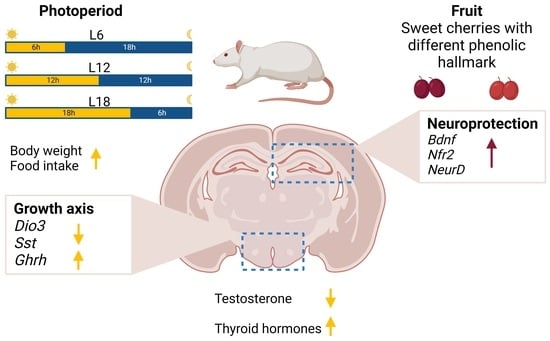
Cherries with Different Geographical Origins Regulate Neuroprotection in a Photoperiod-Dependent Manner in F344 Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit Preparation and Composition
2.2. Experimental Design
2.3. High-Performance Liquid Chromatography Coupled to Triple Quadrupole (LC-QqQ)
2.4. Gene Expression Analysis in the Hypothalamus and Hippocampus
2.5. Statistical Analysis
3. Results
3.1. Effects of Photoperiod on Body Weight, Eating Pattern Index and Serum Testosterone Levels in F344 Rats
3.2. Hypothalamic Gene Expression Responds to Photoperiod
3.3. Sweet Cherry Consumption Exerts a Hippocampal Neuroprotective Effect, Which Was Modulated by Specific Phenolic Hallmarks and Photoperiod
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Helfer, G.; Barrett, P.; Morgan, P.J. A unifying hypothesis for control of body weight and reproduction in seasonally breeding mammals. J. Neuroendocrinol. 2019, 31, e12680. [Google Scholar] [CrossRef] [PubMed]
- Tavolaro, F.M.; Thomson, L.M.; Ross, A.W.; Morgan, P.J.; Helfer, G. Photoperiodic effects on seasonal physiology, reproductive status and hypothalamic gene expression in young male F344 rats. J. Neuroendocrinol. 2015, 27, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Elizondo-Vega, R.J.; Recabal, A.; Oyarce, K. Nutrient sensing by hypothalamic tanycytes. Front. Endocrinol. 2019, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Langlet, F. Tanycyte gene expression dynamics in the regulation of energy homeostasis. Front. Endocrinol. 2019, 10, 286. [Google Scholar] [CrossRef] [PubMed]
- Ibars, M.; Aragonès, G.; Ardid-Ruiz, A.; Gibert-Ramos, A.; Arola-Arnal, A.; Suárez, M.; Bladé, C. Seasonal consumption of polyphenol-rich fruits affects the hypothalamic leptin signaling system in a photoperiod-dependent mode. Sci. Rep. 2018, 8, 13572. [Google Scholar] [CrossRef]
- Arola-Arnal, A.; Cruz-Carrión, Á.; Torres-Fuentes, C.; Ávila-Román, J.; Aragonès, G.; Mulero, M.; Bravo, F.I.; Muguerza, B.; Arola, L.; Suárez, M. Chrononutrition and polyphenols: Roles and diseases. Nutrients 2019, 11, 2602. [Google Scholar] [CrossRef]
- Kimble, R.; Keane, K.M.; Lodge, J.K.; Cheung, W.; Haskell-Ramsay, C.F.; Howatson, G. Polyphenol-rich tart cherries (Prunus Cerasus, cv Montmorency) improve sustained attention, feelings of alertness and mental fatigue and influence the plasma metabolome in middle-aged adults: A randomised, placebo-controlled trial. Br. J. Nutr. 2022, 128, 2409–2420. [Google Scholar] [CrossRef]
- Shukitt-Hale, B.; Kelly, M.; Bielinski, D.; Fisher, D. Tart cherry extracts reduce inflammatory and oxidative stress signaling in microglial cells. Antioxidants 2016, 5, 33. [Google Scholar] [CrossRef]
- Rendeiro, C.; Vauzour, D.; Kean, R.J.; Butler, L.T.; Rattray, M.; Spencer, J.P.E.; Williams, C.M. Blueberry supplementation induces spatial memory improvements and region-specific regulation of hippocampal BDNF mRNA expression in young rats. Psychopharmacology 2012, 223, 319–330. [Google Scholar] [CrossRef]
- Bruna, B.; Lobos, P.; Herrera-Molina, R.; Hidalgo, C.; Paula-Lima, A.; Adasme, T. The signaling pathways underlying BDNF-induced Nrf2 hippocampal nuclear translocation involve ROS, RyR-Mediated Ca2+ signals, ERK and PI3K. Biochem. Biophys. Res. Commun. 2018, 505, 201–207. [Google Scholar] [CrossRef]
