Peroxiredoxin 2: An Important Element of the Antioxidant Defense of the Erythrocyte
Abstract
:1. Introduction
2. Peroxiredoxins
3. Human Erythrocyte Peroxiredoxin 2
3.1. Abundance in Erythrocyte
3.2. Structure of the Prdx2 Dimer
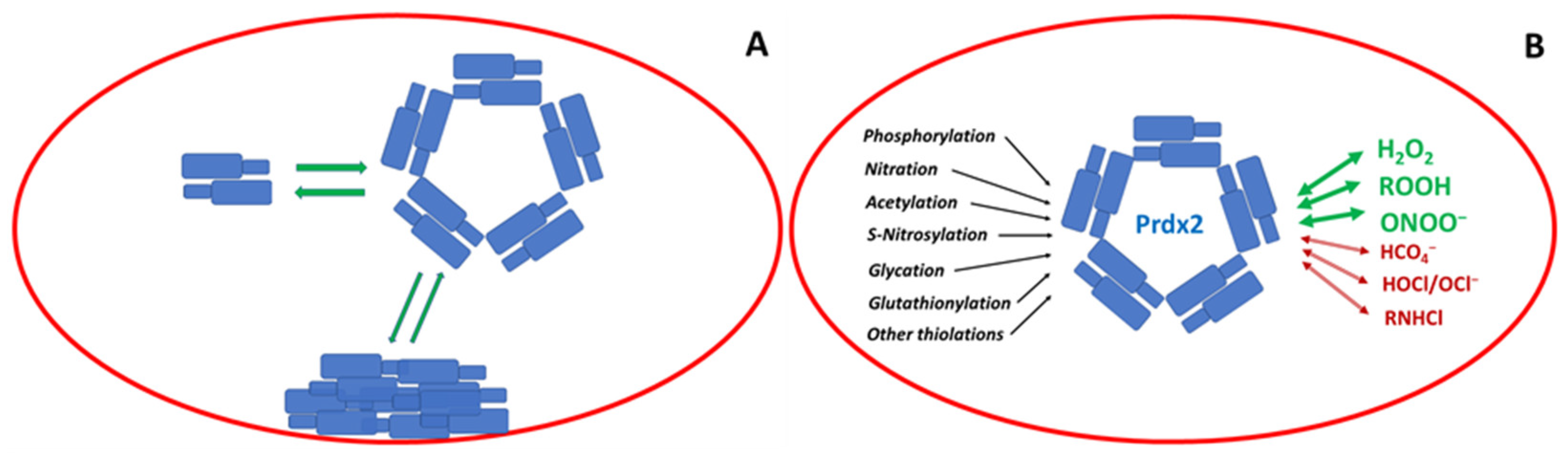
3.3. Formation and Structure of Prdx2 Oligomers
3.4. Binding of Prdx2 to the Erythrocyte Membrane
3.5. Reaction Kinetics of Human Erythrocyte Prdx2
4. Substrates of Prdx2
4.1. Lipid Hydroperoxides
4.2. Other Hydroperoxides
4.3. Peroxynitrite
4.4. Peroxymonocarbonate
4.5. HOCl and Chloramines
5. Post-Translational Modifications of Prdx2
5.1. Phosphorylation
5.2. Nitration
5.3. Acetylation
5.4. S-Nitrosylation
5.5. Glycation
5.6. Glutathionylation
5.7. Formation of Other Mixed Thiols
6. Inhibitors of Prdx2
7. Status and Roles of Prdx 2 in the Erythrocyte
7.1. Oxidation Status In Situ
7.2. Erythrocyte Effects of Knockout of the Prdx2 Gene
7.3. Role of Prdx2 in the Disposal of Hydrogen Peroxide in Erythrocytes
7.4. Miscellaneous Antioxidant Effects in Erythrocytes
7.5. Role of Prdx2 in the Growth of Intracellular Parasites
7.6. Circadian Oscillations of Prdx2 Hyperoxidation
7.7. Chaperone Function
7.8. Effect of Blood Storage on Prdx 2
8. Erythrocyte Prdx2 in Pathologies
8.1. G6PD Deficiency
8.2. Sickle Cell Disease
8.3. β-Thalassemia
8.4. Hereditary Spherocytosis
8.5. Hereditary Ovalocytosis
8.6. Acute Anemia
8.7. Obstructive Sleep Apnea
8.8. Cerebral Hemorrhage
8.9. Neurological Diseases
8.10. Endotoxemia
9. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Hsieh, H.-S.; Jaffé, E. The metabolism of methemoglobin in human erythrocytes. In The Red Blood Cell; Surgenor, D.M., Ed.; Academic Press: New York, NY, USA, 1975; pp. 799–824. [Google Scholar]
- Winterbourn, C.C.; McGrath, B.M.; Carrell, R.W. Reactions involving superoxide and normal and unstable haemoglobins. Biochem. J. 1976, 155, 493–502. [Google Scholar] [CrossRef]
- Díaz-Castillo, A.; Contreras-Puentes, N.; Alvear-Sedán, C.; Moneriz-Pretell, C.; Rodríguez-Cavallo, E.; Mendez-Cuadro, D. Sickle Cell Trait Induces Oxidative Damage on Plasmodium falciparum Proteome at Erythrocyte Stages. Int. J. Mol. Sci. 2019, 20, 5769. [Google Scholar] [CrossRef]
- Matthews, K.; Duffy, S.P.; Myrand-Lapierre, M.E.; Ang, R.R.; Li, L.; Scott, M.D.; Ma, H. Microfluidic analysis of red blood cell deformability as a means to assess hemin-induced oxidative stress resulting from, Plasmodium falciparum intraerythrocytic parasitism. Integr. Biol. 2017, 9, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Free radicals and antioxidants: Updating a personal view. Nutr. Rev. 2012, 70, 257–265. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge., J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Bateman, R.M.; Sharpe, M.D.; Singer, M.; Ellis, C.G. The effect of sepsis on the erythrocyte. Int. J. Mol. Sci. 2017, 18, 1932. [Google Scholar] [CrossRef]
- Laine-Menéndez, S.; Alfaro, C.; Sanchez, J.C.; Franco, F.; Calvo, V.; Romero, A.; Martin-Acosta, P.; Salas, C.; Garcia, J.M.; Provencio, M. Cancer-associated fibroblasts modify lung cancer metabolism involving ROS and TGF-β signaling. Free Radic. Biol. Med. 2019, 130, 163–173. [Google Scholar]
- Kettle, A.J.; Winterbourn, C.C. Myeloperoxidase: A key regulator of neutrophil oxidant production. Redox Rep. 1997, 3, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Radi, R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc. Natl. Acad. Sci. USA 2004, 101, 4003–4008. [Google Scholar] [CrossRef]
- Santo, A.; Zhu, H.; Li, Y.R. Free radicals: From health to disease. React. Oxyg. Species 2016, 2, 245–263. [Google Scholar] [CrossRef]
- Münzel, T.; Daiber, A. Environmental Stressors and Their Impact on Health and Disease with Focus on Oxidative Stress. Antioxid. Redox Signal. 2018, 28, 735–740. [Google Scholar] [CrossRef]
- Alayash, A.I.; Patel, R.P.; Cashon, R.E. Redox reactions of hemoglobin and myoglobin: Biological and toxicological implications. Antioxid. Redox Signal. 2001, 3, 313–327. [Google Scholar] [CrossRef]
- Low, F.M.; Hampton, M.B.; Peskin, A.V.; Winterbourn, C.C. Peroxiredoxin 2 functions as a noncatalytic scavenger of low-level hydrogen peroxide in the erythrocyte. Blood 2007, 109, 2611–2617. [Google Scholar] [CrossRef] [PubMed]
- Pastore, A.; Federici, G.; Bertini, E.; Piemonte, F. Analysis of glutathione: Implication in redox and detoxification. Clin. Chim. Acta 2003, 333, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Ghashghaeinia, M.; Toulany, M.; Saki, M.; Rodemann, H.P.; Mrowietz, U.; Lang, F.; Wieder, T. Potential roles of the NFκB and glutathione pathways in mature human erythrocytes. Cell. Mol. Biol. Lett. 2012, 17, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Kanďár, R.; Štramová, X.; Drábková, P.; Křenková, J. A monitoring of allantoin, uric acid, and malondialdehyde levels in plasma and erythrocytes after ten minutes of running activity. Physiol. Res. 2014, 63, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Tang, L.; Han, J.; Gao, Y.; Tang, B.; Shao, M.; Yuan, W.; Ge, W.; Huang, X.; Yao, T.; et al. Uric Acid Provides Protective Role in Red Blood Cells by Antioxidant Defense: A Hypothetical Analysis. Oxid. Med. Cell. Longev. 2019, 2019, 3435174. [Google Scholar] [CrossRef]
- van’t Erve, T.J.; Wagner, B.A.; Ryckman, K.K.; Raife, T.J.; Buettner, G.R. The concentration of glutathione in human erythrocytes is a heritable trait. Free Radic. Biol. Med. 2013, 65, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.M.; Currie, L.; Campbell, A. The distribution of ascorbic acid between various cellular components of blood, in normal individuals, and its relation to the plasma concentration. Br. J. Nutr. 1982, 47, 473–482. [Google Scholar] [CrossRef]
- May, J.M.; Qu, Z.; Morrow, J.D. Mechanisms of ascorbic acid recycling in human erythrocytes. Biochim. Biophys. Acta 2001, 1528, 159–166. [Google Scholar] [CrossRef]
- Zerez, C.R.; Lachant, N.A.; Lee, S.J.; Tanaka, K.R. Decreased erythrocyte nicotinamide adenine dinucleotide redox potential and abnormal pyridine nucleotide content in sickle cell disease. Blood 1988, 71, 512–515. [Google Scholar] [CrossRef]
- Demarest, T.G.; Truong, G.T.D.; Lovett, J.; Mohanty, J.G.; Mattison, J.A.; Mattson, M.P.; Ferrucci, L.; Bohr, V.A.; Moaddel, R. Assessment of NAD+ metabolism in human cell cultures, erythrocytes, cerebrospinal fluid and primate skeletal muscle. Anal. Biochem. 2019, 572, 1–8. [Google Scholar] [CrossRef]
