The Potential of Mangifera indica L. Peel Extract to Be Revalued in Cosmetic Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Mango Peel Extraction Process
2.3. HPLC-ESI-qTOF-MS Analysis
2.4. HPLC-ESI-qTOF-MS Data Processing
2.5. Evaluation of the Mango Peel Extract Potential
2.5.1. Evaluation of Total Phenolic Content and Antioxidant Capacity
2.5.2. Assessment of ROS/RNS Scavenging Activity
2.5.3. Enzyme Inhibition Activity
2.6. Study of the Effect on Pigmentation in Zebrafishes
2.6.1. Mango Peel Extract Preparation
2.6.2. Preparation and Treatment of Zebrafish Embryos
2.6.3. Pigmentation Assessment
2.6.4. Melanin Quantification
2.7. Cosmetic Formulation and Stability Tests
2.7.1. Cosmetic Formulation
2.7.2. Stability Study
2.8. Statistical Analysis
3. Results and Discussion
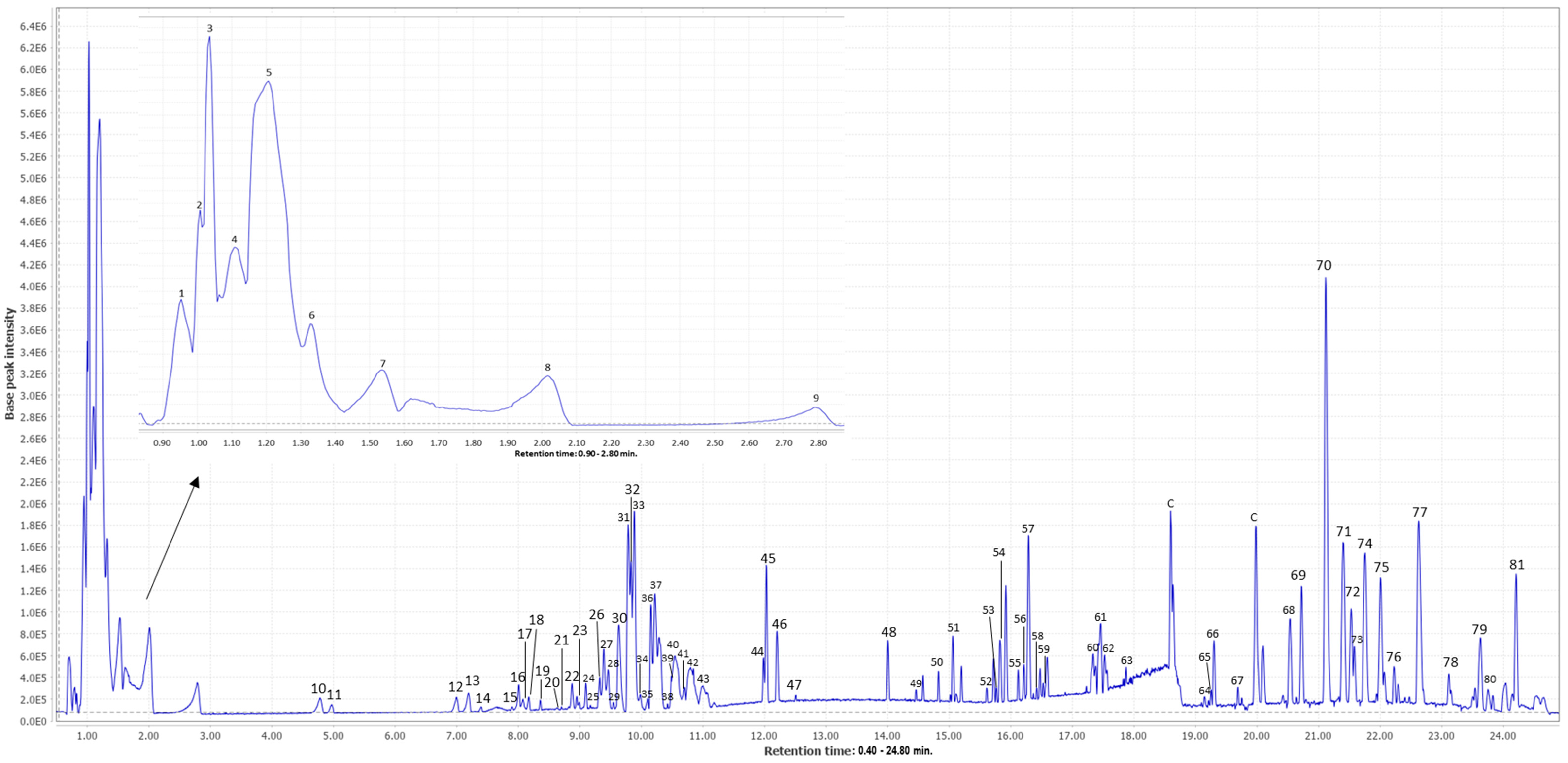
3.1. Characterization of Mango Peel Extracts by HPLC-ESI-qTOF-MS
| Peak | RT (min) | m/z Experimental [M-H]− | m/z Theoretical [M-H]− | Error (ppm) | Level of Annotation | Molecular Formula | Proposed Compound | MS/MS Fragments | References |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.96 | 179.0559 | 179.0561 | −1.18 | 2 | C6H12O6 | Glucose | 71,89 | [38] |
| 2 | 1.01 | 165.0406 | 165.0404 | 1.14 | 2 | C5H10O6 | Arabinonic acid | 75,165 | [39] |
| 3 | 1.04 | 191.0553 | 191.0561 | −4.25 | 2 | C7H12O6 | Quinic acid | 78,85,87,111,158 | [38] |
| 4 | 1.12 | 133.0145 | 133.0142 | 2.17 | 2 | C4H6O5 | Malic acid | 71,89,115,133 | [38] |
| 5 | 1.20 | 191.0215 | 191.0197 | 9.09 | 2 | C6H8O7 | Citric acid isomer 1 | 57,85,87,111,191 | [40] |
| 6 | 1.33 | 191.0199 | 191.0197 | 0.72 | 2 | C6H8O7 | Citric acid isomer 2 | 57,85,87,111,191 | [40] |
| 7 | 1.53 | 331.0680 | 331.0670 | 2.98 | 2 | C13H16O10 | Galloyl glucose isomer 1 | 169,125,271 | [21] |
| 8 | 2.01 | 169.0141 | 169.0142 | −0.66 | 2 | C7H6O5 | Gallic acid | 79,125,169 | [21] |
| 9 | 2.80 | 331.0689 | 331.0670 | 5.70 | 2 | C13H16O10 | Galloyl glucose isomer 2 | 79,125,169 | [21] |
| 10 | 4.79 | 483.0787 | 483.0780 | 1.42 | 2 | C20H20O14 | Digalloyl glucose | 125, 169, 331, 483 | [21] |
| 11 | 4.97 | 443.1923 | 443.1923 | −0.02 | 2 | C21H32O10 | Dihydrophaseic acid glucoside isomer 1 | 59,71,89,101,443 | [41] |
| 12 | 7.00 | 517.2289 | 517.2291 | −0.36 | 2 | C24H38O12 | Vomifoliol xylosyl glucoside | 386,517 | [42] |
| 13 | 7.20 | 519.2463 | 519.2463 | 3.01 | 2 | C24H40O12 | Platanionoside C | 59,89,387 | CNP0116765 |
| 14 | 7.39 | 375.1669 | 375.1660 | 2.37 | 2 | C17H28O9 | Oxo-glucopyranosyloxy-undecenoic acid | 329,375 | CNP0103002 |
| 15 | 7.90 | 635.0891 | 635.0890 | 0.14 | 2 | C27H24O18 | Trigalloylglucose isomer 1 | 125,169,465,483,635 | [40] |
| 16 | 8.01 | 443.1927 | 443.1922 | 1.10 | 2 | C21H32O10 | Dihydrophaseic acid glucoside isomer 2 | 59,443 | [41] |
| 17 | 8.08 | 197.0457 | 197.0455 | 0.95 | 2 | C9H10O5 | Ethyl gallate | 78,124,125,169,197 | [21] |
| 18 | 8.18 | 261.1344 | 261.1343 | 0.34 | 2 | C12H22O6 | Phaseolate | 243,261 | HMDB0031897 |
| 19 | 8.36 | 403.1610 | 403.1609 | 0.22 | 2 | C18H28O10 | Glucopyranosyloxy dimethyl decadienedioic acid | 59,197,241,403 | CNP0365241 |
| 20 | 8.65 | 635.0884 | 635.0890 | −0.96 | 2 | C27H24O18 | Trigalloylglucose isomer 2 | 125,169,331,465,483,635 | [40] |
| 21 | 8.70 | 417.1758 | 417.1766 | −1.95 | 2 | C19H30O10 | Hydroxipropyl methoxyphenox phenoxy propanediol glucoside | 417 | HMDB0040353 |
| 22 | 8.88 | 477.1037 | 477.1038 | −0.23 | 2 | C22H22O12 | Hydroxycinnamoyl galloyl glucopyranoside isomer 1 | 119,163,313, 477 | HMDB0039190 |
| 23 | 8.96 | 477.1036 | 477.1038 | −0.44 | 2 | C22H22O12 | Hydroxycinnamoyl galloyl glucopyranoside isomer 2 | 119,163,477, 313 | HMDB0039190 |
| 24 | 9.11 | 787.0989 | 787.0999 | −1.29 | 2 | C34H28O22 | Tetragalloylglucose isomer 1 | 125,169,465, 617,635 | [21] |
| 25 | 9.15 | 425.1779 | 425.1817 | −9.04 | 2 | C21H30O9 | Abscisic acid glucopyranosyl ester | 147,153,263, 425 | PubChem: 102173239 |
| 26 | 9.32 | 503.2463 | 503.2498 | −7.00 | 2 | C24H40O11 | Megastigmadienediol apiosyl glucoside | 101,161,371, 503 | HMDB0029766 |
| 27 | 9.39 | 787.0972 | 787.0999 | −3.45 | 2 | C34H28O22 | Tetragalloylglucose isomer 2 | 125,169,465, 617,635 | [21] |
| 28 | 9.46 | 787.0897 | 787.0999 | −12.97 | 2 | C34H28O22 | Tetragalloylglucose isomer 3 | 125,169,465, 617, 635 | [21] |
| 29 | 9.55 | 787.0997 | 787.0999 | −0.27 | 2 | C34H28O22 | Tetragalloylglucose isomer 4 | 125,169,465, 617, 635 | [21] |
| 30 | 9.65 | 300.9988 | 300.9990 | −0.70 | 2 | C14H6O8 | Ellagic acid | 242,257, 270,273,300 | [21] |
| 31 | 9.79 | 463.0886 | 463.0882 | 0.84 | 2 | C21H20O12 | Quercetrin isomer 1 | 151,178,255, 271,300 | [21] |
| 32 | 9.84 | 463.0890 | 463.0882 | 1.65 | 2 | C21H20O12 | Quercetrin isomer 2 | 151,178,255, 271,300 | [21] |
| 33 | 9.89 | 939.1012 | 939.1109 | −10.34 | 2 | C41H32O26 | Pentagalloyl glucose isomer 1 | 125,151,169,617,939,769,787 | [21] |
