Aqueous Extract of Psiloxylon mauritianum, Rich in Gallic Acid, Prevents Obesity and Associated Deleterious Effects in Zebrafish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Ethics
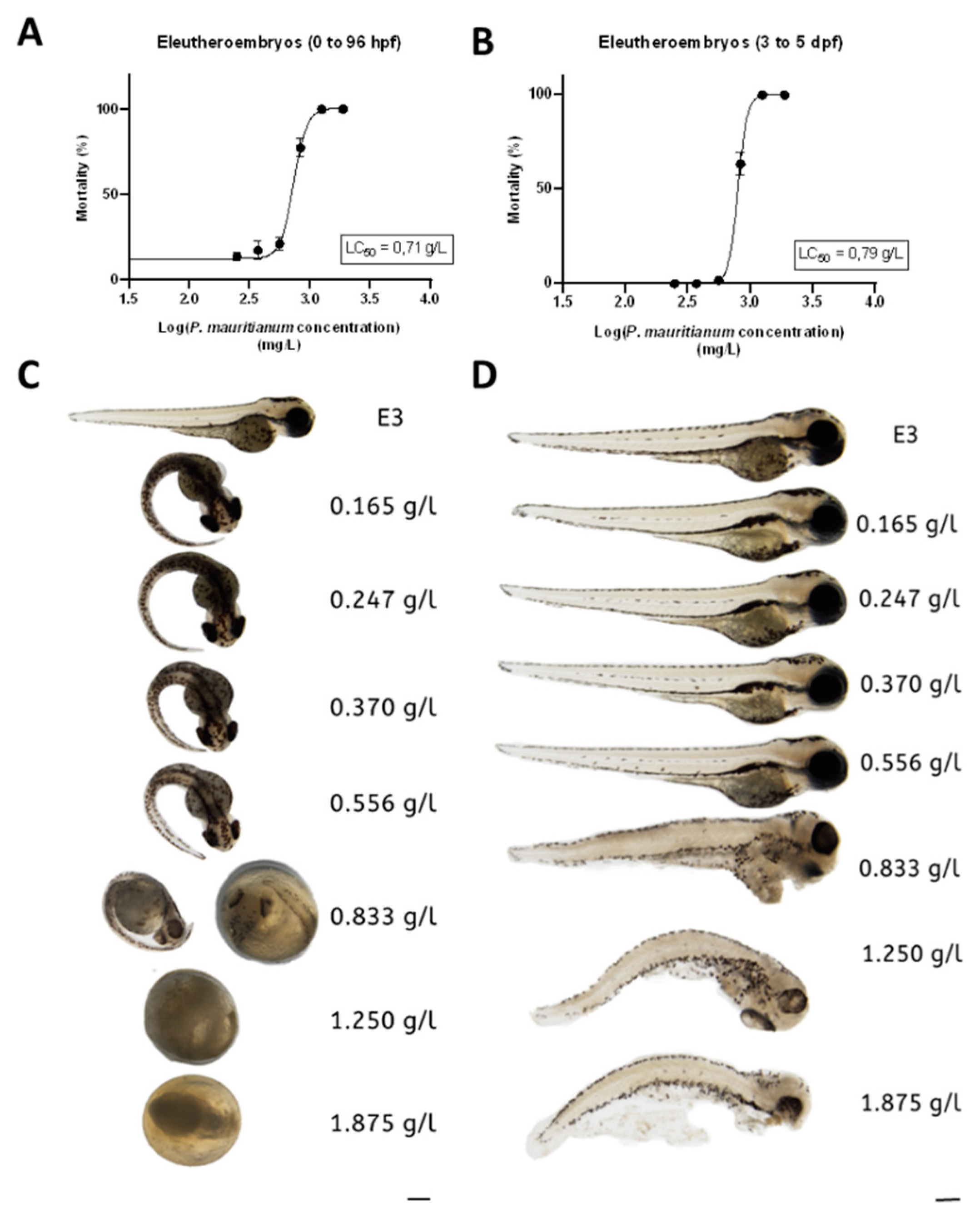
2.2. Eleutheroembryo Toxicity Assay
2.3. Larvae Overfeeding Protocol
