Antioxidant Activity of an Aqueous Extract of Cuttlefish Ink during Fish Muscle Heating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Fish, Starting Ink, and Preparation of the Ink Extract
2.2. Ink Extract/Seabream Muscle System
2.3. Assessment of Lipid Damage
2.4. Determination of Interaction Compound Formation
2.5. FA Profile Analysis
2.6. Statistical Analysis
3. Results
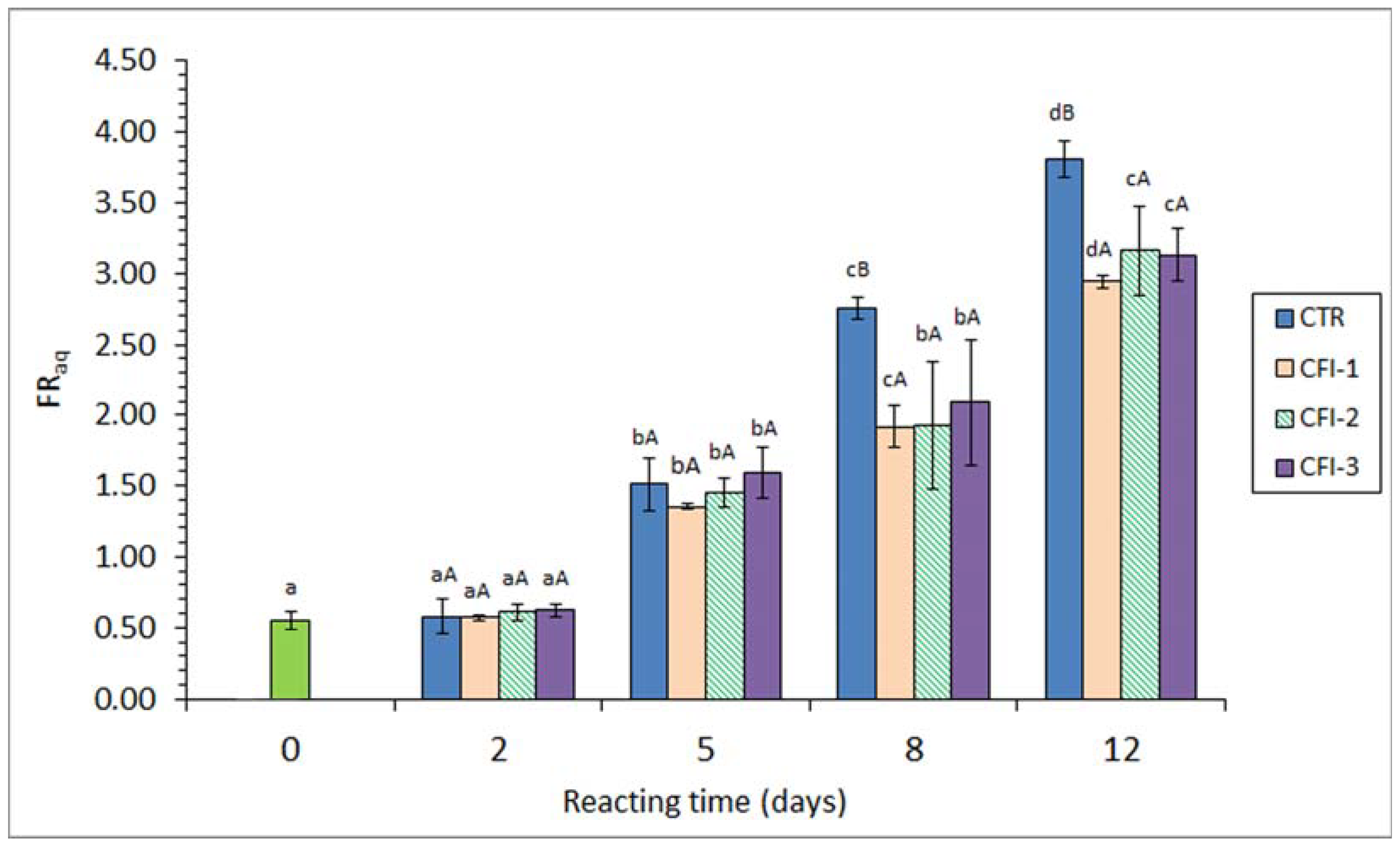
3.1. Determination of Lipid Oxidation
3.2. Determination of Fluorescent Compound Formation
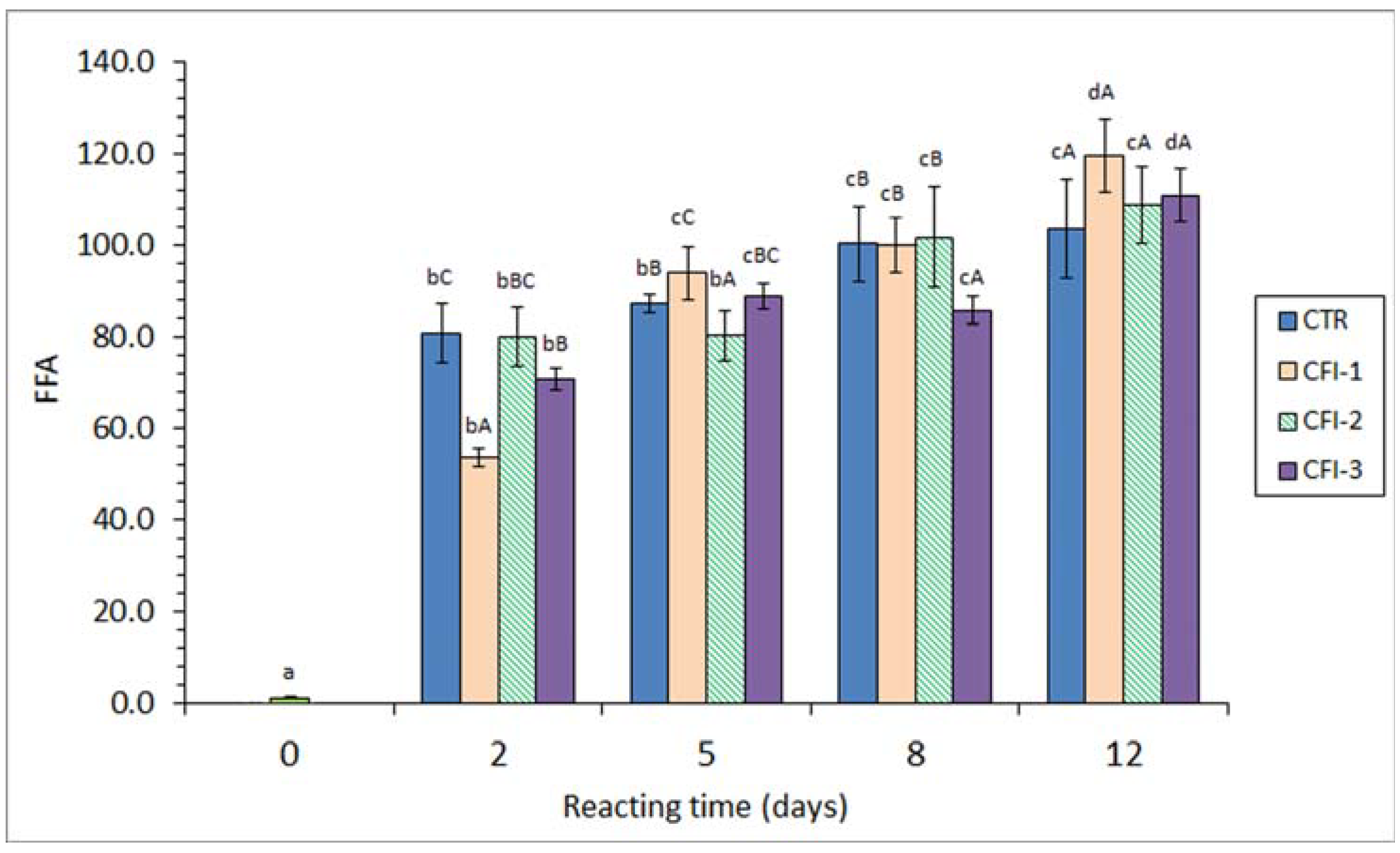
3.3. Determination of Lipid Hydrolysis
3.4. Analysis of the FA Profile
4. Discussion
4.1. Lipid Oxidation Development in the Present Study
4.2. Antioxidant Activity of Ink Extracts
4.3. Lipid Hydrolysis Development during the Present Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tilami, S.K.; Sampels, S. Nutritional value of fish: Lipids, proteins, vitamins, and minerals. Rev. Fish. Sci. 2018, 26, 242–253. [Google Scholar]
- Swanson, S.; Block, R.; Mousa, S. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Ofosu, F.K.; Daliri, E.B.M.; Lee, B.H.; Yu, X. Current trends and future perspectives on omega-3 fatty acids. Res. J. Biol. 2017, 5, 11–20. [Google Scholar]
- Özoğul, Y. Methods for freshness quality and deterioration. In Handbook of Seafood and Seafood Products Analysis; Nollet, L.M., Toldrá, F., Eds.; CRC Press: Boca Raton, FL, USA; Francis and Taylor Group: Abingdon, UK, 2010; pp. 189–214. [Google Scholar]
- Ghali, A.; Dave, D.; Budge, S.; Brooks, M. Fish spoilage mechanisms and preservation: Review. Am. J. Appl. Sci. 2010, 7, 859–877. [Google Scholar] [CrossRef]
- Lukoshkina, M.; Odoeva, G. Kinetics of chemical reactions for prediction of quality of canned fish during storage. App. Biochem. Microb. 2003, 39, 321–327. [Google Scholar] [CrossRef]
- Ling, B.; Tang, J.; Kong, F.; Mitcham, E.J.; Wang, S. Kinetics of food quality changes during thermal processing: A review. Food Bioprocess Technol. 2015, 8, 343–358. [Google Scholar] [CrossRef]
- Mei, J.; Ma, X.; Xie, J. Review on natural preservatives for extending fish shelf life. Foods 2019, 8, 490. [Google Scholar] [CrossRef]
- Aubourg, S.P. Enhancement of lipid stability and acceptability of canned seafood by addition of natural antioxidant compounds to the packing medium. A review. Antioxidants 2023, 12, 245. [Google Scholar] [CrossRef]