- Roch, A.; Magon, N.J.; Maire, J.; Suarna, C.; Ayer, A.; Waldvogel, S.; Imhof, B.A.; Koury, M.J.; Stocker, R.; Schapira, M. Transition to 37 °C reveals importance of NADPH in mitigating oxidative stress in stored RBCs. J. Clin. Investig. 2019, 4, e126376. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, A.; Luthman, M. Tissue distribution and subcellular localization of bovine thioredoxin determined by radioimmunoassay. Biochemistry 1978, 17, 4071–4077. [Google Scholar] [CrossRef]
- Gromer, S.; Urig, S.; Becker, K. The thioredoxin system--from science to clinic. Med. Res. Rev. 2004, 24, 40–89. [Google Scholar] [CrossRef]
- Mueller, S.; Riedel, H.D.; Stremmel, W. Direct evidence for catalase as the predominant H2O2-removing enzyme in human erythrocytes. Blood 1997, 90, 4973–4978. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.D.; Lubin, B.H.; Zuo, L.; Kuypers, F.A. Erythrocyte defense against hydrogen peroxide: Preeminent importance of catalase. J. Lab. Clin. Med. 1991, 118, 7–16. [Google Scholar]
- Cohen, G.; Hochstein, P. Glutathione peroxidase: The primary agent for the elimination of hydrogen peroxide in erythrocytes. Biochemistry 1963, 2, 1420–1428. [Google Scholar] [CrossRef]
- Gaetani, G.F.; Galiano, S.; Canepa, L.; Ferraris, A.M.; Kirkman, H.N. Catalase and glutathione peroxidase are equally active in detoxification of hydrogen peroxide in human erythrocytes. Blood 1989, 73, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Kirkman, H.N.; Rolfo, M.; Ferraris, A.M.; Gaetani, G.F. Mechanisms of protection of catalase by NADPH. Kinetics and stoichiometry. J. Biol. Chem. 1999, 274, 13908–13914. [Google Scholar] [CrossRef]
- Lew, V.L.; Ferreira, H.G. Variable Ca sensitivity of a K-selective channel in intact red-cell membranes. Nature 1976, 263, 336–338. [Google Scholar] [CrossRef] [PubMed]
- Plishker, G.A.; Chevalier, D.; Seinsoth, L.; Moore, R.B. Calcium-activated potassium transport and high molecular weight forms of calpromotin. J. Biol. Chem. 1992, 267, 21839–21843. [Google Scholar] [CrossRef]
- Moore, R.B.; Mankad, M.V.; Shriver, S.K.; Mankad, V.N.; Plishker, G.A. Reconstitution of Ca2+-dependent K+ transport in erythrocyte membrane vesicles requires a cytoplasmic protein. J. Biol. Chem. 1991, 266, 18964–18968. [Google Scholar] [CrossRef]
- Harris, J.R. Some negative contrast staining features of a protein from erythrocyte ghosts. J. Mol. Biol. 1969, 46, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.R.; Naeem, I. Further studies on the characterization of cylindrin and torin, two extrinsic proteins of the erythrocyte membrane. Biochim. Biophys. Acta 1981, 670, 285–290. [Google Scholar] [CrossRef]
- Allen, D.W.; Cadman, S. Calcium-induced erythrocyte membrane changes. The role of adsorption of cytosol proteins and proteases. Biochim. Biophys. Acta 1979, 551, 1–9. [Google Scholar] [CrossRef]
- Shau, H.; Butterfield, L.H.; Chiu, R.; Kim, A. Cloning and sequence analysis of candidate human natural killer enhancing factor genes. Immunogenetics 1994, 40, 129–134. [Google Scholar] [CrossRef]
- Kristensen, P.; Rasmussen, D.E.; Kristensen, B.I. Properties of thiol-specific anti-oxidant protein or calpromotin in solution. Biochem. Biophys. Res. Commun. 1999, 262, 127–131. [Google Scholar] [CrossRef]
- Cha, M.K.; Yun, C.H.; Kim, I.H. Interaction of human thiol-specific antioxidant protein 1 with erythrocyte plasma membrane. Biochemistry 2000, 39, 6944–6950. [Google Scholar] [CrossRef] [PubMed]
- Wood, Z.A.; Poole, L.B.; Karplus, P.A. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science 2003, 300, 650–653. [Google Scholar] [CrossRef]
- Low, F.M.; Hampton, M.B.; Winterbourn, C.C. Peroxiredoxin 2 and peroxide metabolism in the erythrocyte. Antioxid. Redox Signal. 2008, 10, 1621–1630. [Google Scholar] [CrossRef]
- Chae, H.Z.; Kim, H.J.; Kang, S.W.; Rhee, S.G. Characterization of three isoforms of mammalian peroxiredoxin that reduce peroxides in the presence of thioredoxin. Diabetes Res. Clin. Pract. 1999, 45, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Netto, L.E.S.; Chae, H.Z.; Kang, S.W.; Rhee, S.G.; Stadtman, E.R. Removal of hydrogen peroxide by thiol-specific antioxidant enzyme (TSA) is involved with its antioxidant properties. TSA possesses thiol peroxidase activity. J. Biol. Chem. 1996, 271, 15315–15321. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, B.; Hecht, H.J.; Flohé, L. Peroxiredoxins. Biol. Chem. 2002, 383, 347–364. [Google Scholar] [CrossRef]
- Manta, B.; Hugo, M.; Ortiz, C.; Ferrer-Sueta, G.; Trujillo, M.; Denicola, A. The peroxidase and peroxynitrite reductase activity of human erythrocyte peroxiredoxin 2. Arch. Biochem. Biophys. 2009, 484, 146–154. [Google Scholar] [CrossRef]
- Rhee, S.G. Overview on Peroxiredoxin. Mol. Cells 2016, 39, 1–5. [Google Scholar]
- Hall, A.; Parsonage, D.; Poole, L.B.; Karplus, P.A. Structural evidence that peroxiredoxin catalytic power is based on transition-state stabilization. J. Mol. Biol. 2010, 402, 194–209. [Google Scholar] [CrossRef]
- Hall, A.; Karplus, P.A.; Poole, L.B. Typical 2-Cys peroxiredoxins--structures, mechanisms and functions. FEBS J. 2009, 276, 2469–2477. [Google Scholar] [CrossRef]
- Poole, L.B.; Nelson, K.J. Distribution and Features of the Six Classes of Peroxiredoxins. Mol. Cells 2016, 39, 53–59. [Google Scholar]
- Rhee, S.G.; Chae, H.Z.; Kim, K. Peroxiredoxins: A historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic. Biol. Med. 2005, 38, 1543–1552. [Google Scholar] [CrossRef]
- Chae, H.Z.; Chung, S.J.; Rhee, S.G. Thioredoxin-dependent peroxide reductase from yeast. J. Biol. Chem. 1994, 269, 27670–27678. [Google Scholar] [CrossRef]
- Rhee, S.G.; Woo, H.A.; Kil, I.S.; Bae, S.H. Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides. J. Biol. Chem. 2012, 287, 4403–4410. [Google Scholar] [CrossRef] [PubMed]
- Pace, P.E.; Peskin, A.V.; Han, M.H.; Hampton, M.B.; Winterbourn, C.C. Hyperoxidized peroxiredoxin 2 interacts with the protein disulfide- isomerase ERp46. Biochem. J. 2013, 453, 475–485. [Google Scholar] [CrossRef]
- Peskin, A.V.; Pace, P.E.; Behring, J.B.; Paton, L.N.; Soethoudt, M.; Bachschmid, M.M.; Winterbourn, C.C. Glutathionylation of the active site cysteines of peroxiredoxin 2 and recycling by glutaredoxin. J. Biol. Chem. 2016, 291, 3053–3062. [Google Scholar] [CrossRef]
- Peskin, A.V.; Winterbourn, C.C. The Enigma of 2-Cys Peroxiredoxins: What Are Their Roles? Biochemistry 2021, 86, 84–91. [Google Scholar] [CrossRef]
- Sobotta, M.C.; Liou, W.; Stöcker, S.; Talwar, D.; Oehler, M.; Ruppert, T.; Scharf, A.N.; Dick, T.P. Peroxiredoxin-2 and STAT3 form a redox relay for H2O2 signaling. Nat. Chem. Biol. 2015, 11, 64–70. [Google Scholar] [CrossRef]