| 34 | 9.66 | 939.1057 | 939.1109 | −5.55 | 2 | C41H32O26 | Pentagalloyl glucose isomer 2 | 125,151,169,617,769,787,939 | [21] |
| 35 | 10.11 | 655.2623 | - | - | 4 | - | Unknown | - | - |
| 36 | 10.16 | 433.0761 | 433.0776 | −3.49 | 2 | C20H18O11 | Reynuotrin | 271,300,301,433 | [40] |
| 37 | 10.23 | 1091.1130 | 1091.1218 | −8.08 | 2 | C48H36O30 | Hexagalloyl glucose isomer 1 | 169,431,617,769,939 | [40] |
| 38 | 10.43 | 447.0915 | 447.0933 | −4.05 | 2 | C21H20O11 | Quercetrin isomer 3 | 271,151,301,447 | [21] |
| 39 | 10.49 | 491.2120 | 491.2134 | −2.87 | 2 | C22H36O12 | Jasminoside I | 313,445,491 | CNP0216715 |
| 40 | 10.55 | 621.0587 | - | - | 4 | - | Unknown | - | - |
| 41 | 10.70 | 349.0558 | 349.0565 | −2.04 | 2 | C16H14O9 | Ethyl digallate | 124,125,169, 197 | [21] |
| 42 | 10.81 | 697.0682 | - | - | 4 | - | Unknown | - | - |
| 43 | 11.00 | 773.0707 | - | - | 4 | - | Unknown | - | - |
| 44 | 11.99 | 327.2171 | 327.2177 | −1.87 | 2 | C18H32O5 | Corchorifatty acid F isomer 1 | 171,211,327 | HMDB0035919 |
| 45 | 12.03 | 327.2180 | 327.2177 | 0.88 | 2 | C18H32O5 | Corchorifatty acid F isomer 2 | 171,211,327 | HMDB0035919 |
| 46 | 12.21 | 301.0349 | 301.0354 | −1.70 | 2 | C15H10O7 | Quercetin | 151,178 | [43] |
| 47 | 12.51 | 329.2332 | 329.2333 | −0.34 | 2 | C18H34O5 | Trihydroxy octadecenoic acid | 171,211,229,311,329 | HMDB0030936 |
| 48 | 14.01 | 293.2114 | 293.2122 | −2.77 | 2 | C18H30O3 | Hydroxyoctadeca trienoic acid isomer 1 | 59,195,275,235, 293 | HMDB0010203 |
| 49 | 14.46 | 309.2065 | 309.2071 | −2.17 | 2 | C18H30O4 | Hydroperoxyoctadeca trienoic acid | 171,185,209, 291,293,309 | CNP0346715 |
| 50 | 14.83 | 675.3614 | 675.3597 | 2.48 | 3 | C33H56O14 | Gingerglycolipid A isomer 1 | 397,415,675 | [44] |
| 51 | 15.07 | 675.3618 | 675.3597 | 3.01 | 3 | C33H56O14 | Gingerglycolipid A isomer 2 | 397,675 | [44] |
| 52 | 15.61 | 564.3312 | - | - | 4 | - | Unknown | - | - |
| 53 | 15.77 | 476.2787 | 476.2783 | 0.84 | 3 | C23H44NO7P | Octadecadienoyl lysophosphatidyl ethanolamine isomer 1 | 279,476 | HMDB0011507 |
| 54 | 15.82 | 293.2114 | 293.2122 | −2.77 | 2 | C18H30O3 | Hydroxyoctadeca trienoic acid isomer 2 | 171,235,275, 293 | HMDB0010203 |
| 55 | 16.12 | 699.3818 | - | - | 4 | - | Unknown | - | - |
| 56 | 16.22 | 452.2786 | 452.2782 | 0.86 | 2 | C21H44NO7P | Hexadecanoyl lysophosphatidyl ethanolamine isomer 2 | 255,452 | HMDB0011473 |
| 57 | 16.29 | 540.3306 | - | - | 4 | - | Unknown | - | - |
| 58 | 16.42 | 725.3986 | 725.4022 | −4.98 | 2 | C34H62O16 | Dioleic glucoside | 281,397,415,679,725 | [40] |
| 59 | 16.53 | 295.2279 | 295.2278 | 0.30 | 2 | C18H32O3 | Coriolic acid | 183,119,277, 295 | HMDB0062652 |
| 60 | 17.34 | 633.3799 | 633.3797 | 0.61 | 2 | C39H54O7 | hydroxypyracrenic acid isomer 1 | 145,633 | HMDB0029780 |
| 61 | 17.46 | 633.3801 | 633.3797 | 0.30 | 2 | C39H54O7 | hydroxypyracrenic acid isomer 2 | 145,633 | HMDB0029780 |
| 62 | 17.53 | 471.3488 | 471.3480 | 1.67 | 2 | C30H48O4 | Maslinic acid | 99,393,471 | [45] |
| 63 | 17.87 | 445.3170 | 445.3171 | −0.26 | 2 | C24H46O7 | Monogalactosyl stearate | 59,281,445 | CNP0179792 |
| 64 | 19.16 | 617.3867 | 617.3847 | 3.22 | 2 | C39H54O6 | Coumaroylalphitolic acid | 117,145,617 | HMDB0036299 |
| 65 | 19.26 | 299.2596 | 299.2591 | 1.63 | 2 | C18H36O3 | Hydroxyoctadecanoic acid | 59,299 | HMDB0112182 |
| 66 | 19.30 | 279.2334 | 279.2329 | 1.75 | 2 | C18H32O2 | Linoleic acid | 97,219,279 | [40] |
| 67 | 19.75 | 343.2660 | 344.2720 | 4.98 | 2 | C23H36O2 | Benzoyl hexadecanone | 81,343 | HMDB0035582 |
| 68 | 20.54 | 345.2801 | 345.2799 | 0.54 | 2 | C23H38O2 | Heptadecenyl benzenediol | 81,125,303,345 | HMDB0038527 |
| 69 | 20.72 | 981.5788 | 981.5904 | −11.91 | 2 | C51H86N2O16 | Isovalerylspiramycin Iii | 277,935,981 | CNP0328040 |
| 70 | 21.12 | 621.4392 | 621.4372 | 3.20 | 2 | C36H62O8 | Ginsenoside RH2 | 59,161,621 | [46] |
| 71 | 21.40 | 758.5544 | 759.5572 | 6.54 | 2 | C45H78NO6P | (Geranylgeranyl)-Sn-Glycero-Phophoethanolamine isomer 1 | 59,89 | CNP0350471 |
| 72 | 21.53 | 758.5428 | 759.5572 | −8.75 | 2 | C45H78NO6P | (Geranylgeranyl)-Sn-Glycero-Phophoethanolamine isomer 2 | 59,89 | CNP0350471 |
| 73 | 21.59 | 429.3033 | 429.3010 | 5.19 | 2 | C27H42O4 | Plastoquinone 1 | 133,135,429 | CNP0152651 |
| 74 | 21.75 | 819.5288 | 819.5264 | 2.88 | 2 | C45H74O10 | Diethyl tetramethyl propanyl octaoxapentacyclotetracontane tetrone | 277,513,773 | CNP0271090 |
| 75 | 22.00 | 959.5959 | - | - | 4 | - | Unknown | - | - |
| 76 | 22.22 | 795.5314 | - | - | 4 | - | Unknown | - | - |
| 77 | 22.29 | 431.3184 | 431.3167 | 3.94 | 2 | C27H44O4 | Caffeic acid stearyl ester isomer 1 | 133,135,161,179,431 | CNP0292000 |
| 78 | 22.64 | 431.3182 | 431.3167 | 3.45 | 2 | C27H44O4 | Caffeic acid stearyl ester isomer 2 | 133,1351,61,179,431 | CNP0292000 |
| 79 | 23.12 | 797.5463 | - | - | 4 | - | Unknown | - | - |
| 80 | 23.63 | 489.3605 | 489.3585 | 4.06 | 2 | C30H50O5 | Escinidin | 57,125,489 | HMDB0034525 |