- Sánchez-Alonso, I.; Jiménez-Escrig, A.; Saura-Calixto, F.; Borderías, A.J. Effect of grape antioxidant dietary fibre on the prevention of lipid oxidation in minced fish: Evaluation by different methodologies. Food Chem. 2007, 101, 372–378. [Google Scholar] [CrossRef]
- Aubourg, S.P.; Torres-Arreola, W.; Trigo, M.; Ezquerra-Brauer, M. Partial characterization of jumbo squid skin pigment extract and its antioxidant potential in a marine oil system. Eur. J. Lipid Sci. Technol. 2016, 118, 1293–1304. [Google Scholar] [CrossRef]
- Barbosa, R.G.; Trigo, M.; Campos, C.A.; Aubourg, S.P. Preservative effect of algae extracts on lipid composition and rancidity development in brine-canned Atlantic Chub mackerel (Scomber colias). Eur. J. Lipid Sci. Technol. 2019, 121, 1900129. [Google Scholar] [CrossRef]
- Barbosa, R.G.; Trigo, M.; Zhang, B.; Aubourg, S.P. Effect of alga flour extract on lipid damage evolution in heated fish muscle system. Antioxidants 2022, 11, 807. [Google Scholar] [CrossRef] [PubMed]
- Rustad, T.; Storro, I.; Slizyte, R. Possibilities for the utilisation of marine by-products. Int. J. Food Sci. Technol. 2011, 46, 2001–2014. [Google Scholar] [CrossRef]
- Olsen, R.L.; Toppe, J.; Karunasagar, I. Challenges and realistic opportunities in the use of by-products from processing of fish and shellfish. Trends Food Sci. Technol. 2014, 36, 144–152. [Google Scholar] [CrossRef]
- Atef, M.; Ojagh, M. Health benefits and food applications of bioactive compounds from fish byproducts: A review. J. Funct. Foods 2017, 35, 673–681. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Meduiña, A.; Durán, A.I.; Nogueira, M.; Fernández-Compás, A.; Pérez-Martín, R.I.; Rodríguez-Amado, I. Production of valuable compounds and bioactive metabolites from by-products of fish discards using chemical processing, enzymatic hydrolysis, and bacterial fermentation. Mar. Drugs 2019, 17, 139. [Google Scholar] [CrossRef]
- Fahmy, S.R.; Ali, E.M.; Ahmed, N.S. Therapeutic effect of Sepia ink extract against invasive pulmonary aspergillosis in mice. J. Basic App. Zool. 2014, 67, 196–204. [Google Scholar] [CrossRef]
- Solano, F. Melanin and melanin-related polymers as materials with biomedical and biotechnological applications—Cuttlefish ink and mussel foot proteins as inspired biomolecules. Int. J. Mol. Sci. 2017, 18, 1561. [Google Scholar] [CrossRef]