- Cruz-Carrión, Á.; Ruiz de Azua, M.J.; Mulero, M.; Arola-Arnal, A.; Suárez, M. Oxidative stress in rats is modulated by seasonal consumption of sweet cherries from different geographical origins: Local vs. non-local. Nutrients 2020, 12, 2854. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, G.; Bacchetti, T.; Belleggia, A.; Neri, D. Cherry antioxidants: From farm to table. Molecules 2010, 15, 6993–7005. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, L.R.S.; Silva, G.R.; Luís, Â.; Cardoso, H.J.; Correia, S.; Vaz, C.V.; Duarte, A.P.; Socorro, S. Sweet cherries as anti-cancer agents: From bioactive compounds to function. Molecules 2021, 26, 2941. [Google Scholar] [CrossRef] [PubMed]
- Martini, S.; Conte, A.; Tagliazucchi, D. Phenolic compounds profile and antioxidant properties of six sweet cherry (Prunus avium) cultivars. Food Res. Int. 2017, 97, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Carres, L.; Mas-Capdevila, A.; Bravo, F.I.; Mulero, M.; Muguerza, B.; Arola-Arnal, A. Optimization and characterization of Royal Dawn cherry (Prunus avium) phenolics extraction. Sci. Rep. 2019, 9, 17626. [Google Scholar] [CrossRef] [PubMed]
- Ruiz de Azua, M.J.; Manocchio, F.; Cruz-Carrión, Á.; Arola-Arnal, A.; Torres-Fuentes, C.; Bernal, C.A.; Saín, J.; Suarez, M. Fatty acid metabolism in liver and muscle is strongly modulated by photoperiod in Fischer 344 rats. J. Photochem. Photobiol. B Biol. 2023, 238, 112621. [Google Scholar] [CrossRef] [PubMed]
- Chockchaisawasdee, S.; Golding, J.B.; Vuong, Q.V.; Papoutsis, K.; Stathopoulos, C.E. Sweet cherry: Composition, postharvest preservation, processing and trends for its future use. Trends Food Sci. Technol. 2016, 55, 72–83. [Google Scholar] [CrossRef]
- Blando, F.; Oomah, B.D. Sweet and sour cherries: Origin, distribution, nutritional composition and health benefits. Trends Food Sci. Technol. 2019, 86, 517–529. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis, 15th ed.; Helrich, K., Ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990; Volume 1. [Google Scholar]
- Arnous, A.; Makris, D.P.; Kefalas, P. Correlation of pigment and flavanol content with antioxidant properties in selected aged regional wines from Greece. J. Food Compos. Anal. 2002, 15, 655–665. [Google Scholar] [CrossRef]
- Cacace, J.E.; Mazza, G. Optimization of extraction of anthocyanins from black currants with aqueous ethanol. J. Food Sci. 2003, 68, 240–248. [Google Scholar] [CrossRef]
- McLean, S.L.; Yun, H.; Tedder, A.; Helfer, G. The effect of photoperiod and high fat diet on the cognitive response in photoperiod-sensitive F344 rats. Physiol. Behav. 2021, 239, 113496. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Masip, È.; Manocchio, F.; Rodríguez, R.M.; Bravo, F.I.; Torres-Fuentes, C.; Muguerza, B.; Aragonès, G. Photoperiod-dependent effects of grape-seed proanthocyanidins on adipose tissue metabolic markers in healthy rats. Mol. Nutr. Food Res. 2023, 67, 2300035. [Google Scholar] [CrossRef] [PubMed]
- Mariné-Casadó, R.; Domenech-Coca, C.; del Bas, J.M.; Bladé, C.; Caimari, A.; Arola, L. Cherry consumption out of season alters lipid and glucose homeostasis in normoweight and cafeteria-fed obese Fischer 344 rats. J. Nutr. Biochem. 