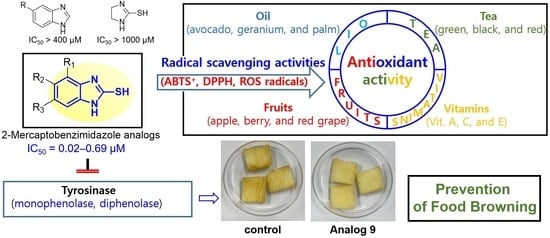
Anti-Browning Effect of 2-Mercaptobenzo[d]imidazole Analogs with Antioxidant Activity on Freshly-Cut Apple Slices and Their Highly Potent Tyrosinase Inhibitory Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemistry
2.1.1. General Methods
2.1.2. Synthesis of 2-MBI Analogs 1–10
General Procedure for the Synthesis of 1, 2, 6, 7, and 9
General Procedure for the Synthesis of 3–5 and 8–10
2.1.3. NMR Data for 2-MBI Analogs 1–10
2.2. Mushroom Tyrosinase Inhibitory Assay
2.3. Kinetic Studies of Tyrosinase Inhibition
2.4. In Silico Docking Simulation
2.4.1. Docking Simulation Using Schrodinger Suite
2.4.2. Docking Simulation Using AutoDock Vina
2.5. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Assay
2.6. 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic Acid (ABTS) Radical Cation Scavenging Assay
2.7. In Vitro Reactive Oxygen Species (ROS) Scavenging Assay
2.8. Anti-Browning Assay of Freshly-Cut Apple Slices
2.8.1. Sample Preparation
2.8.2. Sample Treatment
2.8.3. Browning Color Measurement
2.9. Human Embryonic Kidney Cells (HEK-293) Cell Culture
2.10. Cell Viability Assays
2.11. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of Analogs 1–10
3.2. Inhibitory Activities of Analogs 1–10 against Mushroom Tyrosinase
| Compd | Structure | IC50 (μM) | Compd | Structure | IC50 (μM) | ||
|---|---|---|---|---|---|---|---|
| Monophenolase | Diphenolase | Monophenolase | Diphenolase | ||||
| 1 |  | 0.45 ± 0.10 | 0.15 ± 0.00 | 7 |  | 0.14 ± 0.01 | 0.09 ± 0.01 |
| 2 |  | 0.16 ± 0.02 | 0.10 ± 0.00 | 8 |  | 0.19 ± 0.02 | 0.07 ± 0.01 |
| 3 |  | 0.76 ± 0.03 | 0.69 ± 0.04 | 9 |  | 5.78 ± 0.43 | 0.74 ± 0.17 |
| 4 |  | 0.06 ± 0.01 | 0.03 ± 0.00 | 10 |  | 0.11 ± 0.01 | 0.04 ± 0.02 |
| 5 |  | 0.18 ± 0.01 | 0.08 ± 0.03 | aKA |  | 16.83 ± 3.46 | 19.52 ± 0.68 |
| 6 |  | 0.10 ± 0.01 | 0.02 ± 0.01 | ||||
3.3. Mode of Action of 2-Mercaptobenzimidazole (2-MBI) Analogs
3.4. In Silico Docking Simulation of 2-Mercaptobenzimidazole (2-MBI) Analogs Using Mushroom Tyrosinase
3.5. Antioxidant Effects of 2-Mercaptobenzimidazole (2-MBI) Analogs on 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging, 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) Radical Cation Scavenging, and Reactive Oxygen Species (ROS) Scavenging
3.6. Effect of 2-Mercaptobenzimidazole (2-MBI) Analogs on Browning of Freshly-Cut Apple Slices
3.7. Cytotoxicity of 2-Mercaptobenzimidazole (2-MBI) Analogs in Human Embryonic Kidney Cells (HEK-293) Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- d’Ischia, M.; Wakamatsu, K.; Cicoira, F.; Di Mauro, E.; Garcia-Borron, J.C.; Commo, S.; Galván, I.; Ghanem, G.; Kenzo, K.; Meredith, P.; et al. Melanins and melanogenesis: From pigment cells to human health and technological applications. Pigment Cell Melanoma Res. 2015, 28, 520–544. [Google Scholar] [CrossRef]
- Wang, R.; Chai, W.-M.; Yang, Q.; Wei, M.-K.; Peng, Y. 2-(4-Fluorophenyl)-quinazolin-4(3H)-one as a novel tyrosinase inhibitor: Synthesis, inhibitory activity, and mechanism. Bioorg. Med. Chem. 2016, 24, 4620–4625. [Google Scholar] [CrossRef]