| 81 | 24.21 | 545.2950 | 546.2986 | 7.52 | 2 | C34H42O6 | Dimethyloctadienyl trihydroxy-methoxy xanthenone | 369,527,545 | PubChem: 49798966 |
3.2. Evaluation of Total Phenolic Content and Antioxidant Capacity
3.3. Evaluation of ROS/RNS Scavenging Activity
3.4. Enzyme Inhibition Activity
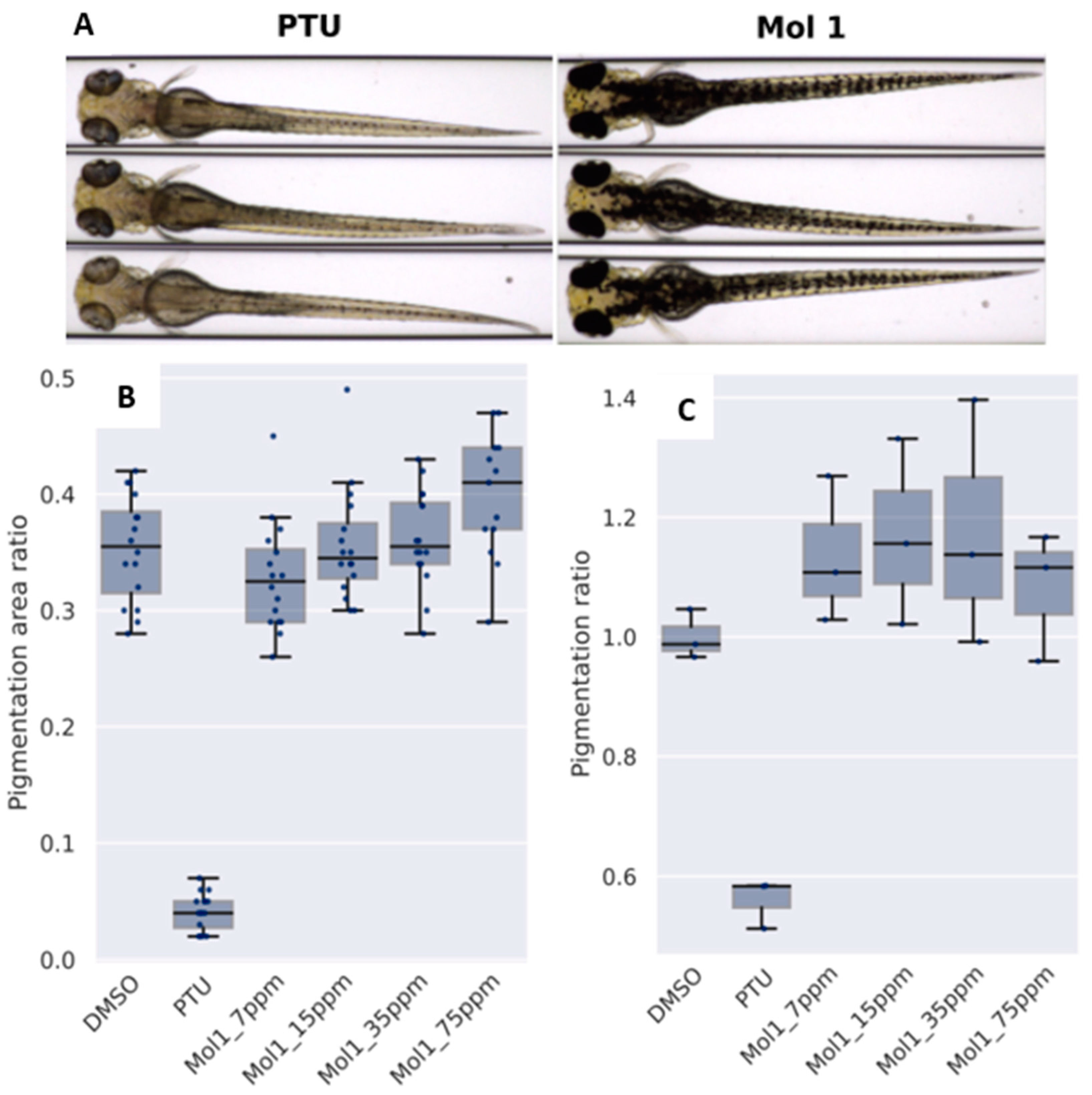
3.5. Study of the Effect on Pigmentation in Zebrafish
3.6. Cosmetic Formulation and Stability Tests
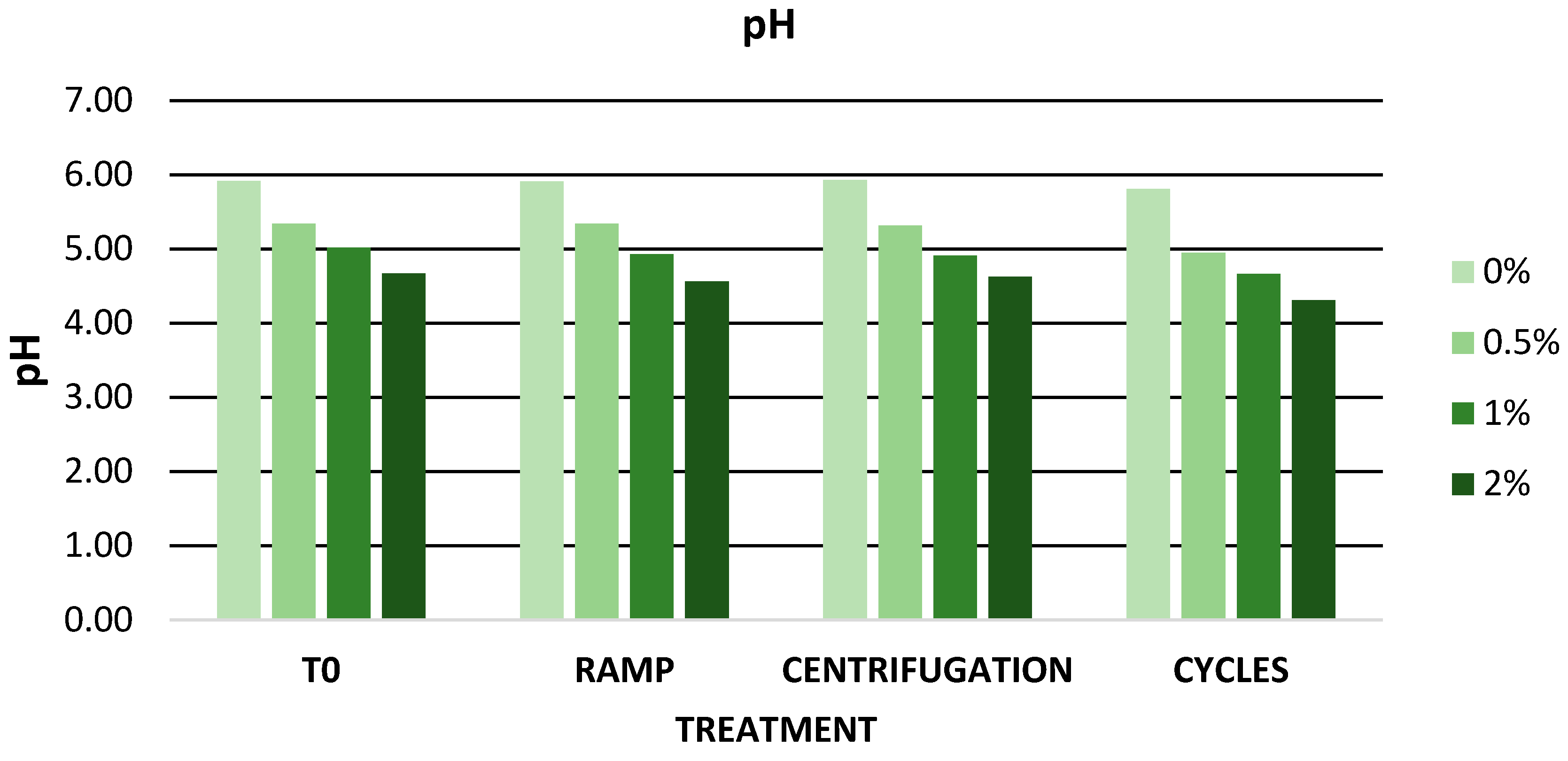
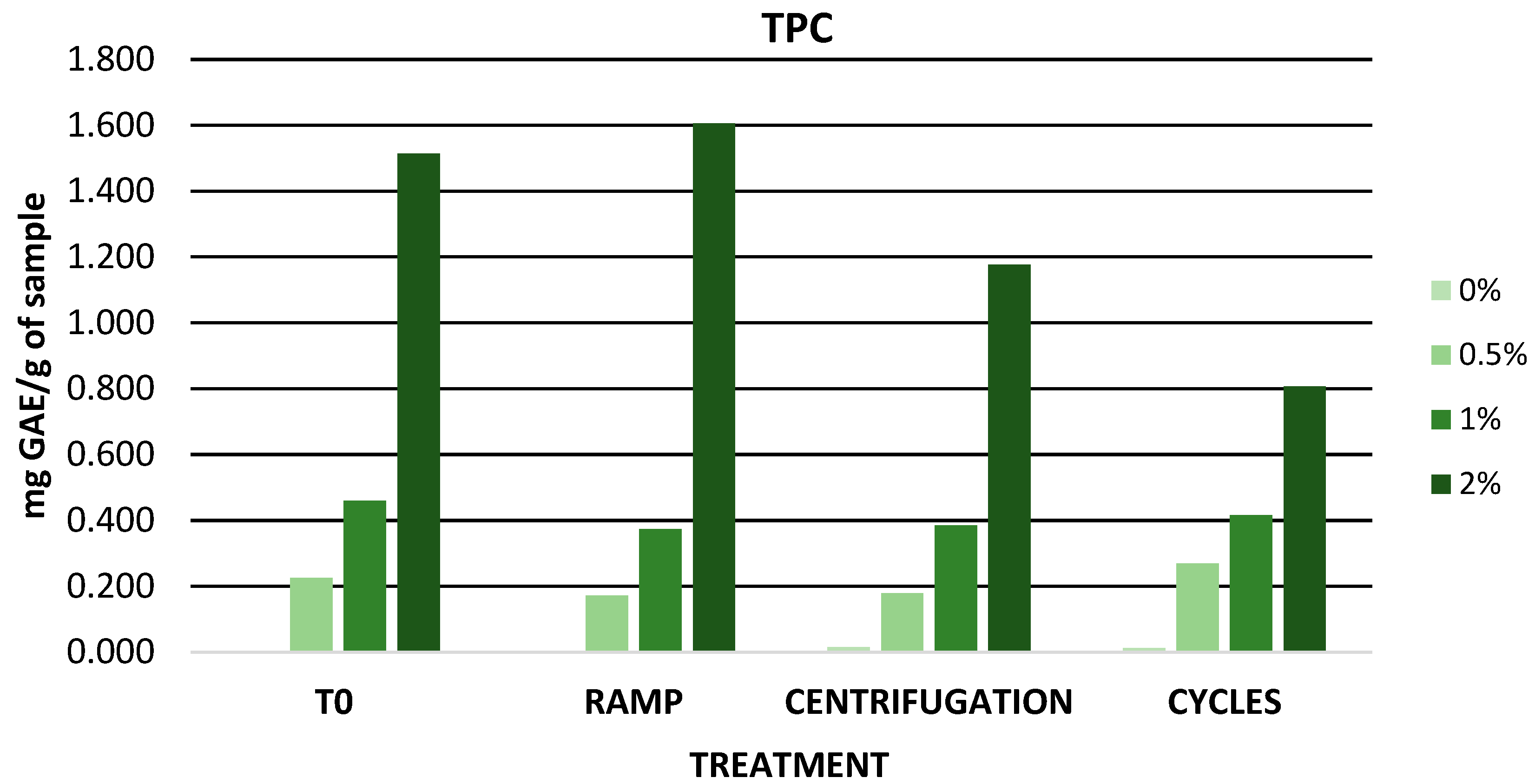
3.6.1. Evaluation of Physicochemical Parameters and TPC of Zero-Time Formulations
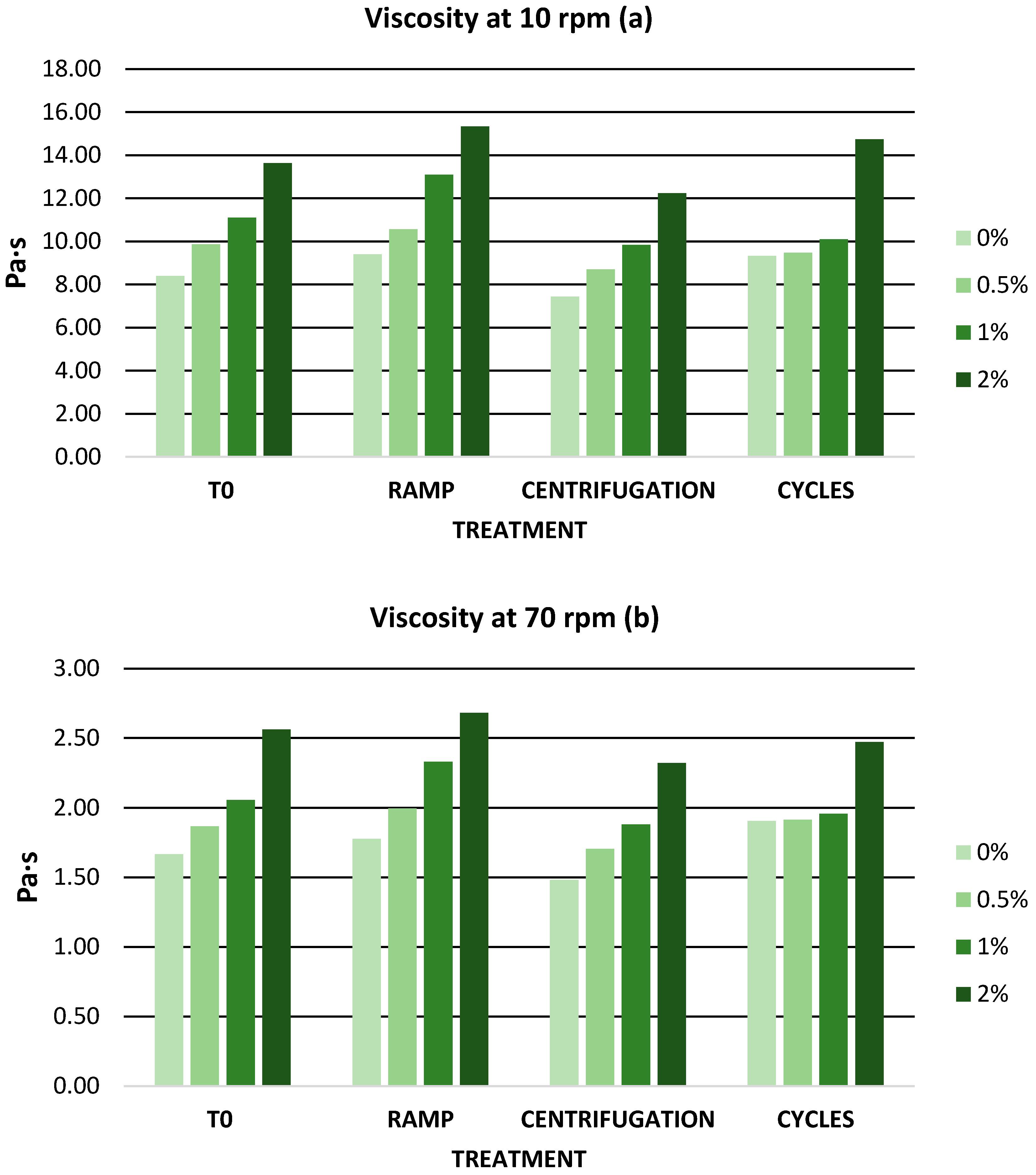
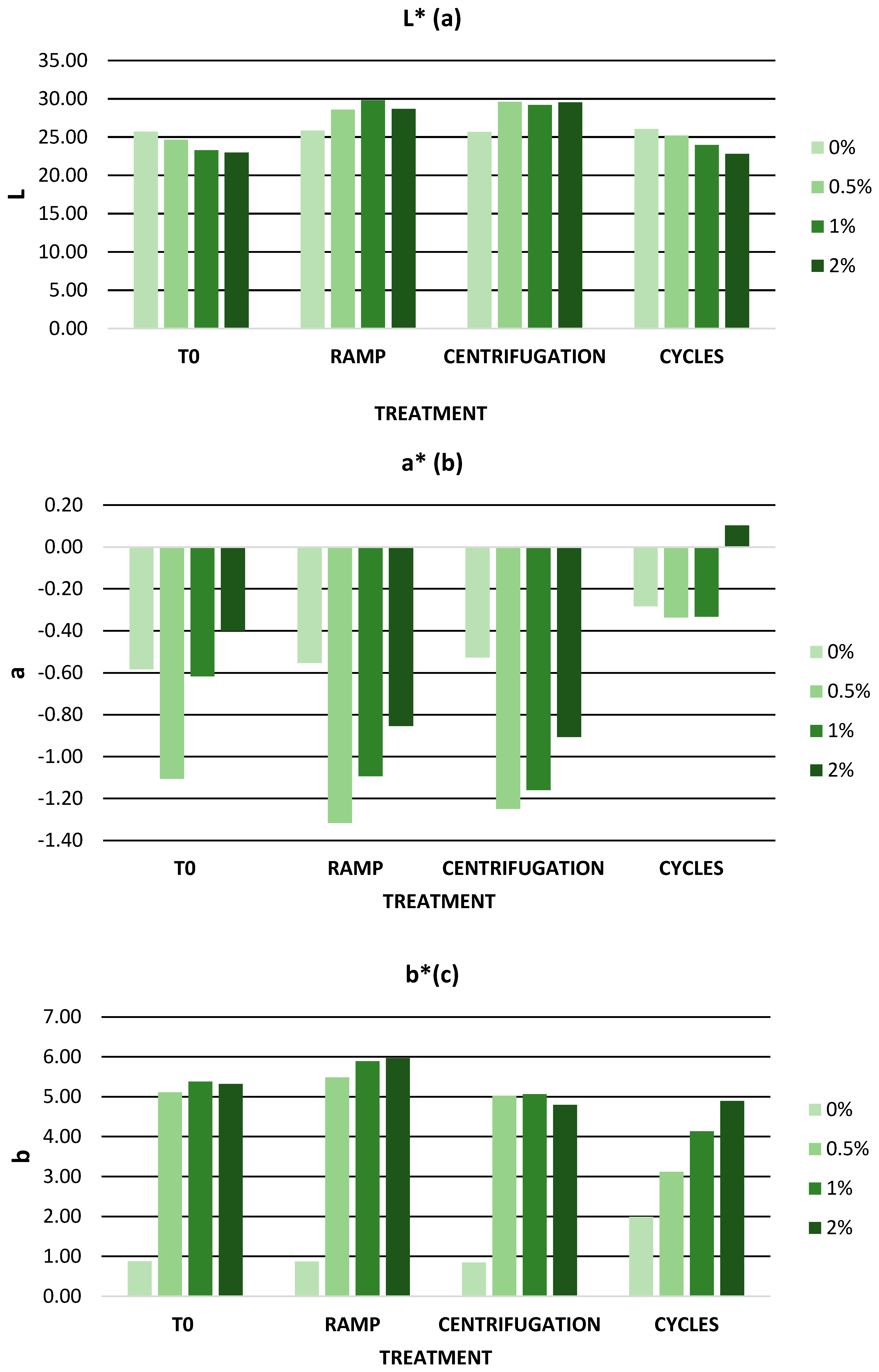
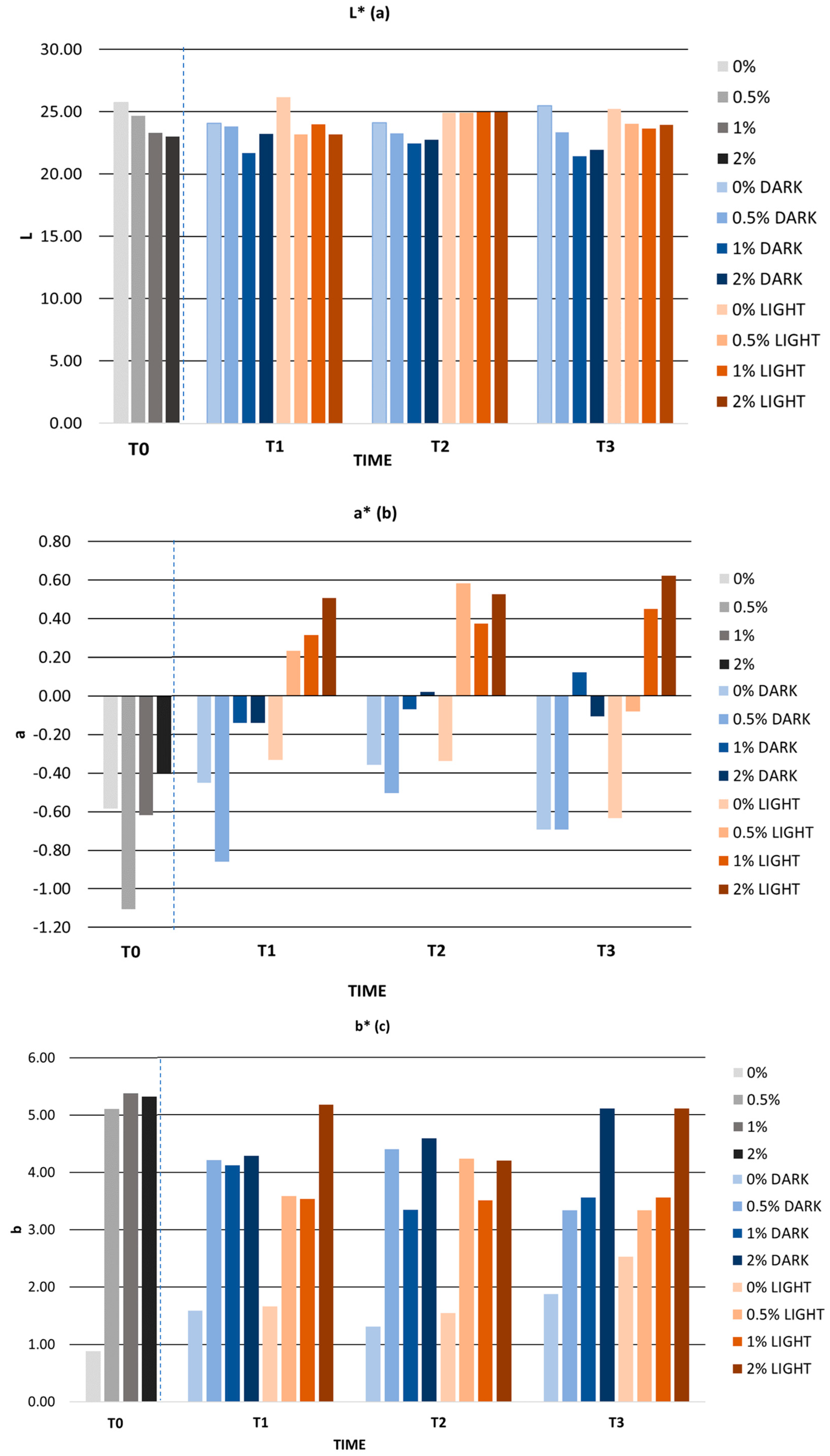
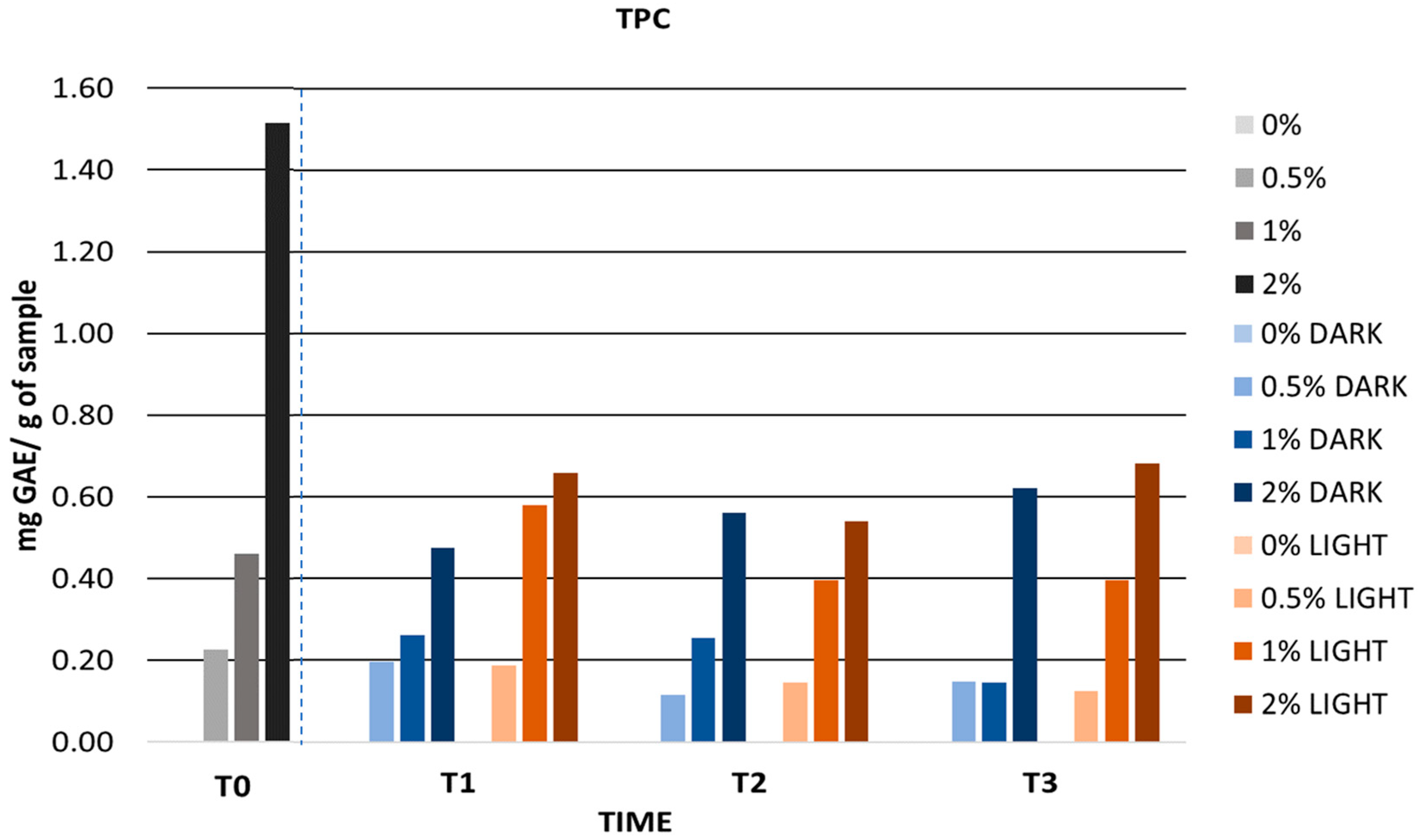
3.6.2. Stability Tests
Accelerated Stability
Long-Term Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R.I.; Simon, A.K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N.; et al. Autophagy in Healthy Aging and Disease. Nat. Aging 2021, 1, 634–650. [Google Scholar] [CrossRef]
- Ahmed, I.A.; Mikail, M.A.; Zamakshshari, N.; Abdullah, A.S.H. Natural Anti-Aging Skincare: Role and Potential. Biogerontology 2020, 21, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, Oxidative Stress and Autophagy in Skin Aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The Skin Aging Exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.A.M.; Michniak-Kohn, B.; Leonardi, G.R. An Overview about Oxidation in Clinical Practice of Skin Aging. An. Bras. Dermatol. 2017, 92, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Kammeyer, A.; Luiten, R.M. Oxidation Events and Skin Aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- Juliano, C.; Magrini, G.A. Cosmetic Functional Ingredients from Botanical Sources for Anti-Pollution Skincare Products. Cosmetics 2018, 5, 19. [Google Scholar] [CrossRef]
- Jesumani, V.; Du, H.; Aslam, M.; Pei, P.; Huang, N. Potential Use of Seaweed Bioactive Compounds in Skincare—A Review. Mar. Drugs 2019, 17, 688. [Google Scholar] [CrossRef] [PubMed]
- García-Villegas, A.; Rojas-García, A.; Villegas-Aguilar, M.d.C.; Fernández-Moreno, P.; Fernández-Ochoa, Á.; Cádiz-Gurrea, M.d.l.L.; Arráez-Román, D.; Segura-Carretero, A. Cosmeceutical Potential of Major Tropical and Subtropical Fruit By-Products for a Sustainable Revalorization. Antioxidants 2022, 11, 203. [Google Scholar] [CrossRef]
- Boo, Y.C. Can Plant Phenolic Compounds Protect the Skin from Airborne Particulate Matter? Antioxidants 2019, 8, 379. [Google Scholar] [CrossRef]
- Ng, S.Y.; Eh Suk, V.R.; Gew, L.T. Plant Polyphenols as Green Sunscreen Ingredients: A Systematic Review. J. Cosmet. Dermatol. 2022, 21, 5409–5444. [Google Scholar] [CrossRef] [PubMed]
- Morocho-Jácome, A.L.; Freire, T.B.; de Oliveira, A.C.; de Almeida, T.S.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. In Vivo SPF from Multifunctional Sunscreen Systems Developed with Natural Compounds—A Review. J. Cosmet. Dermatol. 2021, 20, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- de Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as Natural Antioxidants in Cosmetics Applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef]
- Jahurul, M.H.A.; Zaidul, I.S.M.; Ghafoor, K.; Al-Juhaimi, F.Y.; Nyam, K.L.; Norulaini, N.A.N.; Sahena, F.; Mohd Omar, A.K. Mango (Mangifera indica L.) by-Products and Their Valuable Components: A Review. Food Chem. 2015, 183, 173–180. [Google Scholar] [CrossRef]
- Oliver-Simancas, R.; Muñoz, R.; Díaz-Maroto, M.C.; Pérez-Coello, M.S.; Alañón, M.E. Mango By-Products as a Natural Source of Valuable Odor-Active Compounds. J. Sci. Food Agric. 2020, 100, 4688–4695. [Google Scholar] [CrossRef]
- Vega-Vega, V.; Silva-Espinoza, B.A.; Cruz-Valenzuela, M.R.; Bernal-Mercado, A.T.; González-Aguilar, G.A.; Ruíz-Cruz, S.; Moctezuma, E.; Siddiqui, M.W.; Ayala-Zavala, J.F. Antimicrobial and Antioxidant Properties of Byproduct Extracts of Mango Fruit. J. Appl. Bot. Food Qual. 2013, 86, 205–211. [Google Scholar] [CrossRef]
- Ajila, C.M.; Bhat, S.G.; Prasada Rao, U.J.S. Valuable Components of Raw and Ripe Peels from Two Indian Mango Varieties. Food Chem. 2007, 102, 1006–1011. [Google Scholar] [CrossRef]
- Abbasi, A.M.; Liu, F.; Guo, X.; Fu, X.; Li, T.; Liu, R.H. Phytochemical Composition, Cellular Antioxidant Capacity and Antiproliferative Activity in Mango (Mangifera indica L.) Pulp and Peel. Int. J. Food Sci. Technol. 2017, 52, 817–826. [Google Scholar] [CrossRef]
- Mwaurah, P.W.; Kumar, S.; Kumar, N.; Panghal, A.; Attkan, A.K.; Singh, V.K.; Garg, M.K. Physicochemical Characteristics, Bioactive Compounds and Industrial Applications of Mango Kernel and Its Products: A Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2421–2446. [Google Scholar] [CrossRef]
- Dorta, E.; González, M.; Lobo, M.G.; Sánchez-Moreno, C.; de Ancos, B. Screening of Phenolic Compounds in By-Product Extracts from Mangoes (Mangifera indica L.) by HPLC-ESI-QTOF-MS and Multivariate Analysis for Use as a Food Ingredient. Food Res. Int. 2014, 57, 51–60. [Google Scholar] [CrossRef]