- Momenzadeh, N.; Hajian, S.; Shabankare, A.; Ghavimi, R.; Kabiri-Samani, S.; Kabiri, H.; Hesami-Zadeh, K.; Shabankareh, A.N.T.; Nazaraghay, R.; Nabipour, I.; et al. Photothermic therapy with cuttlefish ink-based nanoparticles in combination with anti-OX40 mAb achieve remission of triple-negative breast cancer. Int. Immunopharm. 2023, 115, 109622. [Google Scholar] [CrossRef]
- Neifar, A.; Abdelmalek, I.B.; Bouajila, G.; Kolsi, R.; Bradai, M.N.; Abdelmouleh, A.; Gargouri, A.; Ayed, N. Purification and incorporation of the black ink of cuttlefish Sepia officinalis in eye cosmetic products. Color. Technol. 2013, 129, 150–154. [Google Scholar] [CrossRef]
- Wang, H.; Xu, T.; Zheng, B.; Cao, M.; Gao, F.; Zhou, G.; Ma, C.; Dang, J.; Yao, W.; Wu, K.; et al. Cuttlefish ink loaded polyamidoxime adsorbent with excellent photothermal conversion and antibacterial activity for highly efficient uranium capture from natural seawater. J. Hazard. Mat. 2022, 433, 128789. [Google Scholar] [CrossRef] [PubMed]
- Derby, C.D. Cephalopod Ink: Production, chemistry, functions and applications. Mar. Drugs 2014, 12, 2700–2730. [Google Scholar] [CrossRef] [PubMed]
- Senan, V.P. Antibacterial activity of methanolic extract of the ink of cuttlefish, Sepia pharaonis, against pathogenic bacterial strains. Int. J. Pharm. Sci. Res. 2015, 6, 1705–1710. [Google Scholar]
- Karim, N.U.; Sadzali, N.L.; Hassan, M. Effects of squid ink as edible coating on squid sp. (Loligo duvauceli) spoilage during chilled storage. Int. Food Res. J. 2016, 23, 1895–1901. [Google Scholar]
- Chen, S.; Xue, C.; Li, Y.; Gao, Z.; Ma, Q. Studies on the free radical scavenging activities of melanin from squid ink. China J. Mar. Drugs 2007, 26, 24–27. [Google Scholar]
- Vate, N.K.; Benjakul, S. Antioxidative activity of melanin-free ink from splendid squid (Loligo formosana). Int. Aquat. Res. 2013, 5, 9. [Google Scholar] [CrossRef]
- Vate, N.K.; Benjakul, S.; Agustini, T.W. Application of melanin-free ink as a new antioxidative gel enhancer in sardine surimi gel. J. Sci. Food Agric. 2015, 95, 2201–2207. [Google Scholar] [CrossRef]
- Liang, Y.; Han, Y.; Dan, J.; Li, R.; Sun, H.; Wang, J.; Zhang, W. A high-efficient and stable artificial superoxide dismutase based on functionalized melanin nanoparticles from cuttlefish ink for food preservation. Food Res. Int. 2023, 163, 112211. [Google Scholar] [CrossRef]
