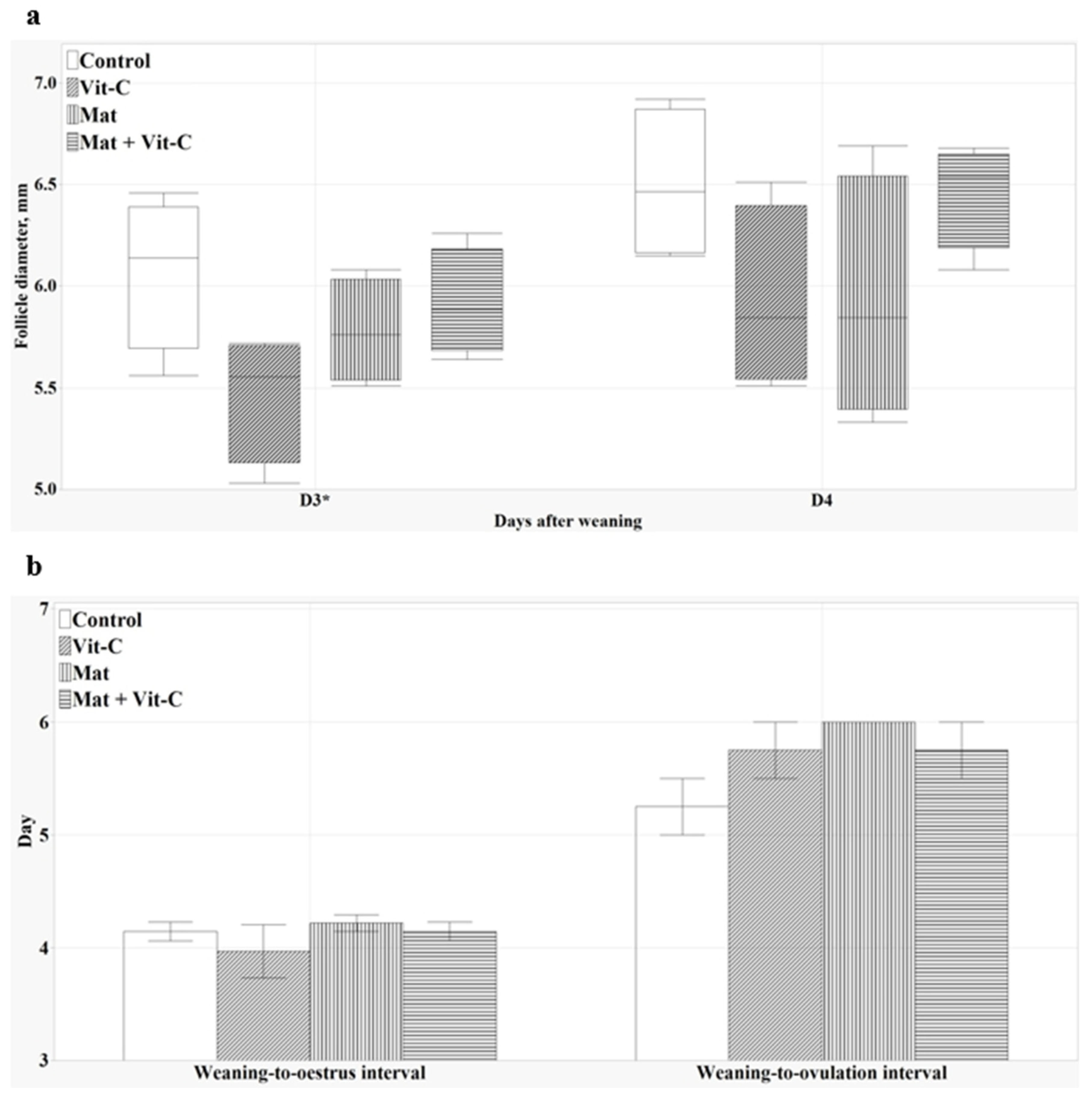
3.6. Follicular Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Tokach, M.D.; Menegat, M.B.; Gourley, K.M.; Goodband, R.D. Review: Nutrient Requirements of the Modern High-Producing Lactating Sow, with an Emphasis on Amino Acid Requirements. Animal 2019, 13, 2967–2977. [Google Scholar] [CrossRef] [PubMed]
- Langendijk, P.; Fleuren, M.; van Hees, H.; van Kempen, T. The Course of Parturition Affects Piglet Condition at Birth and Survival and Growth through the Nursery Phase. Animals 2018, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Weaver, A.C.; Shen, Y.B.; Zhao, Y. Improving Efficiency of Sow Productivity: Nutrition and Health. J. Anim. Sci. Biotechnol. 2013, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Shin, H.; Jo, J.; Lee, G.; Yun, J. Large Litter Size Increases Oxidative Stress and Adversely Affects Nest-Building Behavior and Litter Characteristics in Primiparous Sows. Front. Vet. Sci. 2023, 10, 1219572. [Google Scholar] [CrossRef] [PubMed]
- Berchieri-Ronchi, C.B.; Kim, S.W.; Zhao, Y.; Correa, C.R.; Yeum, K.J.; Ferreira, A.L.A. Oxidative Stress Status of Highly Prolific Sows during Gestation and Lactation. Animal 2011, 5, 1774–1779. [Google Scholar] [CrossRef] [PubMed]
- Szczubiał, M. Effect of Supplementation with Vitamins E, C and β-Carotene on Antioxidative/Oxidative Status Parameters in Sows during the Postpartum Period. Pol. J. Vet. Sci. 2015, 18, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Xing, M.; Gu, X. Research Progress on Oxidative Stress and Its Nutritional Regulation Strategies in Pigs. Animals 2021, 11, 1384. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, S.; Chen, F.; Guan, W.; Zhang, S. Nutritional Strategies to Alleviate Oxidative Stress in Sows. Anim. Nutr. 2022, 9, 60–73. [Google Scholar] [CrossRef]
- Qamar, A.Y.; Naveed, M.I.; Raza, S.; Fang, X.; Roy, P.K.; Bang, S.; Tanga, B.M.; Saadeldin, I.M.; Lee, S.; Cho, J. Role of Antioxidants in Fertility Preservation of Sperm—A Narrative Review. Anim. Biosci. 2023, 36, 385–403. [Google Scholar] [CrossRef]
- Hoang, X.; Shaw, G.; Fang, W.; Han, B. Possible Application of High-Dose Vitamin C in the Prevention and Therapy of Coronavirus Infection. J. Glob. Antimicrob. Resist. 2020, 23, 256–262. [Google Scholar] [CrossRef]
- EL-Gendy, K.S.; Aly, N.M.; Mahmoud, F.H.; Kenawy, A.; El-Sebae, A.K.H. The Role of Vitamin C as Antioxidant in Protection of Oxidative Stress Induced by Imidacloprid. Food Chem. Toxicol. 2010, 48, 215–221. [Google Scholar] [CrossRef]