- Barreto, J.C.; Trevisan, M.T.S.; Hull, W.E.; Erben, G.; De Brito, E.S.; Pfundstein, B.; Würtele, G.; Spiegelhalder, B.; Owen, R.W. Characterization and Quantitation of Polyphenolic Compounds in Bark, Kernel, Leaves, and Peel of Mango (Mangifera indica L.). J. Agric. Food Chem. 2008, 56, 5599–5610. [Google Scholar] [CrossRef] [PubMed]
- Rojas-García, A.; Rodríguez, L.; Cádiz-Gurrea, M.d.l.L.; García-Villegas, A.; Fuentes, E.; Villegas-Aguilar, M.d.C.; Palomo, I.; Arráez-Román, D.; Segura-Carretero, A. Determination of the Bioactive Effect of Custard Apple By-Products by In Vitro Assays. Int. J. Mol. Sci. 2022, 23, 9238. [Google Scholar] [CrossRef] [PubMed]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed Minimum Reporting Standards for Chemical Analysis: Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- De La Luz Cádiz-Gurrea, M.; Fernández-Ochoa, Á.; Leyva-Jiménez, F.J.; Guerrero-Muñoz, N.; Del Carmen Villegas-Aguilar, M.; Pimentel-Moral, S.; Ramos-Escudero, F.; Segura-Carretero, A. LC-MS and Spectrophotometric Approaches for Evaluation of Bioactive Compounds from Peru Cocoa by-Products for Commercial Applications. Molecules 2020, 25, 3177. [Google Scholar] [CrossRef]
- Al-Duais, M.; Müller, L.; Böhm, V.; Jetschke, G. Antioxidant Capacity and Total Phenolics of Cyphostemma Digitatum before and after Processing: Use of Different Assays. Eur. Food Res. Technol. 2009, 228, 813–821. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-Throughput Assay of Oxygen Radical Absorbance Capacity (ORAC) Using a Multichannel Liquid Handling System Coupled with a Microplate Fluorescence Reader in 96-Well Format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-evans, C. Antioxidant activity applying an improved abts radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Gomes, A.; Fernandes, E.; Silva, A.M.S.; Santos, C.M.M.; Pinto, D.C.G.A.; Cavaleiro, J.A.S.; Lima, J.L.F.C. 2-Styrylchromones: Novel Strong Scavengers of Reactive Oxygen and Nitrogen Species. Bioorganic Med. Chem. 2007, 15, 6027–6036. [Google Scholar] [CrossRef]
- Pinto, D.; De La Luz Cádiz-Gurrea, M.; Sut, S.; Ferreira, A.S.; Leyva-Jimenez, F.J.; Dall’acqua, S.; Segura-Carretero, A.; Delerue-Matos, C.; Rodrigues, F. Valorisation of Underexploited Castanea Sativa Shells Bioactive Compounds Recovered by Supercritical Fluid Extraction with CO2: A Response Surface Methodology Approach. J. CO2 Util. 2020, 40, 101194. [Google Scholar] [CrossRef]
- Nema, N.K.; Maity, N.; Sarkar, B.; Mukherjee, P.K. Cucumis Sativus Fruit-Potential Antioxidant, Anti-Hyaluronidase, and Anti-Elastase Agent. Arch. Dermatol. Res. 2011, 303, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Ghalleb, S.; De Vaugelade, S.; Sella, O.; Lavarde, M.; Mielcarek, C.; Pense-Lheritier, A.M.; Pirnay, S. Predictive Microbiology for Cosmetics Based on Physicals, Chemicals and Concentration Parameters. Int. J. Cosmet. Sci. 2015, 37, 70–75. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Rodrigues, R.; de Brito Damasceno, G.A.; Barreto, S.M.A.G.; Fernandes, J.M.; Telaprolu, K.C.; da Rocha-Filho, P.A.; Soares, L.A.L.; Ostrosky, E.A.; de Farias Sales, V.S.; Langassner, S.M.Z.; et al. Vegetable Moisturizing Raw Material from “Caatinga” Brazilian Biome: Safety and Efficacy Evaluations of O/W Cosmetic Emulsions Containing Kalanchoe Brasiliensis Extract. Brazilian J. Pharm. Sci. 2018, 54, 1–10. [Google Scholar] [CrossRef]
- Lee, S.H.; Chow, P.S.; Yagnik, C.K. Developing Eco-Friendly Skin Care Formulations with Microemulsions of Essential Oil. Cosmetics 2022, 9, 30. [Google Scholar] [CrossRef]
- Daudt, R.M.; Back, P.I.; Cardozo, N.S.M.; Marczak, L.D.F.; Külkamp-Guerreiro, I.C. Pinhão Starch and Coat Extract as New Natural Cosmetic Ingredients: Topical Formulation Stability and Sensory Analysis. Carbohydr. Polym. 2015, 134, 573–580. [Google Scholar] [CrossRef]
- Proksch, E. PH in Nature, Humans and Skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef]
- Alañón, M.E.; Pimentel-Moral, S.; Arráez-Román, D.; Segura-Carretero, A. HPLC-DAD-Q-ToF-MS Profiling of Phenolic Compounds from Mango (Mangifera indica L.) Seed Kernel of Different Cultivars and Maturation Stages as a Preliminary Approach to Determine Functional and Nutraceutical Value. Food Chem. 2021, 337, 127764. [Google Scholar] [CrossRef]
- Macedo, C.; Silva, A.M.; Ferreira, A.S.; Cádiz-Gurrea, M.d.l.L.; Fernández-Ochoa, Á.; Segura-Carretero, A.; Delerue-Matos, C.; Costa, P.; Rodrigues, F. Insights into the Polyphenols Extraction from Actinidia Arguta Fruit (Kiwiberry): A Source of pro-Healthy Compounds. Sci. Hortic. 2023, 313, 2–11. [Google Scholar] [CrossRef]