- Barbosa, R.G.; Trigo, M.; Fett, R.; Aubourg, S.P. Impact of a packing medium with alga Bifurcaria bifurcata extract on canned Atlantic mackerel (Scomber scombrus) quality. J. Sci. Food Agric. 2018, 98, 3462–3467. [Google Scholar] [CrossRef]
- Bligh, E.; Dyer, W. A rapid method of total extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Kim, R.; Labella, F. Comparison of analytical methods for monitoring autoxidation profiles of authentic lipids. J. Lipid Res. 1987, 28, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.; McKay, J. The estimation of peroxides in fats and oils by the ferric thiocyanate method. J. Am. Oil Chem. Soc. 1949, 26, 360–363. [Google Scholar] [CrossRef]
- Lowry, R.; Tinsley, I. Rapid colorimetric determination of free fatty acids. J. Am. Oil Chem. Soc. 1976, 53, 470–472. [Google Scholar] [CrossRef]
- Aubourg, S.P.; Medina, I.; Pérez-Martín, R.I. A comparison between conventional and fluorescence detection methods of cooking-induced damage to tuna fish lipids. Z. Lebensm. Unters. Forsch. 1995, 200, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Sokal, R.; Rohlf, F. Biometry, 2nd ed.; W. H. Freeman: San Francisco, CA, USA, 1981; pp. 208–270. [Google Scholar]
- Augimeri, G.; Galluccio, A.; Caparello, G.; Avolio, E.; la Russa, D.; de Rose, D.; Morelli, C.; Barone, I.; Catalano, S.; Ando, S.; et al. Potential antioxidant and anti-inflammatory properties of serum from healthy adolescents with optimal Mediterranean diet adherence: Findings from DIMENU cross-sectional study. Antioxidants 2021, 10, 1172. [Google Scholar] [CrossRef] [PubMed]
- Tibbetts, S.M.; Patelakis, S.J.J. Apparent digestibility coefficients (ADCs) of intact-cell marine microalgae meal (Pavlova sp. 459) for juvenile Atlantic salmon (Salmo salar L.). Aquaculture 2022, 546, 737236. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, M.; Reddy, C.R.; Jha, B. Algal lipids, fatty acids and sterols. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 87–134. [Google Scholar]
- Aubourg, S.P.; Medina, I.; Pérez-Martín, R.I. Polyunsaturated fatty acids in tuna phospholipids: Distribution in the sn-2 location and changes during cooking. J. Agric. Food Chem. 1996, 44, 585–589. [Google Scholar] [CrossRef]
- Selmi, S.; Monser, L.; Sadok, S. The influence of local canning process and storage on pelagic fish from Tunisia: Fatty acids profile and quality indicators. J. Food Proc. Preserv. 2008, 32, 443–457. [Google Scholar] [CrossRef]