- Perkins, A.; Poole, L.B.; Karplus, P.A. Tuning of peroxiredoxin catalysis for various physiological roles. Biochemistry 2014, 53, 7693–7705. [Google Scholar] [CrossRef]
- Peskin, A.V.; Dickerhof, N.; Poynton, R.A.; Paton, L.N.; Pace, P.E.; Hampton, M.B.; Winterbourn, C.C. Hyperoxidation of peroxiredoxins 2 and 3: Rate constants for the reactions of the sulfenic acid of the peroxidatic cysteine. J. Biol. Chem. 2013, 288, 14170–14177. [Google Scholar] [CrossRef]
- Biteau, B.; Labarre, J.; Toledano, M.B. ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature 2003, 425, 980–984. [Google Scholar] [CrossRef]
- Budanov, A.V.; Sablina, A.A.; Feinstein, E.; Koonin, E.V.; Chumakov, P.M. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science 2004, 304, 596–600. [Google Scholar] [CrossRef]
- Jeong, W.; Park, S.J.; Chang, T.S.; Lee, D.Y.; Rhee, S.G. Molecular mechanism of the reduction of cysteine sulfinic acid of peroxiredoxin to cysteine by mammalian sulfiredoxin. J. Biol. Chem. 2006, 281, 14400–14407. [Google Scholar] [CrossRef]
- Chang, T.S.; Jeong, W.; Woo, H.A.; Lee, S.M.; Park, S.; Rhee, S.G. Characterization of mammalian sulfiredoxin and its reactivation of hyperoxidized peroxiredoxin through reduction of cysteine sulfinic acid in the active site to cysteine. J. Biol. Chem. 2004, 279, 50994–51001. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, T.J.; Johnson, L.C.; Lowther, W.T. Structure of the sulphiredoxin-peroxiredoxin complex reveals an essential repair embrace. Nature 2008, 451, 98–101. [Google Scholar] [CrossRef]
- Lowther, W.T.; Haynes, A.C. Reduction of cysteine sulfinic acid in eukaryotic, typical 2-Cys peroxiredoxins by sulfiredoxin. Antioxid. Redox Signal. 2011, 15, 99–109. [Google Scholar] [CrossRef]
- Nelson, K.J.; Perkins, A.; Van Swearingen, A.E.D.; Hartman, S.; Brereton, A.E.; Parsonage, D.; Salsbury, F.R., Jr.; Karplus, P.A.; Poole, L.B. Experimentally Dissecting the Origins of Peroxiredoxin Catalysis. Antioxid. Redox Signal. 2018, 28, 521–553. [Google Scholar] [CrossRef] [PubMed]
- Kumsta, C.; Jakob, U. Redox-regulated chaperones. Biochemistry 2009, 48, 4666–4676. [Google Scholar] [CrossRef]
- Barranco-Medina, S.; Lázaro, J.J.; Dietz, K.J. The oligomeric conformation of peroxiredoxins links redox state to function. FEBS Lett. 2009, 583, 1809–1816. [Google Scholar] [CrossRef]
- Fourquet, S.; Huang, M.E.; D’Autreaux, B.; Toledano, M.B. The dual functions of thiol-based peroxidases in H2O2 scavenging and signaling. Antioxid. Redox Signal. 2008, 10, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W.; Rhee, S.G.; Chang, T.S.; Jeong, W.; Choi, M.H. 2-Cys peroxiredoxin function in intracellular signal transduction: Therapeutic implications. Trends Mol. Med. 2005, 11, 571–578. [Google Scholar] [CrossRef]
- Karplus, P.A. A primer on peroxiredoxin biochemistry. Free Radic. Biol. Med. 2015, 80, 183–190. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Hampton, M.B. Redox biology: Signaling via a peroxiredoxin sensor. Nat. Chem. Biol. 2015, 11, 5–6. [Google Scholar] [CrossRef]
- Phalen, T.J.; Weirather, K.; Deming, P.B.; Anathy, V.; Howe, A.K.; van der Vliet, A.; Jönsson, T.J.; Poole, L.B.; Heintz, N.H. Oxidation state governs structural transitions in peroxiredoxin II that correlate with cell cycle arrest and recovery. J. Cell Biol. 2006, 175, 779–789. [Google Scholar] [CrossRef]
- Day, A.M.; Brown, J.D.; Taylor, S.R.; Rand, J.D.; Morgan, B.A.; Veal, E.A. Inactivation of a peroxiredoxin by hydrogen peroxide is critical for thioredoxin-mediated repair of oxidized proteins and cell survival. Mol. Cell 2012, 45, 398–408. [Google Scholar] [CrossRef]
- Veal, E.A.; Underwood, Z.E.; Tomalin, L.E.; Morgan, B.A.; Pillay, C.S. Hyperoxidation of Peroxiredoxins: Gain or Loss of Function? Antioxid. Redox Signal. 2018, 28, 574–590. [Google Scholar] [CrossRef]
- Link, A.J.; Robison, K.; Church, G.M. Comparing the predicted and observed properties of proteins encoded in the genome of Escherichia coli K-12. Electrophoresis 1997, 18, 1259–1313. [Google Scholar] [CrossRef]
- Fang, J.; Nakamura, T.; Cho, D.H.; Gu, Z.; Lipton, S.A. S-nitrosylation of peroxiredoxin 2 promotes oxidative stress-induced neuronal cell death in Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2007, 104, 18742–18747. [Google Scholar] [CrossRef]
- Rhee, S.G.; Woo, H.A. Multiple functions of peroxiredoxins: Peroxidases, sensors and regulators of the intracellular messenger H2O2, and protein chaperones. Antioxid. Redox Signal. 2011, 15, 781–794. [Google Scholar] [CrossRef]
- Ogasawara, Y.; Ishida, Y.; Takikawa, M.; Funaki, Y.; Suzuki, T.; Koike, S. A simple high performance liquid chromatography method for quantitatively determining the reduced form of peroxiredoxin 2 and the mass spectrometric analysis of its oxidative status. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 997, 136–141, Erratum in J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1020, 170. [Google Scholar] [CrossRef]
- Bryk, A.H.; Wiśniewski, J.R. Quantitative Analysis of Human Red Blood Cell Proteome. J. Proteome Res. 2017, 16, 2752–2761. [Google Scholar] [CrossRef]
- Rabilloud, T.; Berthier, R.; Vincon, M.; Ferbus, D.; Goubin, G.; Lawrence, J.J. Early events in erythroid differentiation: Accumulation of the acidic peroxiredoxin. Biochem. J. 1995, 312, 699–705. [Google Scholar] [CrossRef]
- Schröder, E.; Littlechild, J.A.; Lebedev, A.A.; Errington, N.; Vagin, A.A.; Isupov, M.N. Crystal structure of decameric 2-Cys peroxiredoxin from human erythrocytes at 1.7 A resolution. Structure 2000, 8, 605–615. [Google Scholar] [CrossRef]
- Hall, A.; Nelson, K.; Poole, L.B.; Karplus, P.A. Structure-based insights into the catalytic power and conformational dexterity of peroxiredoxins. Antioxid. Redox. Signal. 2011, 15, 795–815. [Google Scholar] [CrossRef]
- Meissner, U.; Schröder, E.; Scheffler, D.; Martin, A.G.; Harris, J.R. Formation, TEM study and 3D reconstruction of the human erythrocyte peroxiredoxin-2 dodecahedral higher-order assembly. Micron 2007, 38, 29–39. [Google Scholar] [CrossRef]
- Kitano, K.; Niimura, Y.; Nishiyama, Y.; Miki, K. Stimulation of peroxidase activity by decamerization related to ionic strength: AhpC protein from Amphibacillus xylanus. J. Biochem. (Tokyo) 1999, 126, 313–319. [Google Scholar] [CrossRef]
- Teixeira, F.; Castro, H.; Cruz, T.; Tse, E.; Koldewey, P.; Southworth, D.R.; Tomás, A.M.; Jakob, U. Mitochondrial peroxiredoxin functions as crucial chaperone reservoir in Leishmania infantum. Proc. Natl. Acad. Sci. USA 2015, 112, E616–E624. [Google Scholar] [CrossRef]