- Shi, X.; Yang, Y.; Ren, H.; Sun, S.; Mu, L.t.; Chen, X.; Wang, Y.; Zhang, Y.; Wang, L.h.; Sun, C. Identification of Multiple Components in Deep Eutectic Solvent Extract of Acanthopanax Senticosus Root by Ultra-High-Performance Liquid Chromatography with Quadrupole Orbitrap Mass Spectrometry. Phytochem. Lett. 2020, 35, 175–185. [Google Scholar] [CrossRef]
- Pierson, J.T.; Monteith, G.R.; Roberts-Thomson, S.J.; Dietzgen, R.G.; Gidley, M.J.; Shaw, P.N. Phytochemical Extraction, Characterisation and Comparative Distribution across Four Mango (Mangifera indica L.) Fruit Varieties. Food Chem. 2014, 149, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, X.J.; Pan, Y.; Zhou, Z. Identification of the Chemical Compositions of Ponkan Peel by Ultra Performance Liquid Chromatography Coupled with Quadrupole Time-of-Flight Mass Spectrometry. Anal. Methods 2016, 8, 893–903. [Google Scholar] [CrossRef]
- Sun, J.; Janisiewicz, W.J.; Nichols, B.; Jurick, W.M.; Chen, P. Composition of Phenolic Compounds in Wild Apple with Multiple Resistance Mechanisms against Postharvest Blue Mold Decay. Postharvest Biol. Technol. 2017, 127, 68–75. [Google Scholar] [CrossRef]
- Ribeiro, S.M.R.; Barbosa, L.C.A.; Queiroz, J.H.; Knödler, M.; Schieber, A. Phenolic Compounds and Antioxidant Capacity of Brazilian Mango (Mangifera indica L.) Varieties. Food Chem. 2008, 110, 620–626. [Google Scholar] [CrossRef]
- Nastić, N.; Borrás-Linares, I.; Lozano-Sánchez, J.; Švarc-Gajić, J.; Segura-Carretero, A. Optimization of the Extraction of Phytochemicals from Black Mulberry (Morus nigra L.) Leaves. J. Ind. Eng. Chem. 2018, 68, 282–292. [Google Scholar] [CrossRef]
- Goity, L.E.; Queupil, M.J.; Jara, D.; Alegría, S.E.; Peña, M.; Barriga, A.; Aguirre, M.C.; Delporte, C. An HPLC-UV and HPLC-ESI-MS Based Method for Identification of Anti-Inflammatory Triterpenoids from the Extracts of Ugni Molinae. Bol. Latinoam. y del Caribe Plantas Med. y Aromat. 2013, 12, 108–116. [Google Scholar]
- Xu, L.; Xu, J.; Shi, G.; Xiao, S.; Dai, R.; Wu, S.; Sun, B.; Zhang, X.; Zhao, Y. Optimization of Flash Extraction, Separation of Ginsenosides, Identification by HPLC-FT-ICR-MS and Determination of Rare Ginsenosides in Mountain Cultivated Ginseng. RSC Adv. 2020, 10, 44050–44057. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.M.; Guo, X.; Fu, X.; Zhou, L.; Chen, Y.; Zhu, Y.; Yan, H.; Liu, R.H. Comparative Assessment of Phenolic Content and In Vitro Antioxidant Capacity in the Pulp and Peel of Mango Cultivars. Int. J. Mol. Sci. 2015, 16, 13507–13527. [Google Scholar] [CrossRef] [PubMed]
- Agatonovic-Kustrin, S.; Kustrin, E.; Morton, D.W. Phenolic Acids Contribution to Antioxidant Activities and Comparative Assessment of Phenolic Content in Mango Pulp and Peel. S. Afr. J. Bot. 2018, 116, 158–163. [Google Scholar] [CrossRef]
- Rojas-Bravo, M.; Rojas-Zenteno, E.G.; Hernández-Carranza, P.; Ávila-Sosa, R.; Aguilar-Sánchez, R.; Ruiz-López, I.I.; Ochoa-Velasco, C.E. A Potential Application of Mango (Mangifera indica L. Cv Manila) Peel Powder to Increase the Total Phenolic Compounds and Antioxidant Capacity of Edible Films and Coatings. Food Bioprocess Technol. 2019, 12, 1584–1592. [Google Scholar] [CrossRef]
- Martínez-Ramos, T.; Benedito-Fort, J.; Watson, N.J.; Ruiz-López, I.I.; Che-Galicia, G.; Corona-Jiménez, E. Effect of Solvent Composition and Its Interaction with Ultrasonic Energy on the Ultrasound-Assisted Extraction of Phenolic Compounds from Mango Peels (Mangifera indica L.). Food Bioprod. Process. 2020, 122, 41–54. [Google Scholar] [CrossRef]
- Alañón, M.E.; Pimentel-Moral, S.; Arráez-Román, D.; Segura-Carretero, A. Profiling Phenolic Compounds in Underutilized Mango Peel By-Products from Cultivars Grown in Spanish Subtropical Climate over Maturation Course. Food Res. Int. 2021, 140, 109852. [Google Scholar] [CrossRef] [PubMed]
- Umamahesh, K.; Sivudu, S.N.; Vijaya, O.; Reddy, S. Evaluation of Antioxidant Activity, Total Phenolics and Total Flavonoids in Peels of Five Cultivars of Mango (Mangifera Indica) Fruit. J. Med. Plants Stud. 2016, 4, 200–203. [Google Scholar]
- Tacias-Pascacio, V.G.; Castañeda-Valbuena, D.; Fernandez-Lafuente, R.; Berenguer-Murcia, Á.; Meza-Gordillo, R.; Gutiérrez, L.F.; Pacheco, N.; Cuevas-Bernardino, J.C.; Ayora-Talavera, T. Phenolic Compounds in Mango Fruit: A Review. J. Food Meas. Charact. 2022, 16, 619–636. [Google Scholar] [CrossRef]
- Lone, R.; Shuab, R.; Kamili, A.N. (Eds.) Plant Phenolics in Sustainable Agriculture; Springer: Singapore, 2020; Volume 1, ISBN 9789811548901. [Google Scholar]
- Graf, J. Antioxidants and Skin Care: The Essentials. Plast. Reconstr. Surg. 2010, 125, 378–383. [Google Scholar] [CrossRef]
- Rodrigues, F.; Palmeira-de-Oliveira, A.; das Neves, J.; Sarmento, B.; Amaral, M.H.; Oliveira, M.B. Medicago Spp. Extracts as Promising Ingredients for Skin Care Products. Ind. Crops Prod. 2013, 49, 634–644. [Google Scholar] [CrossRef]