- Uchio, R.; Hirose, Y.; Murosaki, S.; Ishigami, A. High Dietary Vitamin C Intake Reduces Glucocorticoid-Induced Immunosuppression and Measures of Oxidative Stress in Vitamin C-Deficient Senescence Marker Protein 30 Knockout Mice. Br. J. Nutr. 2019, 122, 1120–1129. [Google Scholar] [CrossRef]
- Zhao, Y.; Kim, S.W. Oxidative Stress Status and Reproductive Performance of Sows during Gestation and Lactation under Different Thermal Environments. Asian-Australas. J. Anim. Sci. 2020, 33, 722–731. [Google Scholar] [CrossRef]
- Zhao, Y.; Flowers, W.L.; Saraiva, A.; Yeum, K.-J.; Kim, S.W. Effect of Social Ranks and Gestation Housing Systems on Oxidative Stress Status, Reproductive Performance, and Immune Status of Sows. J. Anim. Sci. 2013, 91, 5848–5858. [Google Scholar] [CrossRef]
- Weber, R.; Keil, N.M.; Fehr, M.; Horat, R. Factors Affecting Piglet Mortality in Loose Farrowing Systems on Commercial Farms. Livest. Sci. 2009, 124, 216–222. [Google Scholar] [CrossRef]
- Ringgenberg, N.; Bergeron, R.; Meunier-Salaün, M.C.; Devillers, N. Impact of Social Stress during Gestation and Environmental Enrichment during Lactation on the Maternal Behavior of Sows. Appl. Anim. Behav. Sci. 2012, 136, 126–135. [Google Scholar] [CrossRef]
- Yun, J.; Swan, K.M.; Vienola, K.; Farmer, C.; Oliviero, C.; Peltoniemi, O.; Valros, A. Nest-Building in Sows: Effects of Farrowing Housing on Hormonal Modulation of Maternal Characteristics. Appl. Anim. Behav. Sci. 2013, 148, 77–84. [Google Scholar] [CrossRef]
- Yun, J.; Swan, K.M.; Vienola, K.; Kim, Y.Y.; Oliviero, C.; Peltoniemi, O.A.T.; Valros, A. Farrowing Environment Has an Impact on Sow Metabolic Status and Piglet Colostrum Intake in Early Lactation. Livest. Sci. 2014, 163, 120–125. [Google Scholar] [CrossRef]
- RDA (Rural Development Administration). Korean Feeding Standard for Swine, 4th ed.; National Institute of Animal Science: Jeonju, Republic of Korea, 2022. [Google Scholar]
- Rubio, C.P.; Contreras-Aguilar, M.D.; Quiles, A.; López-Arjona, M.; Cerón, J.J.; Martínez-Subiela, S.; Hevia, M.L.; Escribano, D.; Tecles, F. Biomarkers of Oxidative Stress in Saliva of Sheep: Analytical Performance and Changes after an Experimentally Induced Stress. Res. Vet. Sci. 2019, 123, 71–76. [Google Scholar] [CrossRef]
- Jarvis, S.; Lawrence, A.B.; McLean, K.A.; Deans, L.A.; Chirnside, J.; Calvert, S.K. The Effect of Environment on Behavioural Activity, ACTH, β-Endorphin and Cortisol in Pre-Farrowing Gilts. Anim. Sci. 1997, 65, 465–472. [Google Scholar] [CrossRef]
- Plush, K.J.; McKenny, L.A.; Nowland, T.L.; van Wettere, W.H.E.J. The Effect of Hessian and Straw as Nesting Materials on Sow Behaviour and Piglet Survival and Growth to Weaning. Animal 2021, 15, 100273. [Google Scholar] [CrossRef]