- Pastor-Flores, D.; Talwar, D.; Pedre, B.; Dick, T.P. Real-time monitoring of peroxiredoxin oligomerization dynamics in living cells. Proc. Natl. Acad. Sci. USA 2020, 117, 16313–16323. [Google Scholar] [CrossRef]
- Barranco-Medina, S.; Kakorin, S.; Lázaro, J.J.; Dietz, K.J. Thermodynamics of the dimer-decamer transition of reduced human and plant 2-cys peroxiredoxin. Biochemistry 2008, 47, 7196–7204. [Google Scholar] [CrossRef]
- Wood, Z.A.; Poole, L.B.; Hantgan, R.R.; Karplus, P.A. Dimers to doughnuts: Redox-sensitive oligomerization of 2-cysteine peroxiredoxins. Biochemistry 2002, 41, 5493–5504. [Google Scholar] [CrossRef]
- Ogasawara, Y.; Ohminato, T.; Nakamura, Y.; Ishii, K. Structural and functional analysis of native peroxiredoxin 2 in human red blood cells. Int. J. Biochem. Cell Biol. 2012, 44, 1072–1077. [Google Scholar] [CrossRef]
- Rinalducci, S.; D’Amici, G.M.; Blasi, B.; Zolla, L. Oxidative stress-dependent oligomeric status of erythrocyte peroxiredoxin II (PrxII) during storage under standard blood banking conditions. Biochimie 2011, 93, 845–853. [Google Scholar] [CrossRef]
- Moore, R.B.; Shriver, S.K. Protein 7.2b of human erythrocyte membranes binds to calpromotin. Biochem. Biophys. Res. Commun. 1997, 232, 294–297. [Google Scholar] [CrossRef]
- Rocha, S.; Costa, E.; Coimbra, S.; Nascimento, H.; Catarino, C.; Rocha-Pereira, P.; Quintanilha, A.; Belo, L.; Santos-Silva, A. Linkage of cytosolic peroxiredoxin 2 to erythrocyte membrane imposed by hydrogen peroxide-induced oxidative stress. Blood Cells Mol. Dis. 2009, 43, 68–73. [Google Scholar] [CrossRef]
- Matte, A.; Low, P.S.; Turrini, F.; Bertoldi, M.; Campanella, M.E.; Spano, D.; Pantaleo, A.; Siciliano, A.; De Franceschi, L. Peroxiredoxin-2 expression is increased in beta-thalassemic mouse red cells but is displaced from the membrane as a marker of oxidative stress. Free Radic. Biol. Med. 2010, 49, 457–466. [Google Scholar] [CrossRef]
- Matte, A.; Bertoldi, M.; Mohandas, N.; An, X.; Bugatti, A.; Brunati, A.M.; Rusnati, M.; Tibaldi, E.; Siciliano, A.; Turrini, F.; et al. Membrane association of peroxiredoxin-2 in red cells is mediated by the N-terminal cytoplasmic domain of band 3. Free Radic. Biol. Med. 2013, 55, 27–35. [Google Scholar] [CrossRef]
- Rocha, S.; Vitorino, R.M.; Lemos-Amado, F.M.; Castro, E.B.; Rocha-Pereira, P.; Barbot, J.; Cleto, E.; Ferreira, F.; Quintanilha, A.; Belo, L.; et al. Presence of cytosolic peroxiredoxin 2 in the erythrocyte membrane of patients with hereditary spherocytosis. Blood Cells Mol. Dis. 2008, 41, 5–9. [Google Scholar] [CrossRef]
- Biondani, A.; Turrini, F.; Carta, F.; Matté, A.; Filippini, A.; Siciliano, A.; Beuzard, Y.; De Franceschi, L. Heat-shock protein-27, -70 and peroxiredoxin-II show molecular chaperone function in sickle red cells: Evidence from transgenic sickle cell mouse model. Proteom. Clin. Appl. 2008, 2, 706–719. [Google Scholar] [CrossRef]
- Nagababu, E.; Mohanty, J.G.; Friedman, J.S.; Rifkind, J.M. Role of peroxiredoxin-2 in protecting RBCs from hydrogen peroxide-induced oxidative stress. Free Radic. Res. 2013, 47, 164–171. [Google Scholar] [CrossRef]
- Rocha, S.; Gomes, D.; Lima, M.; Bronze-da-Rocha, E.; Santos-Silva, A. Peroxiredoxin 2, glutathione peroxidase, and catalase in the cytosol and membrane of erythrocytes under H2O2-induced oxidative stress. Free Radic. Res. 2015, 49, 990–1003. [Google Scholar] [CrossRef]
- Bayer, S.B.; Low, F.M.; Hampton, M.B.; Winterbourn, C.C. Interactions between peroxiredoxin 2, hemichrome and the erythrocyte membrane. Free Radic. Res. 2016, 50, 1329–1339. [Google Scholar] [CrossRef]
- Sharma, S.; Zingde, S.M.; Gokhale, S.M. Identification of human erythrocyte cytosolic proteins associated with plasma membrane during thermal stress. J. Membr. Biol. 2013, 246, 591–607. [Google Scholar] [CrossRef]
- Walder, J.A.; Chatterjee, R.; Steck, T.L.; Low, P.S.; Musso, G.F.; Kaiser, E.T.; Rogers, P.H.; Arnone, A. The interaction of hemoglobin with the cytoplasmic domain of band 3 of the human erythrocyte membrane. J. Biol. Chem. 1984, 259, 10238–10246. [Google Scholar] [CrossRef]
- Melo, D.; Ribeiro, S.; Santos-Silva, A.; Rocha, S. Role of peroxiredoxin 2 in erythrocyte antioxidant defense: Peroxidase and chaperone. Free Radic. Biol. Med. 2018, 120, 583. [Google Scholar] [CrossRef]
- Waugh, S.M.; Walder, J.A.; Low, P.S. Partial characterization of the copolymerization reaction of erythrocyte membrane band 3 with hemichromes. Biochemistry 1987, 26, 1777–1783. [Google Scholar] [CrossRef]
- Haruyama, T.; Uchihashi, T.; Yamada, Y.; Kodera, N.; Ando, T.; Konno, H. Negatively Charged Lipids Are Essential for Functional and Structural Switch of Human 2-Cys Peroxiredoxin II. J. Mol. Biol. 2018, 430, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.S.; Cha, M.K.; Yun, C.H.; Kim, H.K.; Kim, K.; Kim, I.H. Purification and characterization of thiol-specific antioxidant protein from human red blood cell: A new type of antioxidant protein. Biochem. Biophys. Res. Commun. 1994, 199, 199–206. [Google Scholar] [CrossRef]
- Ogusucu, R.; Rettori, D.; Munhoz, D.C.; Netto, L.E.; Augusto, O. Reactions of yeast thioredoxin peroxidases I and II with hydrogen peroxide and peroxynitrite: Rate constants by competitive kinetics. Free Radic. Biol. Med. 2007, 42, 326–334. [Google Scholar] [CrossRef]
- Peskin, A.V.; Low, F.M.; Paton, L.N.; Maghzal, G.J.; Hampton, M.B.; Winterbourn, C.C. The high reactivity of peroxiredoxin 2 with H2O2 is not reflected in its reaction with other oxidants and thiol reagents. J. Biol. Chem. 2007, 282, 11885–11892. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Peskin, A.V. Kinetic Approaches to Measuring Peroxiredoxin Reactivity. Mol. Cells 2016, 39, 26–30. [Google Scholar] [PubMed]
- Carvalho, L.A.C.; Truzzi, D.R.; Fallani, T.S.; Alves, S.V.; Toledo, J.C., Jr.; Augusto, O.; Netto, L.E.S.; Meotti, F.C. Urate hydroperoxide oxidizes human peroxiredoxin 1 and peroxiredoxin 2. J. Biol. Chem. 2017, 292, 8705–8715. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C.; Metodiewa, D. Reactivity of biologically important thiol compounds with superoxide and hydrogen peroxide. Free Radic. Biol. Med. 1999, 27, 322–328. [Google Scholar] [CrossRef]
- Mueller, S.; Riedel, H.D.; Stremmel, W. Determination of catalase activity at physiological hydrogen peroxide concentrations. Anal. Biochem. 1997, 245, 55–60. [Google Scholar] [CrossRef]