- Lambert, M.W.; Maddukuri, S.; Karanfilian, K.M.; Elias, M.L.; Lambert, W.C. The physiology of melanin deposition in health and disease. Clin. Dermatol. 2019, 37, 402–417. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Beer, J.Z.; Hearing, V.J. Melanin mediated apoptosis of epidermal cells damaged by ultraviolet radiation: Factors influencing the incidence of skin cancer. Arch. Dermatol. Res. 2008, 300 (Suppl. S1), S43–S50. [Google Scholar] [CrossRef]
- Meredith, P.; Sarna, T. The physical and chemical properties of eumelanin. Pigment Cell Res. 2006, 19, 572–594. [Google Scholar] [CrossRef]
- Vavricka, C.J.; Han, Q.; Mehere, P.; Ding, H.; Christensen, B.M.; Li, J. Tyrosine metabolic enzymes from insects and mammals: A comparative perspective. Insect Sci. 2014, 21, 13–19. [Google Scholar] [CrossRef]
- Zucca, F.A.; Segura-Aguilar, J.; Ferrari, E.; Muñoz, P.; Paris, I.; Sulzer, D.; Sarna, T.; Casella, L.; Zecca, L. Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Prog. Neurobiol. 2017, 155, 96–119. [Google Scholar] [CrossRef]
- Nosanchuk, J.D.; Casadevall, A. The contribution of melanin to microbial pathogenesis. Cell Microbiol. 2003, 5, 203–223. [Google Scholar] [CrossRef]
- Hearing, V.J.; Tsukamoto, K. Enzymatic control of pigmentation in mammals. FASEB J. 1991, 5, 2902–2909. [Google Scholar] [CrossRef]
- Vittorio, S.; Dank, C.; Ielo, L. Heterocyclic Compounds as Synthetic Tyrosinase Inhibitors: Recent Advances. Int. J. Mol. Sci. 2023, 24, 9097. [Google Scholar] [CrossRef]
- Friedman, M. Food Browning and Its Prevention: An Overview. J. Agric. Food Chem. 1996, 44, 631–653. [Google Scholar] [CrossRef]
- Lee, H.K.; Ha, J.W.; Hwang, Y.J.; Boo, Y.C. Identification of L-Cysteinamide as a Potent Inhibitor of Tyrosinase-Mediated Dopachrome Formation and Eumelanin Synthesis. Antioxidants 2021, 10, 1202. [Google Scholar] [CrossRef]
- Jergil, B.; Lindbladh, C.; Rorsman, H.; Rosengren, E. Inactivation of human tyrosinase by cysteine. Protection by dopa and tyrosine. Acta Derm.-Venereol. 1984, 64, 155–157. [Google Scholar] [CrossRef]
- Li, Q.; Mo, J.; Xiong, B.; Liao, Q.; Chen, Y.; Wang, Y.; Xing, S.; He, S.; Lyu, W.; Zhang, N.; et al. Discovery of Resorcinol-Based Polycyclic Structures as Tyrosinase Inhibitors for Treatment of Parkinson’s Disease. ACS Chem. Neurosci. 2022, 13, 81–96. [Google Scholar] [CrossRef]
- Ashooriha, M.; Khoshneviszadeh, M.; Khoshneviszadeh, M.; Moradi, S.E.; Rafiei, A.; Kardan, M.; Emami, S. 1,2,3-Triazole-based kojic acid analogs as potent tyrosinase inhibitors: Design, synthesis and biological evaluation. Bioorg. Chem. 2019, 82, 414–422. [Google Scholar] [CrossRef]
- Lu, Y.; Tonissen, K.F.; Di Trapani, G. Modulating skin colour: Role of the thioredoxin and glutathione systems in regulating melanogenesis. Biosci. Rep. 2021, 41, BSR20210427. [Google Scholar] [CrossRef]