- Huang, D.; Boxin, O.U.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Liang, T.; Yue, W.; Li, Q. Comparison of the Phenolic Content and Antioxidant Activities of Apocynum venetum L. (Luo-Bu-Ma) and Two of Its Alternative Species. Int. J. Mol. Sci. 2010, 11, 4452–4464. [Google Scholar] [CrossRef]
- Safdar, M.N.; Kausar, T.; Nadeem, M. Comparison of Ultrasound and Maceration Techniques for the Extraction of Polyphenols from the Mango Peel. J. Food Process. Preserv. 2017, 41, e13028. [Google Scholar] [CrossRef]
- Feng, C.Y.; Liu, Y.G.; Ma, F.Y.; Qiong, F.; Zhang, X.M. The Antioxidant Activitives of Mango Peel among Different Cultivars. IOP Conf. Ser. Earth Environ. Sci. 2017, 61, 012065. [Google Scholar] [CrossRef]
- Castañeda-Valbuena, D.; Ayora-Talavera, T.; Luján-Hidalgo, C.; Álvarez-Gutiérrez, P.; Martínez-Galero, N.; Meza-Gordillo, R. Ultrasound Extraction Conditions Effect on Antioxidant Capacity of Mango By-Product Extracts. Food Bioprod. Process. 2021, 127, 212–224. [Google Scholar] [CrossRef]
- Sogi, D.S.; Siddiq, M.; Greiby, I.; Dolan, K.D. Total Phenolics, Antioxidant Activity, and Functional Properties of “Tommy Atkins” Mango Peel and Kernel as Affected by Drying Methods. Food Chem. 2013, 141, 2649–2655. [Google Scholar] [CrossRef] [PubMed]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of Phenolic Compounds: A Review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Tsuruta, D. What Are Reactive Oxygen Species, Free Radicals, and Oxidative Stress in Skin Diseases? Int. J. Mol. Sci. 2021, 22, 10799. [Google Scholar] [CrossRef]
- Poljšak, B.; Dahmane, R. Free Radicals and Extrinsic Skin Aging. Dermatol. Res. Pract. 2012, 2012, 135206. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as Potential Antioxidant Therapeutic Agents: Mechanism and Actions. Mutat. Res.—Fundam. Mol. Mech. Mutagen. 2005, 579, 200–213. [Google Scholar] [CrossRef]
- López-López, G.; Moreno, L.; Cogolludo, A.; Galisteo, M.; Ibarra, M.; Duarte, J.; Lodi, F.; Tamargo, J.; Perez-Vizcaino, F. Nitric Oxide (NO) Scavenging and NO Protecting Effects of Quercetin and Their Biological Significance in Vascular Smooth Muscle. Mol. Pharmacol. 2004, 65, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Li, W.; Son, Y.O.; Sun, L.; Lu, J.; Kim, D.; Wang, X.; Yao, H.; Wang, L.; Pratheeshkumar, P.; et al. Quercitrin Protects Skin from UVB-Induced Oxidative Damage. Toxicol. Appl. Pharmacol. 2013, 269, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Leal, C.A.; Garcia-Lara, S. Current Methods for the Discovery of New Active Ingredients from Natural Products for Cosmeceutical Applications. Planta Med. 2019, 85, 535–551. [Google Scholar] [CrossRef]
- Cádiz-Gurrea, M.d.l.L.; Villegas-Aguilar, M.d.C.; Leyva-Jiménez, F.J.; Pimentel-Moral, S.; Fernández-Ochoa, Á.; Alañón, M.E.; Segura-Carretero, A. Revalorization of Bioactive Compounds from Tropical Fruit By-Products and Industrial Applications by Means of Sustainable Approaches. Food Res. Int. 2020, 138, 109786. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin Whitening Agents: Medicinal Chemistry Perspective of Tyrosinase Inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Sancheti, S.A.; Sancheti, S.S. Drug Biochemistry Effect of 1, 2, 3, 4, 6-Penta-O-Galloyl-Β-D-Glucose on Elastase and Hyaluronidase Activities and Its Type Ii. Acta Pol. Pharm. 2010, 67, 145–150. [Google Scholar]
- Li, X.; Xu, R.; Cheng, Z.; Song, Z.; Wang, Z.; Duan, H.; Wu, X.; Ni, T. Comparative Study on the Interaction between Flavonoids with Different Core Structures and Hyaluronidase. Spectrochim. Acta Part A Espectroscopía Mol. y Biomol. 2021, 262, 120079. [Google Scholar] [CrossRef]
- Pientaweeratch, S.; Panapisal, V.; Tansirikongkol, A. Antioxidant, Anti-Collagenase and Anti-Elastase Activities of Phyllanthus emblica, Manilkara zapota and Silymarin: An In Vitro Comparative Study for Anti-Aging Applications. Pharm. Biol. 2016, 54, 1865–1872. [Google Scholar] [CrossRef]
- Fan, M.; Zhang, G.; Hu, X.; Xu, X.; Gong, D. Quercetin as a Tyrosinase Inhibitor: Inhibitory Activity, Conformational Change and Mechanism. Food Res. Int. 2017, 100, 226–233. [Google Scholar] [CrossRef]
- Zeng, H.J.; Ma, J.; Yang, R.; Jing, Y.; Qu, L.B. Molecular Interactions of Flavonoids to Hyaluronidase: Insights from Spectroscopic and Molecular Modeling Studies. J. Fluoresc. 2015, 25, 941–959. [Google Scholar] [CrossRef]
- Lespade, L.; Bercion, S. Theoretical Study of the Mechanism of Inhibition of Xanthine Oxydase by Flavonoids and Gallic Acid Derivatives. J. Phys. Chem. B 2010, 114, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Zu, Y.; Fu, Y.; Kong, Y.; Zhao, J.; Li, X.; Li, J.; Wink, M.; Efferth, T. Antioxidant Activities and Xanthine Oxidase Inhibitory Effects of Extracts and Main Polyphenolic Compounds Obtained from Geranium sibiricum L. J. Agric. Food Chem. 2010, 58, 4737–4743. [Google Scholar] [CrossRef] [PubMed]