- Ng, C.F.; Schafer, F.Q.; Buettner, G.R.; Rodgers, V.G. The rate of cellular hydrogen peroxide removal shows dependency on GSH: Mathematical insight into in vivo H2O2 and GPx concentrations. Free Radic. Res. 2007, 41, 1201–1211. [Google Scholar] [CrossRef]
- Nelson, K.J.; Parsonage, D.; Hall, A.; Karplus, P.A.; Poole, L.B. Cysteine pKa values for the bacterial peroxiredoxin AhpC. Biochemistry 2008, 47, 12860–12868. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P.; Karton, A.; Betz, A.; Peskin, A.V.; Pace, P.; O’Reilly, R.J.; Hampton, M.B.; Radom, L.; Winterbourn, C.C. Model for the exceptional reactivity of peroxiredoxins 2 and 3 with hydrogen peroxide: A kinetic and computational study. J. Biol. Chem. 2011, 286, 18048–18055. [Google Scholar] [CrossRef] [PubMed]
- Peskin, A.V.; Meotti, F.C.; Kean, K.M.; Göbl, C.; Peixoto, A.S.; Pace, P.E.; Horne, C.R.; Heath, S.G.; Crowther, J.M.; Dobson, R.C.J.; et al. Modifying the resolving cysteine affects the structure and hydrogen peroxide reactivity of peroxiredoxin 2. J. Biol. Chem. 2021, 296, 100494. [Google Scholar] [CrossRef]
- Peskin, A.V.; Meotti, F.C.; de Souza, L.F.; Anderson, R.F.; Winterbourn, C.C.; Salvador, A. Intra-dimer cooperativity between the active site cysteines during the oxidation of peroxiredoxin 2. Free Radic. Biol. Med. 2020, 158, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Bolduc, J.A.; Nelson, K.J.; Haynes, A.C.; Lee, J.; Reisz, J.A.; Graff, A.H.; Clodfelter, J.E.; Parsonage, D.; Poole, L.B.; Furdui, C.M.; et al. Novel hyperoxidation resistance motifs in 2-Cys peroxiredoxins. J. Biol. Chem. 2018, 293, 11901–11912. [Google Scholar] [CrossRef]
- Ishida, Y.I.; Ichinowatari, Y.; Nishimoto, S.; Koike, S.; Ishii, K.; Ogasawara, Y. Differential oxidation processes of peroxiredoxin 2 dependent on the reaction with several peroxides in human red blood cells. Biochem. Biophys. Res. Commun. 2019, 518, 685–690. [Google Scholar] [CrossRef]
- Cordray, P.; Doyle, K.; Edes, K.; Moos, P.J.; Fitzpatrick, F.A. Oxidation of 2-Cys-peroxiredoxins by arachidonic acid peroxide metabolites of lipoxygenases and cyclooxygenase-2. J. Biol. Chem. 2007, 282, 32623–32629. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta 2013, 1830, 3289–3303. [Google Scholar] [CrossRef]
- Forcina, G.C.; Dixon, S.J. GPX4 at the Crossroads of Lipid Homeostasis and Ferroptosis. Proteomics 2019, 19, e1800311. [Google Scholar] [CrossRef]
- Stolwijk, J.M.; Stefely, J.A.; Veling, M.T.; van’t Erve, T.J.; Wagner, B.A.; Raife, T.J.; Buettner, G.R. Red blood cells contain enzymatically active GPx4 whose abundance anticorrelates with hemolysis during blood bank storage. Redox Biol. 2021, 46, 102073. [Google Scholar] [CrossRef]
- Meotti, F.C.; Jameson, G.N.; Turner, R.; Harwood, D.T.; Stockwell, S.; Rees, M.D.; Thomas, S.R.; Kettle, A.J. Urate as a physiological substrate for myeloperoxidase: Implications for hyperuricemia and inflammation. J. Biol. Chem. 2011, 286, 12901–12911. [Google Scholar] [CrossRef]
- Seidel, A.; Parker, H.; Turner, R.; Dickerhof, N.; Khalilova, I.S.; Wilbanks, S.M.; Kettle, A.J.; Jameson, G.N. Uric acid and thiocyanate as competing substrates of lactoperoxidase. J. Biol. Chem. 2014, 289, 21937–21949. [Google Scholar] [CrossRef]
- Patrício, E.S.; Prado, F.M.; da Silva, R.P.; Carvalho, L.A.; Prates, M.V.; Dadamos, T.; Bertotti, M.; Di Mascio, P.; Kettle, A.J.; Meotti, F.C. Chemical Characterization of Urate Hydroperoxide, A Pro-oxidant Intermediate Generated by Urate Oxidation in Inflammatory and Photoinduced Processes. Chem. Res. Toxicol. 2015, 28, 1556–1566. [Google Scholar] [CrossRef]
- Gebicki, J.M. Oxidative stress, free radicals and protein peroxides. Arch. Biochem. Biophys. 2016, 595, 33–39. [Google Scholar] [CrossRef]
- Peskin, A.V.; Cox, A.G.; Nagy, P.; Morgan, P.E.; Hampton, M.B.; Davies, M.J.; Winterbourn, C.C. Removal of amino acid, peptide and protein hydroperoxides by reaction with peroxiredoxins 2 and 3. Biochem. J. 2010, 432, 313–321. [Google Scholar] [CrossRef]
- Romero, N.; Denicola, A.; Radi, R. Red blood cells in the metabolism of nitric oxide-derived peroxynitrite. IUBMB Life 2006, 58, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Trindade, D.F.; Cerchiaro, G.; Augusto, O. A role for peroxymonocarbonate in the stimulation of biothiol peroxidation by the bicarbonate/carbon dioxide pair. Chem. Res. Toxicol. 2006, 19, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Bakhmutova-Albert, E.V.; Yao, H.; Denevan, D.E.; Richardson, D.E. Kinetics and mechanism of peroxymonocarbonate formation. Inorg. Chem. 2010, 49, 11287–11296. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.E.; Regino, C.A.; Yao, H.; Johnson, J.V. Methionine oxidation by peroxymonocarbonate, a reactive oxygen species formed from CO2/bicarbonate and hydrogen peroxide. Free Radic. Biol. Med. 2003, 35, 1538–1550. [Google Scholar] [CrossRef]
- Peskin, A.V.; Pace, P.E.; Winterbourn, C.C. Enhanced hyperoxidation of peroxiredoxin 2 and peroxiredoxin 3 in the presence of bicarbonate/CO2. Free Radic. Biol. Med. 2019, 145, 1–7. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Hypochlorous Acid Chemistry in Mammalian Cells-Influence on Infection and Role in Various Pathologies. Int. J. Mol. Sci. 2022, 23, 10735. [Google Scholar] [CrossRef]
- Stacey, M.M.; Peskin, A.V.; Vissers, M.C.; Winterbourn, C.C. Chloramines and hypochlorous acid oxidize erythrocyte peroxiredoxin 2. Free Radic. Biol. Med. 2009, 47, 1468–1476. [Google Scholar] [CrossRef] [PubMed]
- Skoko, J.J.; Attaran, S.; Neumann, C.A. Signals Getting Crossed in the Entanglement of Redox and Phosphorylation Pathways: Phosphorylation of Peroxiredoxin Proteins Sparks Cell Signaling. Antioxidants 2019, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Mattè, A.; Federti, E.; Tibaldi, E.; Di Paolo, M.L.; Bisello, G.; Bertoldi, M.; Carpentieri, A.; Pucci, P.; Iatchencko, I.; Wilson, A.B.; et al. Tyrosine Phosphorylation Modulates Peroxiredoxin-2 Activity in Normal and Diseased Red Cells. Antioxidants 2021, 10, 206. [Google Scholar] [CrossRef] [PubMed]
- Randall, L.M.; Manta, B.; Hugo, M.; Gil, M.; Batthyàny, C.; Trujillo, M.; Poole, L.B.; Denicola, A. Nitration transforms a sensitive peroxiredoxin 2 into a more active and robust peroxidase. J. Biol. Chem. 2014, 289, 15536–15543. [Google Scholar] [CrossRef]
- Randall, L.M.; Dalla Rizza, J.; Parsonage, D.; Santos, J.; Mehl, R.A.; Lowther, W.T.; Poole, L.B.; Denicola, A. Unraveling the effects of peroxiredoxin 2 nitration; role of C-terminal tyrosine 193. Free Radic. Biol. Med. 2019, 141, 492–501. [Google Scholar] [CrossRef]