- Ryu, I.Y.; Choi, I.; Jung, H.J.; Ullah, S.; Choi, H.; Al-Amin, M.; Chun, P.; Moon, H.R. In vitro anti-melanogenic effects of chimeric compounds, 2-(substituted benzylidene)-1,3-indanedione derivatives with a β-phenyl-α, β -unsaturated dicarbonyl scaffold. Bioorg. Chem. 2021, 109, 104688. [Google Scholar] [CrossRef]
- Jung, H.J.; Choi, D.C.; Noh, S.G.; Choi, H.; Choi, I.; Ryu, I.Y.; Chung, H.Y.; Moon, H.R. New Benzimidazothiazolone Derivatives as Tyrosinase Inhibitors with Potential Anti-Melanogenesis and Reactive Oxygen Species Scavenging Activities. Antioxidants 2021, 10, 1078. [Google Scholar] [CrossRef]
- Jeong, Y.; Hong, S.; Jung, H.J.; Ullah, S.; Hwang, Y.; Choi, H.; Ko, J.; Lee, J.; Chun, P.; Chung, H.Y.; et al. Identification of a Novel Class of Anti-Melanogenic Compounds, (Z)-5-(Substituted benzylidene)-3-phenyl-2-thioxothiazolidin-4-one Derivatives, and Their Reactive Oxygen Species Scavenging Activities. Antioxidants 2022, 11, 948. [Google Scholar] [CrossRef]
- Choi, H.; Young Ryu, I.; Choi, I.; Ullah, S.; Jin Jung, H.; Park, Y.; Hwang, Y.; Jeong, Y.; Hong, S.; Chun, P.; et al. Identification of (Z)-2-benzylidene-dihydroimidazothiazolone derivatives as tyrosinase inhibitors: Anti-melanogenic effects and in silico studies. Comput. Struct. Biotechnol. J. 2022, 20, 899–912. [Google Scholar] [CrossRef]
- Son, S.M.; Moon, K.D.; Lee, C.Y. Inhibitory effects of various antibrowning agents on apple slices. Food Chem. 2001, 73, 23–30. [Google Scholar] [CrossRef]
- Wang, G.; He, M.; Huang, Y.; Peng, Z. Synthesis and biological evaluation of new kojic acid-1,3,4-oxadiazole hybrids as tyrosinase inhibitors and their application in the anti-browning of fresh-cut mushrooms. Food Chem. 2023, 409, 135275. [Google Scholar] [CrossRef]
- Koirala, P.; Seong, S.H.; Zhou, Y.; Shrestha, S.; Jung, H.A.; Choi, J.S. Structure–Activity Relationship of the Tyrosinase Inhibitors Kuwanon G, Mulberrofuran G, and Albanol B from Morus Species: A Kinetics and Molecular Docking Study. Molecules 2018, 23, 1413. [Google Scholar] [CrossRef]
- Hwang, Y.; Lee, J.; Jung, H.J.; Ullah, S.; Ko, J.; Jeong, Y.; Park, Y.J.; Kang, M.K.; Yun, H.; Kim, M.S.; et al. A Novel Class of Potent Anti-Tyrosinase Compounds with Antioxidant Activity, 2-(Substituted phenyl)-5-(trifluoromethyl)benzo[d]thiazoles: In Vitro and In Silico Insights. Antioxidants 2022, 11, 1375. [Google Scholar] [CrossRef]
- Larik, F.A.; Saeed, A.; Channar, P.A.; Muqadar, U.; Abbas, Q.; Hassan, M.; Seo, S.-Y.; Bolte, M. Design, synthesis, kinetic mechanism and molecular docking studies of novel 1-pentanoyl-3-arylthioureas as inhibitors of mushroom tyrosinase and free radical scavengers. Eur. J. Med. Chem. 2017, 141, 273–281. [Google Scholar] [CrossRef]
- Hassan, M.; Ashraf, Z.; Abbas, Q.; Raza, H.; Seo, S.-Y. Exploration of Novel Human Tyrosinase Inhibitors by Molecular Modeling, Docking and Simulation Studies. Interdiscip. Sci. Comput. Life Sci. 2018, 10, 68–80. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra Precision Glide: Docking and Scoring Incorporating a Model of Hydrophobic Enclosure for Protein−Ligand Complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef]