- Naseri, M.; Rezaei, M. Lipid changes during long-term storage of canned sprat. J. Aquat. Food Prod. Technol. 2012, 21, 48–58. [Google Scholar] [CrossRef]
- Gómez-Limia, L.; Cobas, N.; Franco, I.; Martínez-Suárez, S. Fatty acid profiles and lipid quality indices in canned European eels: Effects of processing steps, filling medium and storage. Food Res. Int. 2020, 136, 109601. [Google Scholar] [CrossRef]
- Pokorný, J. Browning from lipid-protein interactions. Prog. Food Nutr. Sci. 1981, 5, 421–428. [Google Scholar]
- Aubourg, S.P. Review: Recent advances in assessment of marine lipid oxidation by using fluorescence. J. Am. Oil Chem. Soc. 1999, 76, 409–419. [Google Scholar] [CrossRef]
- Tironi, V.; Tomás, M.; Añón, M.C. Structural and functional changes in myofibrillar proteins of sea salmon (Pseudopercis semifasciata) by interaction with malondialdehyde (RI). J. Food Sci. 2002, 67, 930–935. [Google Scholar] [CrossRef]
- Gómez-Limia, L.; Sanmartín, N.M.; Carballo, J.; Domínguez, R.; Lorenzo, J.M.; Martínez, S. Oxidative stability and antioxidant activity in canned eels: Effect of processing and filling medium. Foods 2021, 10, 790. [Google Scholar] [CrossRef]
- Ortiz-Viedma, J.A.; Aguilera, J.M.; Flores, M.; Lemus-Mondaca, R.; Larrazabal, M.J.; Miranda, J.M.; Aubourg, S.P. Protective effect of red algae (Rhodophyta) extracts on essential dietary components of heat-treated salmon. Antioxidants 2021, 10, 1108. [Google Scholar] [CrossRef]
- Kikugawa, K. Fluorescent products derived from the reaction of primary amines and compounds in peroxidised lipids. Adv. Free Rad. Biol. Med. 1986, 2, 389–417. [Google Scholar] [CrossRef]
- Iio, T.; Yoden, K. Fluorescence formation from hydroperoxide of phosphatidylcholine with amino compound. Lipids 1988, 23, 65–67. [Google Scholar] [CrossRef]
- Xie, J.; Li, H.; Che, H.; Dong, X.; Yang, X.; Xie, W. Extraction, physicochemical characterisation, and bioactive properties of ink melanin from cuttlefish (Sepia esculenta). Int. J. Food Sci. Technol. 2021, 56, 3627–3640. [Google Scholar] [CrossRef]
- Essid, I.; Aroussia, H.; Soufi, E.; Bouriga, N.; Gharbi, S.; Bellagha, S. Improving quality of smoked sardine fillets by soaking in cuttlefish ink. Food Sci. Technol. 2022, 42, e65020. [Google Scholar] [CrossRef]