- Parmigiani, R.B.; Xu, W.S.; Venta-Perez, G.; Erdjument-Bromage, H.; Yaneva, M.; Tempst, P.; Marks, P.A. HDAC6 is a specific deacetylase of peroxiredoxins and is involved in redox regulation. Proc. Natl. Acad. Sci. USA 2008, 105, 9633–9638. [Google Scholar] [CrossRef]
- Engelman, R.; Weisman-Shomer, P.; Ziv, T.; Xu, J.; Arnér, E.S.; Benhar, M. Multilevel regulation of 2-Cys peroxiredoxin reaction cycle by S-nitrosylation. J. Biol. Chem. 2013, 288, 11312–11324. [Google Scholar] [CrossRef]
- Muralidharan, M.; Bhat, V.; Bindu, Y.S.; Mandal, A.K. Glycation profile of minor abundant erythrocyte proteome across varying glycemic index in diabetes mellitus. Anal. Biochem. 2019, 573, 37–43. [Google Scholar] [CrossRef]
- Jeong, W.; Bae, S.H.; Toledano, M.B.; Rhee, S.G. Role of sulfiredoxin as a regulator of peroxiredoxin function and regulation of its expression. Free Radic. Biol. Med. 2012, 53, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Pace, P.E.; Peskin, A.V.; Konigstorfer, A.; Jasoni, C.J.; Winterbourn, C.C.; Hampton, M.B. Peroxiredoxin interaction with the cytoskeletal-regulatory protein CRMP2: Investigation of a putative redox relay. Free Radic. Biol. Med. 2018, 129, 383–393. [Google Scholar] [CrossRef]
- Liu, C.X.; Yin, Q.Q.; Zhou, H.C.; Wu, Y.L.; Pu, J.X.; Xia, L.; Liu, W.; Huang, X.; Jiang, T.; Wu, M.X.; et al. Adenanthin targets peroxiredoxin I and II to induce differentiation of leukemic cells. Nat. Chem. Biol. 2012, 8, 486–493. [Google Scholar] [CrossRef]
- Soethoudt, M.; Peskin, A.V.; Dickerhof, N.; Paton, L.N.; Pace, P.E.; Winterbourn, C.C. Interaction of adenanthin with glutathione and thiol enzymes: Selectivity for thioredoxin reductase and inhibition of peroxiredoxin recycling. Free Radic. Biol. Med. 2014, 77, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Haraldsen, J.D.; Liu, G.; Botting, C.H.; Walton, J.G.; Storm, J.; Phalen, T.J.; Kwok, L.Y.; Soldati-Favre, D.; Heintz, N.H.; Müller, S.; et al. Identification of conoidin A as a covalent inhibitor of peroxiredoxin II. Org. Biomol. Chem. 2009, 7, 3040–3048. [Google Scholar] [CrossRef]
- Brizuela, M.; Huang, H.M.; Smith, C.; Burgio, G.; Foote, S.J.; McMorran, B.J. Treatment of erythrocytes with the 2-cys peroxiredoxin inhibitor, Conoidin A, prevents the growth of Plasmodium falciparum and enhances parasite sensitivity to chloroquine. PLoS ONE 2014, 9, e92411. [Google Scholar] [CrossRef]
- Bruskov, V.I.; Masalimov, Z.K.; Chernikov, A.V. Heat-induced generation of reactive oxygen species in water. Dokl. Biochem. Biophys. 2002, 384, 181–184. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Penkov, N.V.; Baimler, I.V.; Lyakhov, G.A.; Pustovoy, V.I.; Simakin, A.V.; Sarimov, R.M.; Scherbakov, I.A. Effect of Mechanical Shaking on the Physicochemical Properties of Aqueous Solutions. Int. J. Mol. Sci. 2020, 21, 8033. [Google Scholar] [CrossRef]
- Svensson, B.E. Myeloperoxidase oxidation states involved in myeloperoxidase-oxidase oxidation of thiols. Biochem. J. 1988, 256, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Bayer, S.B.; Maghzal, G.; Stocker, R.; Hampton, M.B.; Winterbourn, C.C. Neutrophil-mediated oxidation of erythrocyte peroxiredoxin 2 as a potential marker of oxidative stress in inflammation. FASEB J. 2013, 27, 3315–3322. [Google Scholar] [CrossRef]
- Selvaggio, G.; Coelho, P.M.B.M.; Salvador, A. Mapping the phenotypic repertoire of the cytoplasmic 2-Cys peroxiredoxin-Thioredoxin system. 1. Understanding commonalities and differences among cell types. Redox Biol. 2018, 15, 297–315. [Google Scholar] [CrossRef]
- Poynton, R.A.; Hampton, M.B. Peroxiredoxins as biomarkers of oxidative stress. Biochim. Biophys. Acta 2014, 1840, 906–912. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Stern, A. Human red cells scavenge extracellular hydrogen peroxide and inhibit formation of hypochlorous acid and hydroxyl radical. J. Clin. Investig. 1987, 80, 1486–1491. [Google Scholar] [CrossRef]
- Pearson, A.G.; Pullar, J.M.; Cook, J.; Spencer, E.S.; Vissers, M.C.; Carr, A.C.; Hampton, M.B. Peroxiredoxin 2 oxidation reveals hydrogen peroxide generation within erythrocytes during high-dose vitamin C administration. Redox Biol. 2021, 43, 101980. [Google Scholar] [CrossRef]
- Oh, J.Y.; Bae, C.Y.; Kasztan, M.; Pollock, D.M.; Russell, T.; Lebensburger, J.; Patel, R.P. Peroxiredoxin-2 recycling is slower in denser and pediatric sickle cell red cells. FASEB J. 2022, 36, e22267. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.H.; Lee, K.O.; Chi, Y.H.; Jung, B.G.; Park, S.K.; Park, J.H.; Lee, J.R.; Lee, S.S.; Moon, J.C.; Yun, J.W.; et al. Two enzymes in one; two yeast peroxiredoxins display oxidative stress-dependent switching from a peroxidase to a molecular chaperone function. Cell 2004, 117, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.S.; Yoon, H.J.; Kim, J.Y.; Woo, H.A.; Rhee, S.G. Circadian rhythm of hyperoxidized peroxiredoxin II is determined by hemoglobin autoxidation and the 20S proteasome in red blood cells. Proc. Natl. Acad. Sci. USA 2014, 111, 12043–12048. [Google Scholar] [CrossRef]
- Cho, C.S.; Lee, S.; Lee, G.T.; Woo, H.A.; Choi, E.J.; Rhee, S.G. Irreversible inactivation of glutathione peroxidase 1 and reversible inactivation of peroxiredoxin II by H2O2 in red blood cells. Antioxid. Redox Signal. 2010, 12, 1235–1246. [Google Scholar] [CrossRef]
- Lee, T.H.; Kim, S.U.; Yu, S.L.; Kim, S.H.; Park, D.S.; Moon, H.B.; Dho, S.H.; Kwon, K.S.; Kwon, H.J.; Han, Y.H.; et al. Peroxiredoxin II is essential for sustaining life span of erythrocytes in mice. Blood 2003, 101, 5033–5038. [Google Scholar] [CrossRef] [PubMed]
- Matté, A.; Pantaleo, A.; Ferru, E.; Turrini, F.; Bertoldi, M.; Lupo, F.; Siciliano, A.; Ho Zoon, C.; De Franceschi, L. The novel role of peroxiredoxin-2 in red cell membrane protein homeostasis and senescence. Free Radic. Biol. Med. 2014, 76, 80–88. [Google Scholar] [CrossRef]
- Nagababu, E.; Rifkind, J.M. Heme degradation by reactive oxygen species. Antioxid. Redox Signal. 2004, 6, 967–978. [Google Scholar] [PubMed]
- Johnson, R.M.; Ho, Y.S.; Yu, D.Y.; Kuypers, F.A.; Ravindranath, Y.; Goyette, G.W. The effects of disruption of genes for peroxiredoxin-2, glutathione peroxidase-1, and catalase on erythrocyte oxidative metabolism. Free Radic. Biol. Med. 2010, 48, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Ogata, M. Acatalasemia. Hum Genet. 1991, 86, 331–340. [Google Scholar] [CrossRef]
- Fukuda, K.; Shindo, H.; Yamashita, K.; Mizuhira, V. Catalase activity of erythrocytes from beagle dogs: An appearance of hereditary acatalasemia. Acta Histochem. Cytochem. 1982, 15, 685–690. [Google Scholar] [CrossRef]