- Damm, B.I.; Pedersen, L.J.; Marchant-Forde, J.N.; Gilbert, C.L. Does Feed-Back from a Nest Affect Periparturient Behaviour, Heart Rate and Circulatory Cortisol and Oxytocin in Gilts? Appl. Anim. Behav. Sci. 2003, 83, 55–76. [Google Scholar] [CrossRef]
- Jarvis, S.; D’Eath, R.B.; Robson, S.K.; Lawrence, A.B. The Effect of Confinement during Lactation on the Hypothalamic-Pituitary- Adrenal Axis and Behaviour of Primiparous Sows. Physiol. Behav. 2006, 87, 345–352. [Google Scholar] [CrossRef]
- Yun, J.; Valros, A. Benefits of Prepartum Nest-Building Behaviour on Parturition and Lactation in Sows-a Review. Asian-Australas. J. Anim. Sci. 2015, 28, 1519–1524. [Google Scholar] [CrossRef]
- Godoy, L.D.; Rossignoli, M.T.; Delfino-Pereira, P.; Garcia-Cairasco, N.; Umeoka, E.H. de L. A Comprehensive Overview on Stress Neurobiology: Basic Concepts and Clinical Implications. Front. Behav. Neurosci. 2018, 12, 127. [Google Scholar] [CrossRef]
- Zulueta, A.; Esteve, M.J.; Frasquet, I.; Frígola, A. Vitamin C, Vitamin A, Phenolic Compounds and Total Antioxidant Capacity of New Fruit Juice and Skim Milk Mixture Beverages Marketed in Spain. Food Chem. 2007, 103, 1365–1374. [Google Scholar] [CrossRef]
- Arts, M.J.T.J.; Haenen, G.R.M.M.; Voss, H.P.; Bast, A. Antioxidant Capacity of Reaction Products Limits the Applicability of the Trolox Equivalent Antioxidant Capacity (TEAC) Assay. Food Chem. Toxicol. 2004, 42, 45–49. [Google Scholar] [CrossRef]
- Eliasson, C.; Isberg, S. Production and Composition of Sow Milk; Literature Review, Second cycle, A1N; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2011. [Google Scholar]
- Butler, W.R. Inhibition of Ovulation in the Postpartum Cow and the Lactating Sow. Livest. Prod. Sci. 2005, 98, 5–12. [Google Scholar] [CrossRef]
- Valros, A.; Rundgren, M.; Špinka, M.; Saloniemi, H.; Hultén, F.; Uvnäs-Moberg, K.; Tománek, M.; Krejcí, P.; Algers, B. Oxytocin, Prolactin and Somatostatin in Lactating Sows: Associations with Mobilisation of Body Resources and Maternal Behaviour. Livest. Prod. Sci. 2004, 85, 3–13. [Google Scholar] [CrossRef]
- Idriss, H.T.; Naismith, J.H. TNF alpha and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef]
- Mahdavi Sharif, P.; Jabbari, P.; Razi, S.; Keshavarz-Fathi, M.; Rezaei, N. Importance of TNF-Alpha and Its Alterations in the Development of Cancers. Cytokine 2020, 130, 155066. [Google Scholar] [CrossRef]
- Liu, H.; Yi, R.; Bi, Y.; Li, J.; Li, X.; Xu, S.; Bao, J. Physiology, Immunity, Stereotyped Behavior, and Production Performance of the Lactating Sows in the Enriched Environment. Int. J. Appl. Res. Vet. Med. 2018, 16, 44–51. [Google Scholar]