- Zayas, J.F. Functionality of Proteins in Food; Springer: Berlin, Germany, 1997; 373p. [Google Scholar]
- Tian, W.; Song, X.; Wang, F.; Jiang, W. Study on the preparation and biological activities of low molecular weight squid ink polysaccharide from Sepiella maindroni. Int. J. Biol. Macromol. 2023, 237, 124040. [Google Scholar] [CrossRef] [PubMed]
- Fatimah Zaharah, M.Y.; Rabeta, M.S. Antioxidant and antimicrobial activities of squid ink powder. Food Res. 2018, 2, 82–88. [Google Scholar]
- Sadok, S.; Abdelmoulah, A.; El Abed, A. Combined effect of sepia soaking and temperature on the shelf life of peeled shrimp Penaeus kerathurus. Food Chem. 2004, 88, 115–122. [Google Scholar] [CrossRef]
- Labuza, T. Kinetics of lipid oxidation in foods. CRC Crit. Rev. Food Technol. 1971, 2, 355–405. [Google Scholar] [CrossRef]
- Miyashita, K.; Tagagi, T. Study on the oxidative rate and prooxidant activity of free fatty acids. J. Am. Oil Chem. Soc. 1986, 63, 1380–1384. [Google Scholar] [CrossRef]
- Mohan, C.O.; Remya, S.; Murthy, L.N.; Ravishankar, C.N.; Kumar, K.A. Effect of filling medium on cooking time and quality of canned yellowfin tuna (Thunnus albacares). Food Control 2015, 50, 320–327. [Google Scholar] [CrossRef]
- Naseri, M.; Rezaei, M.; Moieni, S.; Hosseini, H.; Eskandari, S. Effects of different filling media on the oxidation and lipid quality of canned silver carp (Hypophthalmichthys molitrix). Int. J. Food Sci. 2011, 46, 1149–1156. [Google Scholar] [CrossRef]

| Quality Index | Heating Time (Days) | CFI Concentration | |||
|---|---|---|---|---|---|
| CTR | CFI-1 | CFI-2 | CFI-3 | ||
| Conjugated diene formation *** | 0 | 0.27 a (0.01) | 0.27 a (0.01) | 0.27 a (0.01) | 0.27 a (0.01) |
| 2 | 0.39 bB (0.05) | 0.31 bA (0.01) | 0.30 bA (0.00) | 0.31 bA (0.00) | |
| 5 | 0.50 cB (0.04) | 0.35 cA (0.02) | 0.33 cA (0.01) | 0.33 bA (0.01) | |
| 8 | 0.53 cB (0.02) | 0.52 dB (0.04) | 0.41 dAB (0.08) | 0.38 cA (0.04) | |
| 12 | 0.46 bcA (0.05) | 0.49 dA (0.04) | 0.46 dA (0.02) | 0.44 cA (0.05) | |
| Conjugated triene formation *** | 0 | 0.02 a (0.00) | 0.02 a (0.00) | 0.02 a (0.00) | 0.02 a (0.00) |
| 2 | 0.04 aA (0.01) | 0.03 aA (0.00) | 0.03 aA (0.00) | 0.03 aA (0.00) | |
| 5 | 0.07 bB (0.01) | 0.04 abA (0.01) | 0.04 aA (0.00) | 0.04 aA (0.00) | |
| 8 | 0.11 cB (0.01) | 0.08 bcAB (0.03) | 0.05 abA (0.03) | 0.04 abA (0.01) | |
| 12 | 0.10 bcA (0.02) | 0.09 cA (0.02) | 0.08 bA (0.01) | 0.08 bA (0.02) | |