2.4. Diet-Induced Overweight/Obesity (DIO) Protocol
2.5. Plant Material and Preparation of the Aqueous Plant Extract for Toxicity Tests and Preventive/Therapeutic Treatments
2.6. Body Weight, Body Mass Index (BMI) and Fasting Blood Glucose Measurements
2.7. Tissue Preparation
2.8. Oil Red O (ORO) Staining
2.9. RNA Extraction and Reverse Transcription
2.10. Gene Expression Analysis by qPCR
2.11. Immunostaining
2.12. Investigation of BBB Permeability (Evans Blue Dye Injection)
2.13. Protein Extraction and Dot Blot
2.14. Microscopy
2.15. Cell Counting
2.16. Instrumentation and LC-MS/MS Conditions
2.17. Statistical Analysis
3. Results
3.1. Polyphenol Composition of the Aqueous Extract of P. mauritianum
3.2. Toxicity of the Aqueous Extract of P. mauritianum
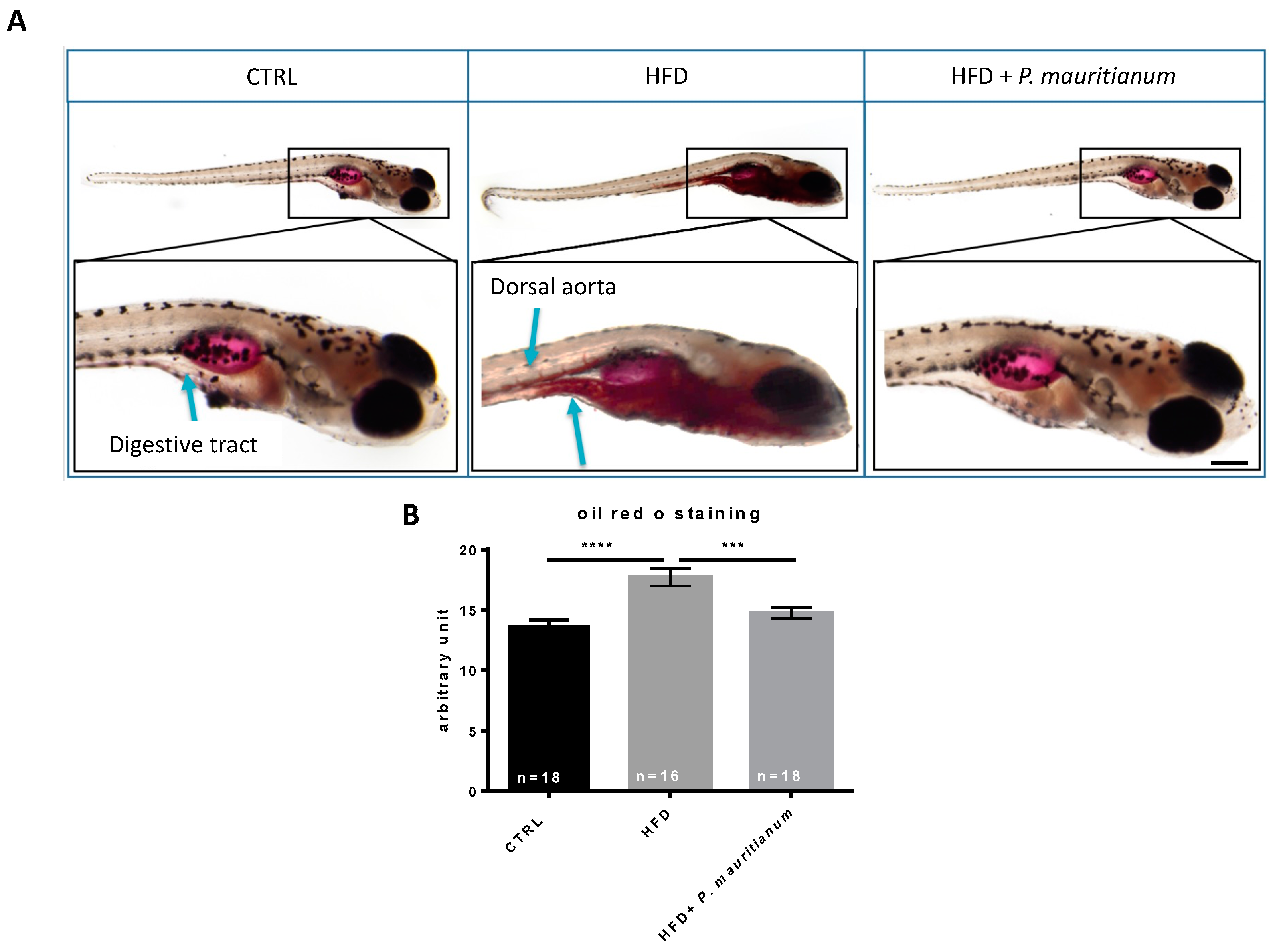
3.3. Aqueous Extract of P. mauritianum Exhibits Lipid-Lowering Effects in High-Fat-Diet-Fed Larvae
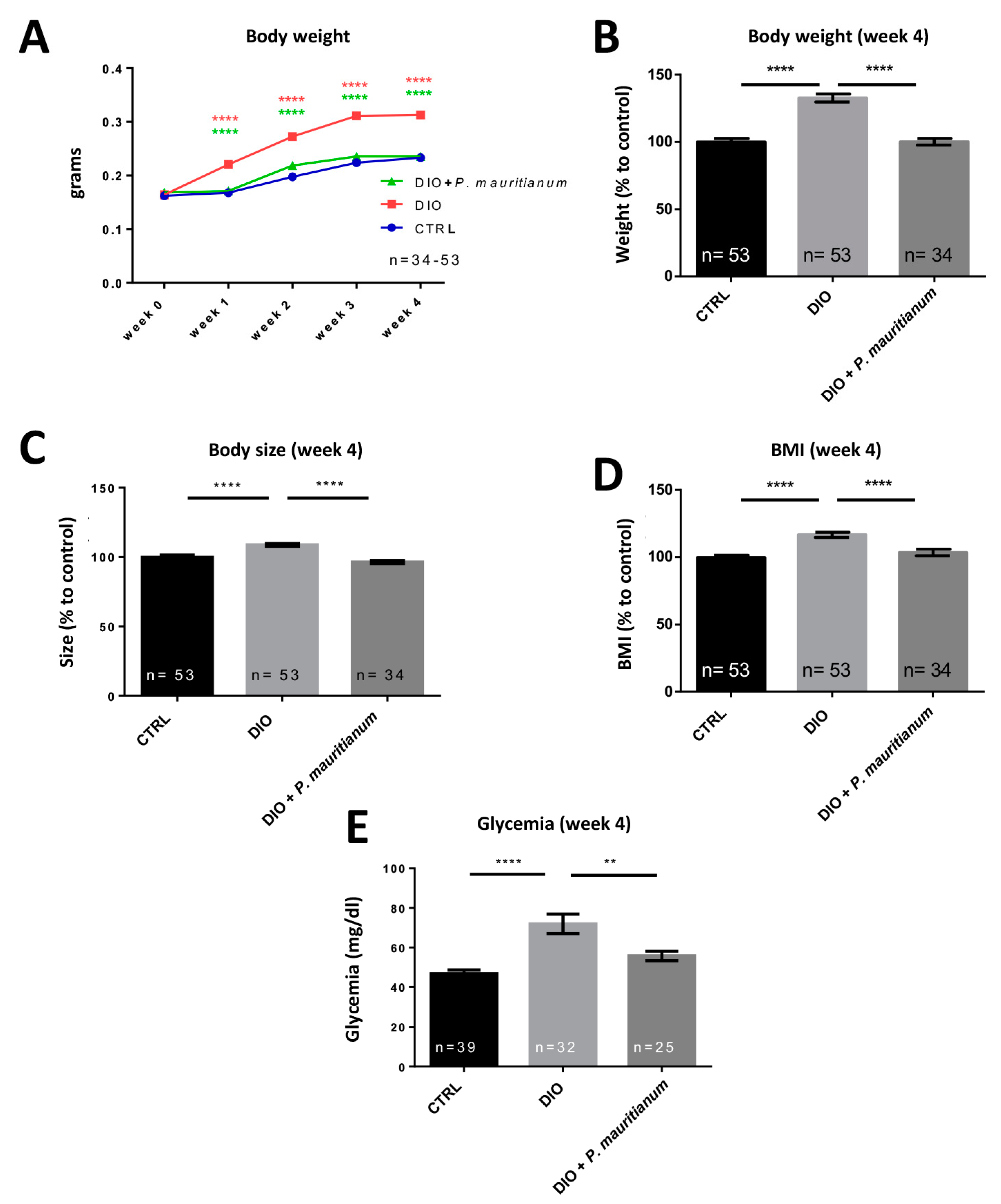