2019, 63, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Domenech-Coca, C.; Mariné-Casadó, R.; Caimari, A.; Arola, L.; del Bas, J.M.; Bladé, C.; Rodriguez-Naranjo, M.I. Dual liquid-liquid extraction followed by LC-MS/MS method for the simultaneous quantification of melatonin, cortisol, triiodothyronine, thyroxine and testosterone levels in serum: Applications to a photoperiod study in rats. J. Chromatogr. B 2019, 1108, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Helfer, G.; Ross, A.W.; Russell, L.; Thomson, L.M.; Shearer, K.D.; Goodman, T.H.; McCaffery, P.J.; Morgan, P.J. Photoperiod regulates vitamin A and Wnt/β-catenin signaling in F344 Rats. Endocrinology 2012, 153, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.W.; Helfer, G.; Russell, L.; Darras, V.M.; Morgan, P.J. Thyroid hormone signalling genes are regulated by photoperiod in the hypothalamus of F344 rats. PLoS ONE 2011, 6, e21351. [Google Scholar] [CrossRef] [PubMed]
- Helfer, G.; Ross, A.W.; Morgan, P.J. Neuromedin U partly mimics thyroid-stimulating hormone and triggers Wnt/β-Catenin signalling in the photoperiodic response of F344 rats. J. Neuroendocrinol. 2013, 25, 1264–1272. [Google Scholar] [CrossRef]
- Habauzit, V.; Morand, C. Evidence for a protective effect of polyphenols-containing foods on cardiovascular health: An update for clinicians. Ther. Adv. Chronic Dis. 2012, 3, 87–106. [Google Scholar] [CrossRef]
- Lapuente, M.; Estruch, R.; Shahbaz, M.; Casas, R. Relation of fruits and vegetables with major cardiometabolic risk factors, Markers of oxidation, and inflammation. Nutrients 2019, 11, 2381. [Google Scholar] [CrossRef]
- Cosme, F.; Pinto, T.; Aires, A.; Morais, M.C.; Bacelar, E.; Anjos, R.; Ferreira-Cardoso, J.; Oliveira, I.; Vilela, A.; Gonçalves, B. Red fruits composition and their health benefits—A Review. Foods 2022, 11, 644. [Google Scholar] [CrossRef]
- Haminiuk, C.W.I.; Maciel, G.M.; Plata-Oviedo, M.S.V.; Peralta, R.M. Phenolic compounds in fruits—An overview. Int. J. Food Sci. Technol. 2012, 47, 2023–2044. [Google Scholar] [CrossRef]
- Figueira, I.; Menezes, R.; Macedo, D.; Costa, I.; dos Santos, C.N. Polyphenols beyond barriers: A glimpse into the brain. Curr. Neuropharmacol. 2017, 15, 562–594. [Google Scholar] [CrossRef] [PubMed]
- Ardid-Ruiz, A.; Harazin, A.; Barna, L.; Walter, F.R.; Bladé, C.; Suárez, M.; Deli, M.A.; Aragonès, G. The effects of Vitis vinifera L. phenolic compounds on a blood-brain barrier culture model: Expression of leptin receptors and protection against cytokine-induced damage. J. Ethnopharmacol. 2020, 247, 112253. [Google Scholar] [CrossRef] [PubMed]
- Moosavi, F.; Hosseini, R.; Saso, L.; Firuzi, O. Modulation of neurotrophic signaling pathways by polyphenols. Drug Des. Dev. Ther. 2015, 10, 23–42. [Google Scholar] [CrossRef]
- Jurkowski, M.P.; Bettio, L.; Woo, E.K.; Patten, A.; Yau, S.-Y.; Gil-Mohapel, J. Beyond the hippocampus and the SVZ: Adult neurogenesis throughout the brain. Front. Cell. Neurosci. 2020, 14, 576444. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D. Dietary polyphenols as modulators of brain functions: Biological actions and molecular mechanisms underpinning their beneficial effects. Oxid. Med. Cell. Longev. 2012, 2012, 914273. [Google Scholar] [CrossRef] [PubMed]
- Dardente, H.; Migaud, M. Thyroid hormone and hypothalamic stem cells in seasonal functions. Vitam. Horm. 2021, 116, 91–131. [Google Scholar] [CrossRef]
- Ross, A.W.; Johnson, C.E.; Bell, L.M.; Reilly, L.; Duncan, J.S.; Barrett, P.; Heideman, P.D.; Morgan, P.J. Divergent regulation of hypothalamic neuropeptide Y and Agouti-related protein by photoperiod in F344 rats with differential food intake and growth. J. Neuroendocrinol. 2009, 21, 610–619. [Google Scholar] [CrossRef]
- Helfer, G.; Dumbell, R. Endocrine drivers of photoperiod response. Curr. Opin. Endocr. Metab. Res. 2020, 11, 49–54. [Google Scholar] [CrossRef]