- Nakamura, K.; Watanabe, M.; Sawai-Tanimoto, S.; Ikeda, T. A low catalase activity in dog erythrocytes is due to a very low content of catalase protein despite having a normal specific activity. Int. J. Biochem. Cell Biol. 1998, 30, 823–831. [Google Scholar] [CrossRef]
- Melo, D.; Coimbra, S.; Rocha, S.; Santos-Silva, A. Inhibition of erythrocyte’s catalase, glutathione peroxidase or peroxiredoxin 2-Impact on cytosol and membrane. Arch. Biochem. Biophys. 2023, 739, 109569. [Google Scholar] [CrossRef]
- Dei Zotti, F.; Verdoy, R.; Brusa, D.; Lobysheva, I.I.; Balligand, J.L. Redox regulation of nitrosyl-hemoglobin in human erythrocytes. Redox Biol. 2020, 34, 101399. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Sharma, N.; Sharma, G.P.; Mishra, N. Redox interactome in malaria parasite Plasmodium falciparum. Parasitol. Res. 2021, 120, 423–434. [Google Scholar] [CrossRef]
- Koncarevic, S.; Rohrbach, P.; Deponte, M.; Krohne, G.; Prieto, J.H.; Yates, J., 3rd; Rahlfs, S.; Becker, K. The malarial parasite Plasmodium falciparum imports the human protein peroxiredoxin 2 for peroxide detoxification. Proc. Natl. Acad. Sci. USA 2009, 106, 13323–13328. [Google Scholar] [CrossRef]
- Edgar, R.S.; Green, E.W.; Zhao, Y.; van Ooijen, G.; Olmedo, M.; Qin, X.; Xu, Y.; Pan, M.; Valekunja, U.K.; Feeney, K.A.; et al. Peroxiredoxins are conserved markers of circadian rhythms. Nature 2012, 485, 459–464. [Google Scholar] [CrossRef]
- O’Neill, J.S.; Reddy, A.B. Circadian clocks in human red blood cells. Nature 2011, 469, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.E.; Hipp, M.S.; Bracher, A.; Hayer-Hartl, M.; Hartl, F.U. Molecular chaperone functions in protein folding and proteostasis. Annu. Rev. Biochem. 2013, 82, 323–355. [Google Scholar] [CrossRef]
- Moon, J.C.; Hah, Y.S.; Kim, W.Y.; Jung, B.G.; Jang, H.H.; Lee, J.R.; Kim, S.Y.; Lee, Y.M.; Jeon, M.G.; Kim, C.W.; et al. Oxidative stress-dependent structural and functional switching of a human 2-Cys peroxiredoxin isotype II that enhances HeLa cell resistance to H2O2-induced cell death. J. Biol. Chem. 2005, 280, 28775–28784. [Google Scholar] [CrossRef]
- Lee, W.; Choi, K.S.; Riddell, J.; Ip, C.; Ghosh, D.; Park, J.H.; Park, Y.M. Human peroxiredoxin 1 and 2 are not duplicate proteins: The unique presence of CYS83 in Prx1 underscores the structural and functional differences between Prx1 and Prx2. J. Biol. Chem. 2007, 282, 22011–22022. [Google Scholar] [CrossRef]
- Hanzén, S.; Vielfort, K.; Yang, J.; Roger, F.; Andersson, V.; Zamarbide-Forés, S.; Andersson, R.; Malm, L.; Palais, G.; Biteau, B.; et al. Lifespan Control by Redox-Dependent Recruitment of Chaperones to Misfolded Proteins. Cell 2016, 166, 140–151. [Google Scholar] [CrossRef]
- Stuhlmeier, K.M.; Kao, J.J.; Wallbrandt, P.; Lindberg, M.; Hammarström, B.; Broell, H.; Paigen, B. Antioxidant protein 2 prevents methemoglobin formation in erythrocyte hemolysates. Eur. J. Biochem. 2003, 270, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Chakrabarti, A. Hemoglobin interacting proteins and implications of spectrin hemoglobin interaction. J. Proteomics 2015, 128, 469–475. [Google Scholar] [CrossRef]
- Han, Y.H.; Kim, S.U.; Kwon, T.H.; Lee, D.S.; Ha, H.L.; Park, D.S.; Woo, E.J.; Lee, S.H.; Kim, J.M.; Chae, H.B.; et al. Peroxiredoxin II is essential for preventing hemolytic anemia from oxidative stress through maintaining hemoglobin stability. Biochem. Biophys. Res. Commun. 2012, 426, 427–432. [Google Scholar] [CrossRef]
- Ma, Q.; An, L.; Tian, H.; Liu, J.; Zhang, L.; Li, X.; Wei, C.; Xie, C.; Ding, H.; Qin, W.; et al. Interactions between human hemoglobin subunits and peroxiredoxin 2. Front. Biosci. 2019, 24, 1085–1096. [Google Scholar]
- Ferru, E.; Pantaleo, A.; Carta, F.; Mannu, F.; Khadjavi, A.; Gallo, V.; Ronzoni, L.; Graziadei, G.; Cappellini, M.D.; Turrini, F. Thalassemic erythrocytes release microparticles loaded with hemichromes by redox activation of p72Syk kinase. Haematologica 2014, 99, 570–578. [Google Scholar] [CrossRef]
- Bayer, S.B.; Hampton, M.B.; Winterbourn, C.C. Accumulation of oxidized peroxiredoxin 2 in red blood cells and its prevention. Transfusion 2015, 55, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Marques, M.B.; Xu, X.; Li, J.; Genschmer, K.; Gaggar, A.; Jansen, J.O.; Holcomb, J.B.; Pittet, J.F.; Patel, R.P. Damage to red blood cells during whole blood storage. J. Trauma Acute Care Surg. 2020, 89, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Harper, V.M.; Oh, J.Y.; Stapley, R.; Marques, M.B.; Wilson, L.; Barnes, S.; Sun, C.W.; Townes, T.; Patel, R.P. Peroxiredoxin-2 recycling is inhibited during erythrocyte storage. Antioxid. Redox Signal. 2015, 22, 294–307. [Google Scholar] [CrossRef]
- Amen, F.; Machin, A.; Touriño, C.; Rodríguez, I.; Denicola, A.; Thomson, L. N-acetylcysteine improves the quality of red blood cells stored for transfusion. Arch. Biochem. Biophys. 2017, 621, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Rinalducci, S.; D’Amici, G.M.; Blasi, B.; Vaglio, S.; Grazzini, G.; Zolla, L. Peroxiredoxin-2 as a candidate biomarker to test oxidative stress levels of stored red blood cells under blood bank conditions. Transfusion 2011, 51, 1439–1449. [Google Scholar] [CrossRef]
- Chen, D.; Schubert, P.; Devine, D.V. Identification of potential protein quality markers in pathogen inactivated and gamma-irradiated red cell concentrates. Proteom. Clin. Appl. 2017, 11, 1600121. [Google Scholar] [CrossRef] [PubMed]
- Tzounakas, V.L.; Kriebardis, A.G.; Papassideri, I.S.; Antonelou, M.H. Donor-variation effect on red blood cell storage lesion: A close relationship emerges. Proteom. Clin. Appl. 2016, 10, 791–804. [Google Scholar] [CrossRef]
- Cheah, F.C.; Peskin, A.V.; Wong, F.L.; Ithnin, A.; Othman, A.; Winterbourn, C.C. Increased basal oxidation of peroxiredoxin 2 and limited peroxiredoxin recycling in glucose-6-phosphate dehydrogenase-deficient erythrocytes from newborn infants. FASEB J. 2014, 28, 3205–3210. [Google Scholar] [CrossRef] [PubMed]
- Detterich, J.A.; Liu, H.; Suriany, S.; Kato, R.M.; Chalacheva, P.; Tedla, B.; Shah, P.M.; Khoo, M.C.; Wood, J.C.; Coates, T.D.; et al. Erythrocyte and plasma oxidative stress appears to be compensated in patients with sickle cell disease during a period of relative health, despite the presence of known oxidative agents. Free Radic. Biol. Med. 2019, 141, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Saha, S.; Karmakar, S.; Chakravarty, S.; Banerjee, D.; Dash, B.P.; Chakrabarti, A. 2D DIGE based proteomics study of erythrocyte cytosol in sickle cell disease: Altered proteostasis and oxidative stress. Proteomics 2013, 13, 3233–3242. [Google Scholar] [CrossRef]