- Ochocka, R.; Hering, A.; Stefanowicz-Hajduk, J.; Cal, K.; Barańska, H. The Effect of Mangiferin on Skin: Penetration, Permeation and Inhibition of ECM Enzymes. PLoS ONE 2017, 12, e0181542. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Xie, L.; Lin, Q.; Tong, C.; Fu, Q.; Xu, J.; Xiao, J.; Shi, S. Profiling of Tyrosinase Inhibitors in Mango Leaves for a Sustainable Agro-Industry. Food Chem. 2020, 312, 126042. [Google Scholar] [CrossRef] [PubMed]
- Namngam, C.; Pinsirodom, P. Antioxidant Properties, Selected Enzyme Inhibition Capacities, and a Cosmetic Cream Formulation of Thai Mango Seed Kernel Extracts. Trop. J. Pharm. Res. 2017, 16, 9–16. [Google Scholar] [CrossRef]
- Rojas-García, A.; Fuentes, E.; Cádiz-Gurrea, M.d.l.L.; Rodriguez, L.; Villegas-Aguilar, M.D.C.; Palomo, I.; Arráez-Román, D.; Segura-Carretero, A. Biological Evaluation of Avocado Residues as a Potential Source of Bioactive Compounds. Antioxidants 2022, 11, 1049. [Google Scholar] [CrossRef]
- Agalou, A.; Thrapsianiotis, M.; Angelis, A.; Papakyriakou, A.; Skaltsounis, A.L.; Aligiannis, N.; Beis, D. Identification of Novel Melanin Synthesis Inhibitors from Crataegus Pycnoloba Using an in Vivo Zebrafish Phenotypic Assay. Front. Pharmacol. 2018, 9, 265. [Google Scholar] [CrossRef]
- Kim, Y.M.; Lee, E.C.; Lim, H.M.; Seo, Y.K. Rice Bran Ash Mineral Extract Increases Pigmentation through the P-ERK Pathway in Zebrafish (Danio Rerio). Int. J. Mol. Sci. 2019, 20, 2172. [Google Scholar] [CrossRef]
- Kumar, K.J.S.; Vani, M.G.; Wang, S.Y.; Liao, J.W.; Hsu, L.S.; Yang, H.L.; Hseu, Y.C. In Vitro and in Vivo Studies Disclosed the Depigmenting Effects of Gallic Acid: A Novel Skin Lightening Agent for Hyperpigmentary Skin Diseases. BioFactors 2013, 39, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Lee, J.; Jeong, Y.T.; Byun, G.H.; Kim, J.H. Melanogenesis Inhibition Activity of Floralginsenoside A from Panax Ginseng Berry. J. Ginseng Res. 2017, 41, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, M.; Hellen, L.; Hirvonen, J.; Yliruusi, J. Rheological Properties of Creams with Four Different Surfactant Combinations—Effect of Storage Time and Conditions. Int. J. Pharm. 2001, 221, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Ahshawat, M.S.; Saraf, S.; Saraf, S. Preparation and Characterization of Herbal Creams for Improvement of Skin Viscoelastic Properties. Int. J. Cosmet. Sci. 2008, 30, 183–193. [Google Scholar] [CrossRef]
- Nunes, A.; Gonçalves, L.; Marto, J.; Martins, A.M.; Silva, A.N.; Pinto, P.; Martins, M.; Fraga, C.; Ribeiro, H.M. Investigations of Olive Oil Industry By-Products Extracts with Potential Skin Benefits in Topical Formulations. Pharmaceutics 2021, 13, 465. [Google Scholar] [CrossRef]
- Liang, Z.; Sang, M.; Fan, P.; Wu, B.; Wang, L.; Yang, S.; Li, S. CIELAB Coordinates in Response to Berry Skin Anthocyanins and Their Composition in Vitis. J. Food Sci. 2011, 76, C490–C497. [Google Scholar] [CrossRef] [PubMed]
- Gil-Muñoz, R.; Gómez-Plaza, E.; Martínez, A.; López-Roca, J.M. Evolution of the CIELAB and Other Spectrophotometric Parameters during Wine Fermentation. Influence of Some Pre and Postfermentative Factors. Food Res. Int. 1997, 30, 699–705. [Google Scholar] [CrossRef]
- Zheng, H.; Lu, H. A Least-Squares Support Vector Machine (LS-SVM) Based on Fractal Analysis and CIELab Parameters for the Detection of Browning Degree on Mango (Mangifera indica L.). Comput. Electron. Agric. 2012, 83, 47–51. [Google Scholar] [CrossRef]
- Censi, R.; Peregrina, D.V.; Lacava, G.; Agas, D.; Lupidi, G.; Sabbieti, M.G.; Di Martino, P. Cosmetic Formulation Based on an Açai Extract. Cosmetics 2018, 5, 48. [Google Scholar] [CrossRef]
- Schmid-Wendtner, M.H.; Korting, H.C. The PH of the Skin Surface and Its Impact on the Barrier Function. Skin Pharmacol. Physiol. 2006, 19, 296–302. [Google Scholar] [CrossRef]
- Pinto, D.; Lameirão, F.; Delerue-Matos, C.; Rodrigues, F.; Costa, P. Characterization and Stability of a Formulation Containing Antioxidants-Enriched Castanea Sativa Shells Extract. Cosmetics 2021, 8, 49. [Google Scholar] [CrossRef]
- Salem, Y.; Rajha, H.N.; Franjieh, D.; Hoss, I.; Manca, M.L.; Manconi, M.; Castangia, I.; Perra, M.; Maroun, R.G.; Louka, N. Stability and Antioxidant Activity of Hydro-Glyceric Extracts Obtained from Different Grape Seed Varieties Incorporated in Cosmetic Creams. Antioxidants 2022, 11, 1348. [Google Scholar] [CrossRef]
- Mapoung, S.; Semmarath, W.; Arjsri, P.; Umsumarng, S.; Srisawad, K.; Thippraphan, P.; Yodkeeree, S.; Limtrakul, P. Determination of Phenolic Content, Antioxidant Activity, and Tyrosinase Inhibitory Effects of Functional Cosmetic Creams Available on the Thailand Market. Plants 2021, 10, 1383. [Google Scholar] [CrossRef]
- Campanero, M.A. Evaluación De La Estabilidad De Productos Cosméticos: Necesidad Y Procedimiento. Ind. Cosmética 2019, 10, 48–52. [Google Scholar]
- ONUDI. Recomendaciones Para El Desarrollo de Estudios de Estabilidad de Productos Cosméticos; Giraldo, H.J.M., Sánchez, M.F., Hernandez, G.N., Eds.; ONUDI: Bogotá, Colombia, 2018; ISBN 9789585985131. [Google Scholar]