- Nakao, N.; Ono, H.; Yamamura, T.; Anraku, T.; Takagi, T.; Higashi, K.; Yasuo, S.; Katou, Y.; Kageyama, S.; Uno, Y.; et al. Thyrotrophin in the pars tuberalis triggers photoperiodic response. Nature 2008, 452, 317–322. [Google Scholar] [CrossRef]
- Hanon, E.A.; Lincoln, G.A.; Fustin, J.-M.; Dardente, H.; Masson-Pévet, M.; Morgan, P.J.; Hazlerigg, D.G. Ancestral TSH mechanism signals summer in a photoperiodic mammal. Curr. Biol. 2008, 18, 1147–1152. [Google Scholar] [CrossRef]
- Yoshimura, T. Thyroid hormone and seasonal regulation of reproduction. Front. Neuroendocrinol. 2013, 34, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Santi, D.; Spaggiari, G.; Brigante, G.; Setti, M.; Tagliavini, S.; Trenti, T.; Simoni, M. Semi-annual seasonal pattern of serum thyrotropin in adults. Sci. Rep. 2019, 9, 10786. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Horiguchi, K.; Akuzawa, M.; Sakamaki, K.; Shimomura, Y.; Kobayashi, I.; Andou, Y.; Yamada, M. Seasonal variation in thyroid function in over 7000 healthy subjects in an iodine-sufficient area and literature review. J. Endocr. Soc. 2022, 6, bvac054. [Google Scholar] [CrossRef]
- Bellastella, G.; Scappaticcio, L.; Caiazzo, F.; Tomasuolo, M.; Carotenuto, R.; Caputo, M.; Arena, S.; Caruso, P.; Maiorino, M.I.; Esposito, K. Mediterranean diet and thyroid: An interesting alliance. Nutrients 2022, 14, 4130. [Google Scholar] [CrossRef]
- de Souza dos Santos, M.C.; Gonçalves, C.F.L.; Vaisman, M.; Ferreira, A.C.F.; de Carvalho, D.P. Impact of flavonoids on thyroid function. Food Chem. Toxicol. 2011, 49, 2495–2502. [Google Scholar] [CrossRef] [PubMed]
- Habza-Kowalska, E.; Kaczor, A.A.; Bartuzi, D.; Piłat, J.; Gawlik-Dziki, U. Some dietary phenolic compounds can activate thyroid peroxidase and inhibit lipoxygenase-preliminary study in the model systems. Int. J. Mol. Sci. 2021, 22, 5108. [Google Scholar] [CrossRef]
- Dumbell, R. An appetite for growth: The role of the hypothalamic—Pituitary—Growth hormone axis in energy balance. J. Neuroendocrinol. 2022, 34, e13133. [Google Scholar] [CrossRef]
- Caputo, M.; Pigni, S.; Agosti, E.; Daffara, T.; Ferrero, A.; Filigheddu, N.; Prodam, F. Regulation of GH and GH Signaling by Nutrients. Cells 2021, 10, 1376. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Laudisio, D.; Di Somma, C.; Pugliese, G.; Salzano, C.; Colao, A.; Savastano, S. Somatotropic axis and obesity: Is there any role for the Mediterranean diet? Nutrients 2019, 11, 2228. [Google Scholar] [CrossRef]
- Dardente, H.; Wood, S.; Ebling, F.; Sáenz de Miera, C. An integrative view of mammalian seasonal neuroendocrinology. J. Neuroendocrinol. 2019, 31, e12729. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, M.B.; Heideman, P.D. Reduced body mass, food intake, and testis size in response to short photoperiod in adult F344 rats. BMC Physiol. 2002, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Duong, P.; Tenkorang, M.A.A.; Trieu, J.; McCuiston, C.; Rybalchenko, N.; Cunningham, R.L. Neuroprotective and neurotoxic outcomes of androgens and estrogens in an oxidative stress environment. Biol. Sex Differ. 2020, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Huang, J.; Song, D.; Deng, R.; Wei, J.; Zhang, Z. Increased consumption of fruit and vegetables is related to a reduced risk of cognitive impairment and dementia: Meta-analysis. Front. Aging Neurosci. 2017, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Thangthaeng, N.; Poulose, S.M.; Gomes, S.M.; Miller, M.G.; Bielinski, D.F.; Shukitt-Hale, B. Tart cherry supplementation improves working memory, hippocampal inflammation, and autophagy in aged rats. Age 2016, 38, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Moratilla-Rivera, I.; Sánchez, M.; Valdés-González, J.A.; Gómez-Serranillos, M.P. Natural products as modulators of Nrf2 signaling pathway in neuroprotection. Int. J. Mol. Sci. 2023, 24, 3748. [Google Scholar] [CrossRef] [PubMed]