- Chen, Y.; Luo, G.; Yuan, J.; Wang, Y.; Yang, X.; Wang, X.; Li, G.; Liu, Z.; Zhong, N. Vitamin C Mitigates Oxidative Stress and Tumor Necrosis Factor-Alpha in Severe Community-Acquired Pneumonia and LPS-Induced Macrophages. Mediators Inflamm. 2014, 2014, 426740. [Google Scholar] [CrossRef]
- Mainau, E.; Manteca, X. Pain and Discomfort Caused by Parturition in Cows and Sows. Appl. Anim. Behav. Sci. 2011, 135, 241–251. [Google Scholar] [CrossRef]
- Nathan, C.; Ding, A. Nonresolving Inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Lawrence, A.B.; Petherick, J.C.; Mclean, K.A.; Deans, L.; Chirnside, J.; Vaughan, A.; Gilbert, C.L.; Forsling, M.L.; Russell, J.A. The Effects of Chronic Environmental Stress on Parturition and on Oxytocin and Vasopressin Secretion in the Pig. Anim. Reprod. Sci. 1995, 38, 251–264. [Google Scholar] [CrossRef]
- Rushen, J.; Schwarze, N.; Ladewig, J.; Foxcroft, G.; Schwarze, N.; Ladewig, J.; FOXCROFT Opioid, G. Opioid Modulation of the Effects of Repeated Stress on ACTH, Cortisol, Prolactin, and Growth Hormone in Pigs. Physiol. Behav. 1993, 53, 923–928. [Google Scholar] [CrossRef]
- Damm, B.I.; Vestergaard, K.S.; Schrøder-Petersen, D.L.; Ladewig, J. The Effects of Branches on Prepartum Nest Building in Gilts with Access to Straw. Appl. Anim. Behav. Sci. 2000, 69, 113–124. [Google Scholar] [CrossRef]
- Heimer, K.A.; Hart, A.M.; Martin, L.G.; Rubio-Wallace, S. Examining the Evidence for the Use of Vitamin C in the Prophylaxis and Treatment of the Common Cold. J. Am. Acad. Nurse Pract. 2009, 21, 295–300. [Google Scholar] [CrossRef]
- Oliviero, C.; Heinonen, M.; Valros, A.; Peltoniemi, O. Environmental and Sow-Related Factors Affecting the Duration of Farrowing. Anim. Reprod. Sci. 2010, 119, 85–91. [Google Scholar] [CrossRef]
- Chambial, S.; Dwivedi, S.; Shukla, K.K.; John, P.J.; Sharma, P. Vitamin C in Disease Prevention and Cure: An Overview. Indian J. Clin. Biochem. 2013, 28, 314–328. [Google Scholar] [CrossRef]
- Mahan, D.C.; Ching, S.; Dabrowski, K. Developmental Aspects and Factors Influencing the Synthesis and Status of Ascorbic Acid in the Pig. Annu. Rev. Nutr. 2004, 24, 79–103. [Google Scholar] [CrossRef]
- Wischner, D.; Kemper, N.; Krieter, J. Nest-Building Behaviour in Sows and Consequences for Pig Husbandry. Livest. Sci. 2009, 124, 1–8. [Google Scholar] [CrossRef]
- Markowska-Daniel, I.; Pomorska-Mól, M. Shifts in immunoglobulins levels in the porcine mammary secretions during whole lactation period. Bull. Vet. Inst. Pulawy 2010, 54, 345–349. [Google Scholar]