- Plyduang, T.; Atipairin, A.; Yoon, A.S.; Sermkaew, N.; Sakdiset, P.; Sawatdee, S. Formula Development of Red Palm (Elaeis Guineensis) Fruit Extract Loaded with Solid Lipid Nanoparticles Containing Creams and Its Anti-Aging Efficacy in Healthy Volunteers. Cosmetics 2022, 9, 3. [Google Scholar] [CrossRef]
- Brochier, B.; Mercali, G.D.; Marczak, L.D.F. Effect of Ohmic Heating Parameters on Peroxidase Inactivation, Phenolic Compounds Degradation and Color Changes of Sugarcane Juice. Food Bioprod. Process. 2018, 111, 62–71. [Google Scholar] [CrossRef]
- Maisuthisakul, P.; Harnsilawat, T. Characterization and Stabilization of the Extract from Mango Seed Kernel in a Cosmetic Emulsion. Kasetsart J.—Nat. Sci. 2011, 45, 521–529. [Google Scholar]
- Chuarienthong, P.; Lourith, N.; Leelapornpisid, P. Clinical Efficacy Comparison of Anti-Wrinkle Cosmetics Containing Herbal Flavonoids. Int. J. Cosmet. Sci. 2010, 32, 99–106. [Google Scholar] [CrossRef]
- Moraes, C.A.P.; Arêas, E.P.G.; Velasco, M.V.R. Assessment of Functional Stability of Photoprotective Formulations Containing Rutin Succinate. Cosmetics 2017, 4, 27. [Google Scholar] [CrossRef]
- Balboa, E.; Conde, E.; Constenla, A.; Falqué, E.; Domínguez, H. Sensory Evaluation and Oxidative Stability of a Suncream Formulated with Thermal Spring Waters from Ourense (NW Spain) and Sargassum Muticum Extracts. Cosmetics 2017, 4, 19. [Google Scholar] [CrossRef]
- Hwang, D.; Kim, H.; Shin, H.; Jeong, H.; Kim, J.; Kim, D. Cosmetic Effects of Prunus Padus Bark Extract. Korean J. Chem. Eng. 2014, 31, 2280–2285. [Google Scholar] [CrossRef]
- Huma, S.; Khan, H.M.S.; Sohail, M.; Akhtar, N.; Rasool, F.; Majeed, F.; Daniyal, M. Development, In-Vitro Characterization and Assessment of Cosmetic Potential of Beta Vulgaris Extract Emulsion. J. Herb. Med. 2020, 23, 100372. [Google Scholar] [CrossRef]
- Pinto, D.; Braga, N.; Rodrigues, F.; Oliveira, M.B.P.P. Castanea Sativa Bur: An Undervalued by-Product but a Promising Cosmetic Ingredient. Cosmetics 2017, 4, 50. [Google Scholar] [CrossRef]


| Commercial Name | INCI Name | CAS Code | Function | % |
|---|---|---|---|---|
| Demineralized water | Water | 7732-18-5 | Aqueous phase | 89.97, 89.47, 88.97, 87.97 |
| Glycerine | Glycerine | 56-81-5 | Moisturizer/Emollient | 5 |
| Vegetal extract | - | - | Active ingredient | 0, 0.5, 1, 2 |
| Xanthan gum | Xanthan gum | 11138-66-2 | Emulsifier/Gelling agent | 2 |
| Ethanol | Alcohol | 64-17-5 | Solvent/Preservative | 3 |
| Citric acid | Citric acid | 77-92-9/5949-29-1 | Ph regulator | 0.03 |
| Methodology | MP Extract | EPI | GA | KA | ELA | PHE |
|---|---|---|---|---|---|---|
| TPC (mg GAE/g DE) | 42.20 ± 0.07 | - | - | - | - | - |
| FRAP (mmol FeSO4/g DE) | 0.659 ± 0.004 | - | - | - | - | - |
| TEAC (μmol TE/g DE) | 484.3 ± 0.7 | - | - | - | - | - |
| ORAC (mmol TE/g DE) | 0.393 ± 0.008 | - | - | - | - | - |
| O2•− (mg/L) 1 | 950 ± 10 | 70 ± 5 | 50 ± 3 | - | - | - |
| •NO (mg/L) 1 | 7.5 ± 0.3 | 0.87 ± 0.02 | 1.4 ± 0.3 | - | - | - |
| HOCl (mg/L) 1 | 50 ± 10 | 0.18 ± 0.01 | 3.8 ± 0.3 | - | - | - |
| Tyrosinase | 170 ± 16 1 | - | - | 49 ± 6 2 | - | - |
| Elastase | 880 ± 10 1 | - | - | - | 53 ± 5 3 | - |
| HYALase (mg/L) | 27 ± 2 1 | 167 ± 6 4 | 102 ± 4 4 | - | - | - |
| Collagenase (mg/L) 1 | 260 ± 20 | - | - | - | - | 83 ± 2 |
| XOD (mg/L) 1 | 2 ± 1 | 9 ± 1 | - | - | - | - |
| Parameters | 0% | 0.5% | 1% | 2% |
|---|---|---|---|---|
| Viscosity (Pa·s) | ||||
| 10 rpm | 8.4 ± 0 | 9.87 ± 0.12 | 11.1 ± 0 | 13.63 ± 0.06 |
| 70 rpm | 1.67 ± 0.01 | 1.87 ± 0.02 | 2.06 ± 0.02 | 2.56 ± 0.01 |
| Color | ||||
| L* | 25.73 ± 0.02 | 24.65 ± 0.02 | 23.30 ± 0.02 | 23.00 ± 0.02 |
| a* | −0.58 ± 0.01 | −1.11 ± 0.04 | −0.62 ± 0.02 | −0.4 ± 0.03 |
| b* | 0.88 ± 0.06 | 5.11 ± 0.03 | 5.38 ± 0.04 | 5.32 ± 0.02 |
| pH | 5.92 ± 0.01 | 5.34 ± 0.02 | 5.01 ± 0.05 | 4.67 ± 0.02 |
| TPC (mg GA/g DE) | 0 | 0.23 ± 0.05 | 0.46 ± 0.05 | 1.51 ± 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Villegas, A.; Fernández-Ochoa, Á.; Rojas-García, A.; Alañón, M.E.; Arráez-Román, D.; Cádiz-Gurrea, M.d.l.L.; Segura-Carretero, A. The Potential of Mangifera indica L. Peel Extract to Be Revalued in Cosmetic Applications. Antioxidants 2023, 12, 1892. https://doi.org/10.3390/antiox12101892
García-Villegas A, Fernández-Ochoa Á, Rojas-García A, Alañón ME, Arráez-Román D, Cádiz-Gurrea MdlL, Segura-Carretero A. The Potential of Mangifera indica L. Peel Extract to Be Revalued in Cosmetic Applications. Antioxidants. 2023; 12(10):1892. https://doi.org/10.3390/antiox12101892
Chicago/Turabian StyleGarcía-Villegas, Abigail, Álvaro Fernández-Ochoa, Alejandro Rojas-García, María Elena Alañón, David Arráez-Román, María de la Luz Cádiz-Gurrea, and Antonio Segura-Carretero. 2023. "The Potential of Mangifera indica L. Peel Extract to Be Revalued in Cosmetic Applications" Antioxidants 12, no. 10: 1892. https://doi.org/10.3390/antiox12101892