| Peroxide value (meq active oxygen·kg−1 lipids) | 0 | 0.50 a (0.10) | 0.50 a (0.10) | 0.50 a (0.10) | 0.50 a (0.10) |
| 2 | 10.72 bC (1.74) | 4.14 bB (1.01) | 2.13 bAB (0.94) | 1.59 bA (0.38) | |
| 5 | 32.19 dC (3.82) | 7.63 cB (1.45) | 5.11 cB (1.90) | 2.71 cA (0.22) | |
| 8 | 24.61 cA (3.18) | 40.11 eB (4.78) | 25.51 eA (2.14) | 24.55 eA (2.12) | |
| 12 | 9.07 bA (4.49) | 22.11 dB (2.74) | 21.24 dB (2.06) | 16.77 dAB (4.75) | |
| FA Ratio | Heating Time (Days) | CFI Concentration | |||
|---|---|---|---|---|---|
| CTR | CFI-1 | CFI-2 | CFI-3 | ||
| Polyene index | 0 | 0.56 b (0.01) | 0.56 a (0.01) | 0.56 a (0.01) | 0.56 a (0.01) |
| 2 | 0.56 bA (0.01) | 0.56 aA (0.01) | 0.56 aA (0.00) | 0.56 aA (0.01) | |
| 5 | 0.54 abA (0.01) | 0.56 aAB (0.00) | 0.57 aB (0.01) | 0.56 aAB (0.00) | |
| 8 | 0.53 aA (0.01) | 0.55 aAB (0.01) | 0.56 aAB (0.02) | 0.56 aB (0.01) | |
| 12 | 0.53 aA (0.01) | 0.54 aA (0.01) | 0.56 aA (0.02) | 0.55 aA (0.02) | |
| Total MUFAs + Total PUFAs/Total STFAs | 0 | 3.48 (0.11) | 3.48 (0.11) | 3.48 (0.11) | 3.48 (0.11) |
| 2 | 3.49 (0.17) | 3.47 (0.27) | 3.49 (0.05) | 3.49 (0.09) | |
| 5 | 3.45 (0.26) | 3.47 (0.11) | 3.49 (0.15) | 3.49 (0.08) | |
| 8 | 3.42 (0.15) | 3.45 (0.47) | 3.47 (0.44) | 3.46 (0.16) | |
| 12 | 3.44 (0.26) | 3.43 (0.31) | 3.45 (0.11) | 3.46 (0.39) | |
| Total ω3 FAs/Total ω6 FAs | 0 | 0.40 (0.01) | 0.40 (0.01) | 0.40 (0.01) | 0.40 (0.01) |
| 2 | 0.40 (0.01) | 0.40 (0.01) | 0.39 (0.01) | 0.40 (0.02) | |
| 5 | 0.39 (0.01) | 0.40 (0.01) | 0.41 (0.01) | 0.40 (0.01) | |
| 8 | 0.39 (0.01) | 0.40 (0.01) | 0.40 (0.02) | 0.40 (0.01) | |
| 12 | 0.40 (0.01) | 0.40 (0.01) | 0.41 (0.01) | 0.40 (0.02) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trigo, M.; Paz, D.; Bote, A.; Aubourg, S.P. Antioxidant Activity of an Aqueous Extract of Cuttlefish Ink during Fish Muscle Heating. Antioxidants 2023, 12, 1996. https://doi.org/10.3390/antiox12111996
Trigo M, Paz D, Bote A, Aubourg SP. Antioxidant Activity of an Aqueous Extract of Cuttlefish Ink during Fish Muscle Heating. Antioxidants. 2023; 12(11):1996. https://doi.org/10.3390/antiox12111996
Chicago/Turabian StyleTrigo, Marcos, David Paz, Antía Bote, and Santiago P. Aubourg. 2023. "Antioxidant Activity of an Aqueous Extract of Cuttlefish Ink during Fish Muscle Heating" Antioxidants 12, no. 11: 1996. https://doi.org/10.3390/antiox12111996