- Moore, R.B.; Shriver, S.K.; Jenkins, L.D.; Mankad, V.N.; Shah, A.K.; Plishker, G.A. Calpromotin, a cytoplasmic protein, is associated with the formation of dense cells in sickle cell anemia. Am. J. Hematol. 1997, 56, 100–106. [Google Scholar] [CrossRef]
- Romanello, K.S.; Teixeira, K.K.L.; Silva, J.P.M.O.; Nagamatsu, S.T.; Bezerra, M.A.C.; Domingos, I.F.; Martins, D.A.P.; Araujo, A.S.; Lanaro, C.; Breyer, C.A.; et al. Global analysis of erythroid cells redox status reveals the involvement of Prdx1 and Prdx2 in the severity of beta thalassemia. PLoS ONE 2018, 13, e0208316. [Google Scholar] [CrossRef]
- Franco, S.S.; De Falco, L.; Ghaffari, S.; Brugnara, C.; Sinclair, D.A.; Matte’, A.; Iolascon, A.; Mohandas, N.; Bertoldi, M.; An, X.; et al. Resveratrol accelerates erythroid maturation by activation of FoxO3 and ameliorates anemia in beta-thalassemic mice. Haematologica 2014, 99, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.; Rocha-Pereira, P.; Cleto, E.; Ferreira, F.; Belo, L.; Santos-Silva, A. Linkage of typically cytosolic peroxidases to erythrocyte membrane—A possible mechanism of protection in Hereditary Spherocytosis. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183172. [Google Scholar] [CrossRef] [PubMed]
- Flatt, J.F.; Stevens-Hernandez, C.J.; Cogan, N.M.; Eggleston, D.J.; Haines, N.M.; Heesom, K.J.; Picard, V.; Thomas, C.; Bruce, L.J. Expression of South East Asian Ovalocytic Band 3 Disrupts Erythroblast Cytokinesis and Reticulocyte Maturation. Front. Physiol. 2020, 11, 357. [Google Scholar] [CrossRef]
- Sadvakassova, G.; Tiedemann, K.; Steer, K.J.D.; Mikolajewicz, N.; Stavnichuk, M.; In-Kyung Lee, I.; Sabirova, Z.; Schranzhofer, M.; Komarova, S.V. Active hematopoiesis triggers exosomal release of PRDX2 that promotes osteoclast formation. Physiol. Rep. 2021, 9, e14745. [Google Scholar] [CrossRef]
- Koike, S.; Sudo, H.; Turudome, S.; Ueyama, M.; Tanaka, Y.; Kimura, H.; Ishida, Y.I.; Ogasawara, Y. Hyperoxidized Peroxiredoxin 2 Is a Possible Biomarker for the Diagnosis of Obstructive Sleep Apnea. Antioxidants 2022, 11, 2486. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, A.; Vaz, F.; Torres, V.M.; Valentim-Coelho, C.; Silva, R.; Prosinecki, V.; Alexandre, B.M.; Carvalho, A.S.; Matthiesen, R.; Malhotra, A.; et al. Evening and morning peroxiredoxin-2 redox/oligomeric state changes in obstructive sleep apnea red blood cells: Correlation with polysomnographic and metabolic parameters. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 621–629. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, X.S.; Zhou, X.M.; Gao, Y.Y.; Chen, C.L.; Liu, J.P.; Ye, Z.N.; Zhang, Z.H.; Wu, L.Y.; Li, W.; et al. Peroxiredoxin 1/2 protects brain against H2O2-induced apoptosis after subarachnoid hemorrhage. FASEB J. 2019, 33, 3051–3062. [Google Scholar] [CrossRef] [PubMed]
- Connor, D.E., Jr.; Chaitanya, G.V.; Chittiboina, P.; McCarthy, P.; Scott, L.K.; Schrott, L.; Minagar, A.; Nanda, A.; Alexander, J.S. Variations in the cerebrospinal fluid proteome following traumatic brain injury and subarachnoid hemorrhage. Pathophysiology 2017, 24, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, X.S.; Zhang, Z.H.; Zhou, X.M.; Gao, Y.Y.; Liu, G.J.; Wang, H.; Wu, L.Y.; Li, W.; Hang, C.H. Peroxiredoxin 2 activates microglia by interacting with Toll-like receptor 4 after subarachnoid hemorrhage. J. Neuroinflamm. 2018, 15, 87. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Keep, R.F.; Ye, F.; Holste, K.G.; Wan, S.; Xi, G.; Hua, Y. The Fate of Erythrocytes after Cerebral Hemorrhage. Transl. Stroke Res. 2022, 13, 655–664. [Google Scholar] [CrossRef]
- Bian, L.; Zhang, J.; Wang, M.; Keep, R.F.; Xi, G.; Hua, Y. Intracerebral Hemorrhage-Induced Brain Injury in Rats: The Role of Extracellular Peroxiredoxin 2. Transl. Stroke Res. 2020, 11, 288–295. [Google Scholar] [CrossRef]
- Peng, Z.; Pang, C.; Li, X.J.; Zhang, H.S.; Zhang, J.T.; Zhu, Q.; Xu, H.J.; Gao, Y.Y.; Zhuang, Z.; Li, W.; et al. Peroxiredoxin 2 Is a Potential Objective Indicator for Severity and the Clinical Status of Subarachnoid Hemorrhage Patients. Dis. Markers 2023, 2023, 5781180. [Google Scholar] [CrossRef]
- Martinez-Pinna, R.; Burillo, E.; Madrigal-Matute, J.; Lopez, J.A.; Camafeita, E.; Torres-Fonseca, M.M.; Llamas-Granda, P.; Egido, J.; Michel, J.B.; Blanco-Colio, L.M.; et al. Label-free proteomic analysis of red blood cell membrane fractions from abdominal aortic aneurysm patients. Proteom. Clin. Appl. 2014, 8, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Yoshikawa, A.; Kinumi, T.; Ogawa, Y.; Saito, Y.; Ohara, K.; Yamamoto, H.; Imai, Y.; Niki, E. Hydroxyoctadecadienoic acid and oxidatively modified peroxiredoxins in the blood of Alzheimer’s disease patients and their potential as biomarkers. Neurobiol. Aging 2009, 30, 174–185. [Google Scholar] [CrossRef]
- Reed, T.T.; Pierce, W.M., Jr.; Turner, D.M.; Markesbery, W.R.; Allan Butterfield, D. Proteomic identification of nitrated brain proteins in early Alzheimer’s disease inferior parietal lobule. J. Cell. Mol. Med. 2009, 13, 2019–2029. [Google Scholar] [CrossRef] [PubMed]
- Abruzzo, P.M.; Matté, A.; Bolotta, A.; Federti, E.; Ghezzo, A.; Guarnieri, T.; Marini, M.; Posar, A.; Siciliano, A.; De Franceschi, L.; et al. Plasma peroxiredoxin changes and inflammatory cytokines support the involvement of neuro-inflammation and oxidative stress in Autism Spectrum Disorder. J. Transl. Med. 2019, 17, 332. [Google Scholar] [CrossRef]

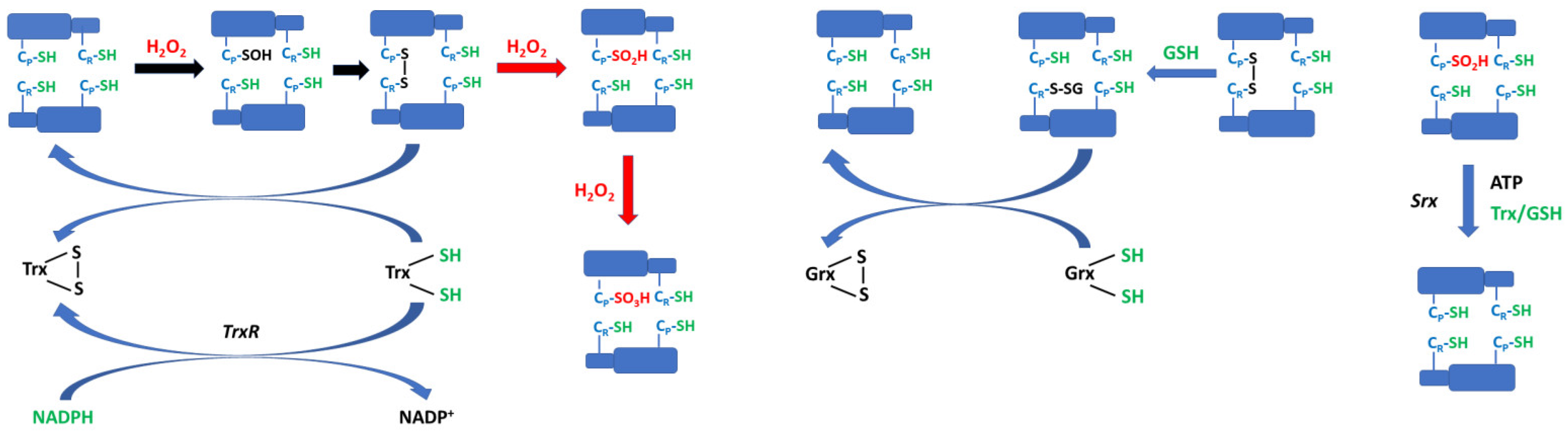

| Reaction | Rate Constant |
|---|---|
| Prdx-SH + H2O2 → Prdx-SOH | 2.7 × 107 M−1 s−1 |
| Prdx-SOH + H2O2 → Prdx-SO2H | 12,000 M−1 s−1 |
| Prdx-SOH → Prdx(SS) | 2 s−1 |
| Prdx-SOH + GSH → Prdx-SSG | 500 M−1 s−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadowska-Bartosz, I.; Bartosz, G. Peroxiredoxin 2: An Important Element of the Antioxidant Defense of the Erythrocyte. Antioxidants 2023, 12, 1012. https://doi.org/10.3390/antiox12051012
Sadowska-Bartosz I, Bartosz G. Peroxiredoxin 2: An Important Element of the Antioxidant Defense of the Erythrocyte. Antioxidants. 2023; 12(5):1012. https://doi.org/10.3390/antiox12051012
Chicago/Turabian StyleSadowska-Bartosz, Izabela, and Grzegorz Bartosz. 2023. "Peroxiredoxin 2: An Important Element of the Antioxidant Defense of the Erythrocyte" Antioxidants 12, no. 5: 1012. https://doi.org/10.3390/antiox12051012