- Nuntapaitoon, M.; Suwimonteerabutr, J.; Am-in, N.; Tienthai, P.; Chuesiri, P.; Kedkovid, R.; Tummaruk, P. Impact of Parity and Housing Conditions on Concentration of Immunoglobulin G in Sow Colostrum. Trop. Anim. Health Prod. 2019, 51, 1239–1246. [Google Scholar] [CrossRef]
- Studnitz, M.; Jensen, M.B.; Pedersen, L.J. Why Do Pigs Root and in What Will They Root? A Review on the Exploratory Behaviour of Pigs in Relation to Environmental Enrichment. Appl. Anim. Behav. Sci. 2007, 107, 183–197. [Google Scholar] [CrossRef]
- Godyń, D.; Nowicki, J.; Herbut, P. Effects of Environmental Enrichment on Pig Welfare—A Review. Animals 2019, 9, 383. [Google Scholar] [CrossRef]
- Algers, B.; Uvnäs-Moberg, K. Maternal Behavior in Pigs. Horm. Behav. 2007, 52, 78–85. [Google Scholar] [CrossRef]
- Baxter, M.R. Ethology in environmental design for animal production. Appl. Anim. Ethol. 1983, 9, 207–220. [Google Scholar] [CrossRef]
- Rosvold, E.M.; Newberry, R.C.; Framstad, T.; Andersen, I.L. Nest-Building Behaviour and Activity Budgets of Sows Provided with Different Materials. Appl. Anim. Behav. Sci. 2018, 200, 36–44. [Google Scholar] [CrossRef]
- Yun, J.; Han, T.; Björkman, S.; Nystén, M.; Hasan, S.; Valros, A.; Oliviero, C.; Kim, Y.; Peltoniemi, O. Factors affecting piglet mortality during the first 24 h after the onset of parturition in large litters: Effects of farrowing housing on behaviour of postpartum sows. Animal 2019, 13, 1045–1053. [Google Scholar] [CrossRef]
- Fiebich, B.L.; Lieb, K.; Kammerer, N.; Hüll, M. Synergistic Inhibitory Effect of Ascorbic Acid and Acetylsalicylic Acid on Prostaglandin E2 Release in Primary Rat Microglia. J. Neurochem. 2003, 86, 173–178. [Google Scholar] [CrossRef]
- Rosenkrans, C.F.; Paria, B.C.; Davis, D.L.; Milliken, G. In vitro synthesis of prostaglandin E and F2α by pig endometrium in the presence of estradiol, catechol estrogen and ascorbic acid. J. Anim. Sci. 1990, 68, 435–443. [Google Scholar] [CrossRef]
- Lawrence, A.B.; McLean, K.A.; Jarvis, S.; Gilbert, C.L.; Petherick, J.C. Stress and Parturition in the Pig. Reprod. Domest. Anim. 1997, 32, 231–236. [Google Scholar] [CrossRef]
- Oliviero, C.; Heinonen, M.; Valros, A.; Hälli, O.; Peltoniemi, O.A.T. Effect of the Environment on the Physiology of the Sow during Late Pregnancy, Farrowing and Early Lactation. Anim. Reprod. Sci. 2008, 105, 365–377. [Google Scholar] [CrossRef]
- Timoshnikov, V.A.; Kobzeva, T.V.; Polyakov, N.E.; Kontoghiorghes, G.J. Redox Interactions of Vitamin c and Iron: Inhibition of the pro-Oxidant Activity by Deferiprone. Int. J. Mol. Sci. 2020, 21, 3967. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Sharma, R.K. Role of Oxidative Stress in Female Reproduction. Reprod. Biol. Endocrinol. 2005, 3, 28. [Google Scholar] [CrossRef]