- Bagherzadeh, K.; Shirgahi Talari, F.; Sharifi, A.; Ganjali, M.R.; Saboury, A.A.; Amanlou, M. A new insight into mushroom tyrosinase inhibitors: Docking, pharmacophore-based virtual screening, and molecular modeling studies. J. Biomol. Struct. Dyn. 2015, 33, 487–501. [Google Scholar] [CrossRef]
- Matos, M.J.; Varela, C.; Vilar, S.; Hripcsak, G.; Borges, F.; Santana, L.; Uriarte, E.; Fais, A.; Di Petrillo, A.; Pintus, F.; et al. Design and discovery of tyrosinase inhibitors based on a coumarin scaffold. RSC Adv. 2015, 5, 94227–94235. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- LeBel, C.P.; Bondy, S.C. Sensitive and rapid quantitation of oxygen reactive species formation in rat synaptosomes. Neurochem. Int. 1990, 17, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Chaiprasongsuk, A.; Panich, U. Role of Phytochemicals in Skin Photoprotection via Regulation of Nrf2. Front. Pharmacol. 2022, 13, 823881. [Google Scholar] [CrossRef] [PubMed]
- Htike, T.; Saengrayap, R.; Aunsri, N.; Tontiwattanakul, K.; Chaiwong, S. Investigation and Evaluation of Impact Bruising in Guava Using Image Processing and Response Surface Methodology. Horticulturae 2021, 7, 411. [Google Scholar] [CrossRef]
- Castañer, M.; Gil, M.I.; Ruíz, M.V.; Artés, F. Browning susceptibility of minimally processed Baby and Romaine lettuces. Eur. Food Res. Technol. 1999, 209, 52–56. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Park, H.S.; Jung, H.J.; Park, Y.J.; Kang, M.K.; Kim, H.J.; Yoon, D.; Ullah, S.; Kang, D.; Park, Y.; et al. Anti-Browning Effect of 2-Mercaptobenzo[d]imidazole Analogs with Antioxidant Activity on Freshly-Cut Apple Slices and Their Highly Potent Tyrosinase Inhibitory Activity. Antioxidants 2023, 12, 1814. https://doi.org/10.3390/antiox12101814
Lee J, Park HS, Jung HJ, Park YJ, Kang MK, Kim HJ, Yoon D, Ullah S, Kang D, Park Y, et al. Anti-Browning Effect of 2-Mercaptobenzo[d]imidazole Analogs with Antioxidant Activity on Freshly-Cut Apple Slices and Their Highly Potent Tyrosinase Inhibitory Activity. Antioxidants. 2023; 12(10):1814. https://doi.org/10.3390/antiox12101814
Chicago/Turabian StyleLee, Jieun, Hye Soo Park, Hee Jin Jung, Yu Jung Park, Min Kyung Kang, Hye Jin Kim, Dahye Yoon, Sultan Ullah, Dongwan Kang, Yujin Park, and et al. 2023. "Anti-Browning Effect of 2-Mercaptobenzo[d]imidazole Analogs with Antioxidant Activity on Freshly-Cut Apple Slices and Their Highly Potent Tyrosinase Inhibitory Activity" Antioxidants 12, no. 10: 1814. https://doi.org/10.3390/antiox12101814
APA StyleLee, J., Park, H. S., Jung, H. J., Park, Y. J., Kang, M. K., Kim, H. J., Yoon, D., Ullah, S., Kang, D., Park, Y., Chun, P., Chung, H. Y., & Moon, H. R. (2023). Anti-Browning Effect of 2-Mercaptobenzo[d]imidazole Analogs with Antioxidant Activity on Freshly-Cut Apple Slices and Their Highly Potent Tyrosinase Inhibitory Activity. Antioxidants, 12(10), 1814. https://doi.org/10.3390/antiox12101814