- Babazadeh, A.; Vahed, F.M.; Liu, Q.; Siddiqui, S.A.; Kharazmi, M.S.; Jafari, S.M. Natural bioactive molecules as neuromedicines for the treatment/prevention of neurodegenerative diseases. ACS Omega 2023, 8, 3667–3683. [Google Scholar] [CrossRef]
- Kapoor, R.; Fanibunda, S.E.; Desouza, L.A.; Guha, S.K.; Vaidya, V.A. Perspectives on thyroid hormone action in adult neurogenesis. J. Neurochem. 2015, 133, 599–616. [Google Scholar] [CrossRef]
- Ikeno, T.; Weil, Z.M.; Nelson, R.J. Photoperiod affects the diurnal rhythm of hippocampal neuronal morphology of siberian hamsters. Chronobiol. Int. 2013, 30, 1089–1100. [Google Scholar] [CrossRef]
- Molendijk, M.L.; Haffmans, J.P.M.; Bus, B.A.A.; Spinhoven, P.; Penninx, B.W.J.H.; Prickaerts, J.; Voshaar, R.C.O.; Elzinga, B.M. Serum BDNF concentrations show strong seasonal variation and correlations with the amount of ambient sunlight. PLoS ONE 2012, 7, e48046. [Google Scholar] [CrossRef]
- Hu, J.; Li, X.; Wu, N.; Zhu, C.; Jiang, X.; Yuan, K.; Li, Y.; Sun, J.; Bai, W. Anthocyanins prevent AAPH-induced steroidogenesis disorder in leydig cells by counteracting oxidative stress and StAR abnormal expression in a structure-dependent manner. Antioxidants 2023, 12, 508. [Google Scholar] [CrossRef] [PubMed]



| Parameters | L6 Photoperiod | L12 Photoperiod | L18 Photoperiod | 2wA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | Ch1 | Ch2 | C | Ch1 | Ch2 | C | Ch1 | Ch2 | ||
| Final BW (g) | 367.7 ± 13.7 ab | 369.5 ± 20.0 b | 370.8 ± 21.3 ab | 389.8 ± 25.4 a | 393.7 ± 14.9 a | 381.8 ± 17.1 ab | 383.7 ± 27.8 a | 387.8 ± 20.7 ab | 376.1 ± 24.0 ab | P |
| EPI (kcal/h of dark) | 3.00 ± 0.04 a | 3.16 ± 0.19 a | 3.06 ± 0.25 a | 4.62 ± 0.27 b | 4.68 ± 0.27 b | 4.48 ± 0.26 b | 9.15 ± 0.22 c | 9.02 ± 0.22 c | 9.19 ± 0.47 c | P |
| Testosterone (ng/mL) | 2.98 ± 0.72 a | 4.16 ± 1.02 b | 3.45 ± 1.43 ab | 2.95 ± 0.98 a | 4.20 ± 1.09 b | 3.68 ± 1.83 ab | 1.63 ± 1.0 c | 1.86 ± 0.98 c | 2.43 ± 1.39 ac | P, PxF |
| Testes (g) | 3.08 ± 0.16 | 3.13 ± 0.14 | 3.15 ± 0.12 | 3.15 ± 0.13 | 3.20 ± 0.1 | 3.26 ± 0.14 | 3.05 ± 0.17 | 3.2 ± 0.15 | 3.2 ± 0.17 | |
| T4/T3 | 42.46 ± 2.64 a | 50.68 ± 4.89 b | 47.76 ± 4.85 ab | 48.31 ± 4.41 b | 47.45 ± 5.90 ab | 47.89 ± 5.40 b | 51.25 ± 6.14 b | 51.97 ± 6.47 b | 47.6 ± 5.22 ab | PxF |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manocchio, F.; Bravo, F.I.; Helfer, G.; Muguerza, B. Cherries with Different Geographical Origins Regulate Neuroprotection in a Photoperiod-Dependent Manner in F344 Rats. Antioxidants 2024, 13, 72. https://doi.org/10.3390/antiox13010072
Manocchio F, Bravo FI, Helfer G, Muguerza B. Cherries with Different Geographical Origins Regulate Neuroprotection in a Photoperiod-Dependent Manner in F344 Rats. Antioxidants. 2024; 13(1):72. https://doi.org/10.3390/antiox13010072
Chicago/Turabian StyleManocchio, Francesca, Francisca Isabel Bravo, Gisela Helfer, and Begoña Muguerza. 2024. "Cherries with Different Geographical Origins Regulate Neuroprotection in a Photoperiod-Dependent Manner in F344 Rats" Antioxidants 13, no. 1: 72. https://doi.org/10.3390/antiox13010072