- Tarín, J.J.; Pérez-Albalá, S.; Cano, A. Oral Antioxidants Counteract the Negative Effects of Female Aging on Oocyte Quantity and Quality in the Mouse. Mol. Reprod. Dev. 2002, 61, 385–397. [Google Scholar] [CrossRef]
- Kere, M.; Siriboon, C.; Lo, N.-W.; Nguyen, N.T.; Ju, J.-C. Ascorbic acid improves the developmental competence of porcine oocytes after parthenogenetic activation and somatic cell nuclear transplantation. J. Reprod. Dev. 2013, 59, 78–84. [Google Scholar] [CrossRef]
- Shaeib, F.; Banerjee, J.; Maitra, D.; Diamond, M.P.; Abu-Soud, H.M. Impact of Hydrogen Peroxide-Driven Fenton Reaction on Mouse Oocyte Quality. Free Radic. Biol. Med. 2013, 58, 154–159. [Google Scholar] [CrossRef] [PubMed]

| Control | Vit-C | Mat | Mat + Vit-C | SEM | p Value | |||
|---|---|---|---|---|---|---|---|---|
| N | 9 | 8 | 10 | 8 | Treatment | Mat 1 | Vit-C 2 | |
| AOPP level (μmol/L) 3 | ||||||||
| Day −4 | 9.38 a | 4.79 b | 5.41 b | 7.85 ab | 0.67 | 0.047 | 0.694 | 0.651 |
| Day 1 | 7.54 | 7.33 | 6.11 | 5.43 | 0.67 | 0.521 | 0.175 | 0.709 |
| Day 7 | 8.24 a | 3.10 ab | 2.81 b | 3.60 ab | 0.91 | 0.046 | 0.086 | 0.495 |
| Day 28 | 2.62 | 2.03 | 4.14 | 7.14 | 0.69 | 0.292 | 0.093 | 0.905 |
| Day −4–28 | 7.37 a | 4.83 ab | 4.66 b | 5.81 ab | 0.39 | 0.042 | 0.193 | 0.401 |
| TEAC level (μM) 3 | ||||||||
| Day −4 | 255.54 | 246.57 | 262.47 | 248.21 | 6.97 | 0.827 | 0.648 | 0.414 |
| Day 1 | 210.33 ab | 242.50 ab | 180.46 b | 254.88 a | 10.07 | 0.040 | 0.361 | 0.014 |
| Day 7 | 219.44 | 208.34 | 211.20 | 218.26 | 7.54 | 0.956 | 0.977 | 0.870 |
| Day 28 | 187.62 | 226.34 | 204.55 | 178.89 | 11.73 | 0.464 | 0.405 | 0.971 |
| Day −4–28 | 218.23 | 230.93 | 214.67 | 225.06 | 4.89 | 0.478 | 0.343 | 0.217 |
| H2O2 level (μM) 3 | ||||||||
| Day −4 | 5.51 | 9.67 | 24.36 | 8.11 | 3.69 | 0.285 | 0.142 | 0.787 |
| Day 1 | 14.58 | 16.92 | 10.29 | 19.40 | 1.77 | 0.788 | 0.405 | 0.557 |
| Day 7 | 9.04 | 11.01 | 8.36 | 5.91 | 1.25 | 0.786 | 0.828 | 0.408 |
| Day 28 | 17.55 | 24.05 | 14.56 | 13.96 | 1.99 | 0.273 | 0.074 | 0.958 |
| Day −4–28 | 12.23 | 15.76 | 13.58 | 12.09 | 1.09 | 0.614 | 0.896 | 0.985 |
| TNF-α level (pg/mL) 3 | ||||||||
| Day −4 | 63.28 | 99.21 | 61.11 | 63.40 | 9.84 | 0.570 | 0.414 | 0.244 |
| Day 1 | 104.85 | 61.40 | 101.40 | 97.11 | 11.71 | 0.370 | 0.312 | 0.256 |
| Day 7 | 51.74 | 47.17 | 60.35 | 43.03 | 4.52 | 0.662 | 0.858 | 0.268 |
| Day 28 | 43.36 | 41.77 | 44.33 | 62.84 | 5.28 | 0.801 | 0.433 | 0.724 |
| Day −4–28 | 65.81 | 61.20 | 66.80 | 66.60 | 4.41 | 0.834 | 0.630 | 0.652 |
| Control | Vit-C | Mat | Mat + Vit-C | SEM | p Value | |||
|---|---|---|---|---|---|---|---|---|
| N | 9 | 8 | 10 | 8 | Treatment | Mat 1 | Vit-C 2 | |
| Concentrations (pg/mL) 3 | ||||||||
| Oxytocin | 135.20 | 138.03 | 153.51 | 114.94 | 12.53 | 0.677 | 0.876 | 0.569 |
| Prolactin | 66.68 | 64.48 | 76.11 | 63.51 | 3.95 | 0.600 | 0.575 | 0.304 |
| Concentrations (pg/mL) 4 | ||||||||
| IgG | 49.06 | 48.79 | 66.65 | 47.70 | 5.22 | 0.613 | 0.385 | 0.608 |
| IgM | 136.39 | 112.40 | 161.57 | 128.03 | 7.24 | 0.212 | 0.279 | 0.077 |
| IgA | 329.99 | 373.08 | 297.46 | 388.89 | 20.02 | 0.487 | 0.916 | 0.219 |
| Control | Vit-C | Mat | Mat + Vit-C | SEM | p Value | |||
|---|---|---|---|---|---|---|---|---|
| N | 9 | 8 | 10 | 8 | Treatment | Mat 1 | Vit-C 2 | |
| Farrowing process (min) | ||||||||
| Farrowing duration | 205.64 c | 242.57 a | 203.56 c | 214.37 b | 16.64 | <0.0001 | 0.008 | <0.0001 |
| Birth interval | 14.40 | 17.05 | 13.97 | 15.47 | 1.19 | 0.277 | 0.474 | 0.078 |
| Birth-to-udder touch interval | 25.52 a | 25.55 a | 17.92 b | 21.97 ab | 1.46 | 0.003 | 0.002 | 0.045 |
| Litter characteristics | ||||||||
| Total births, n | 15.00 | 15.50 | 16.40 | 14.63 | 0.27 | 0.788 | 0.789 | 0.622 |
| Live births, n | 14.56 | 15.16 | 15.10 | 13.00 | 0.31 | 0.640 | 0.603 | 0.542 |
| Stillbirths, n | 0.44 | 0.38 | 1.30 | 1.63 | 0.20 | 0.053 | 0.006 | 0.965 |
| Piglet birth weight, kg | 1.50 | 1.44 | 1.39 | 1.53 | 0.03 | 0.270 | 0.773 | 0.358 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, H.; Lee, J.; Kim, J.; Lee, G.; Yun, J. Effects of Nesting Material Provision and High-Dose Vitamin C Supplementation during the Peripartum Period on Prepartum Nest-Building Behavior, Farrowing Process, Oxidative Stress Status, Cortisol Levels, and Preovulatory Follicle Development in Hyperprolific Sows. Antioxidants 2024, 13, 210. https://doi.org/10.3390/antiox13020210
Shin H, Lee J, Kim J, Lee G, Yun J. Effects of Nesting Material Provision and High-Dose Vitamin C Supplementation during the Peripartum Period on Prepartum Nest-Building Behavior, Farrowing Process, Oxidative Stress Status, Cortisol Levels, and Preovulatory Follicle Development in Hyperprolific Sows. Antioxidants. 2024; 13(2):210. https://doi.org/10.3390/antiox13020210
Chicago/Turabian StyleShin, Hyeonwook, Juho Lee, Junsik Kim, Geonil Lee, and Jinhyeon Yun. 2024. "Effects of Nesting Material Provision and High-Dose Vitamin C Supplementation during the Peripartum Period on Prepartum Nest-Building Behavior, Farrowing Process, Oxidative Stress Status, Cortisol Levels, and Preovulatory Follicle Development in Hyperprolific Sows" Antioxidants 13, no. 2: 210. https://doi.org/10.3390/antiox13020210