3.4. Preventive Effect of Aqueous Extract of P. mauritianum on Overfeeding-Induced Weight Gain in Adult Zebrafish
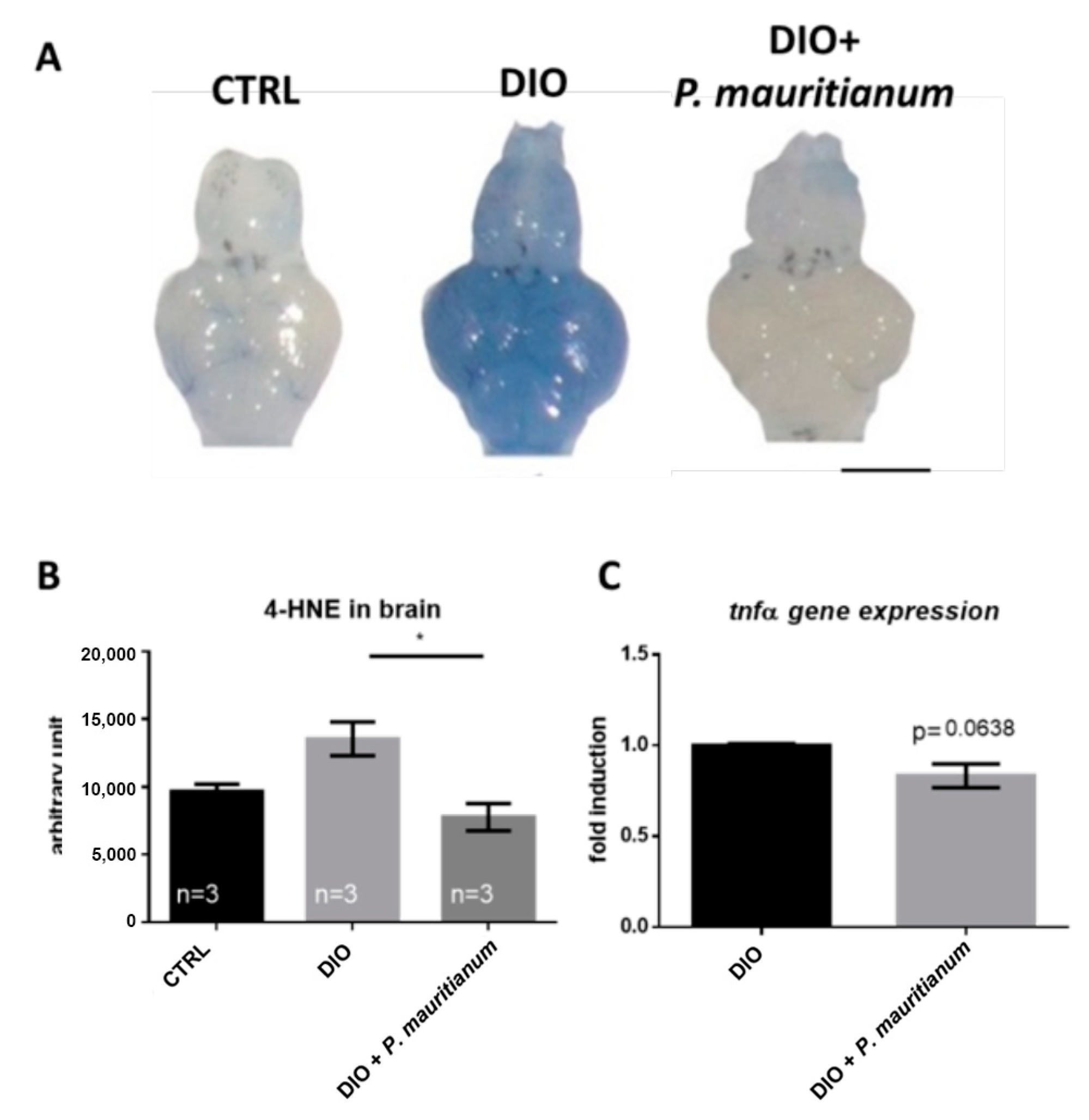
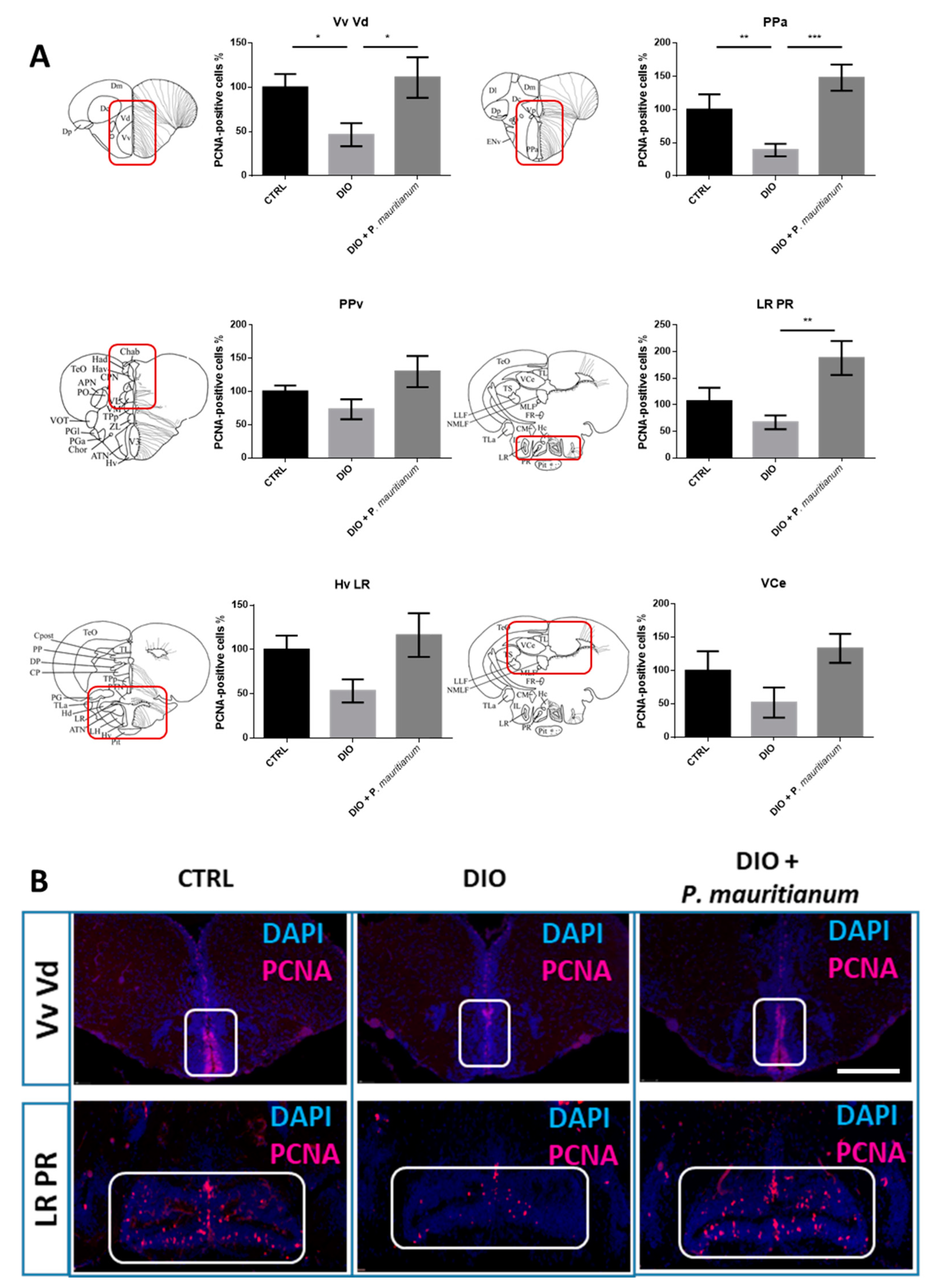
3.5. P. mauritianum Prevents Brain Homeostasis Disruption Induced by Overfeeding
3.6. Effect of P. mauritianum on Feeding Behavior and Feces Production
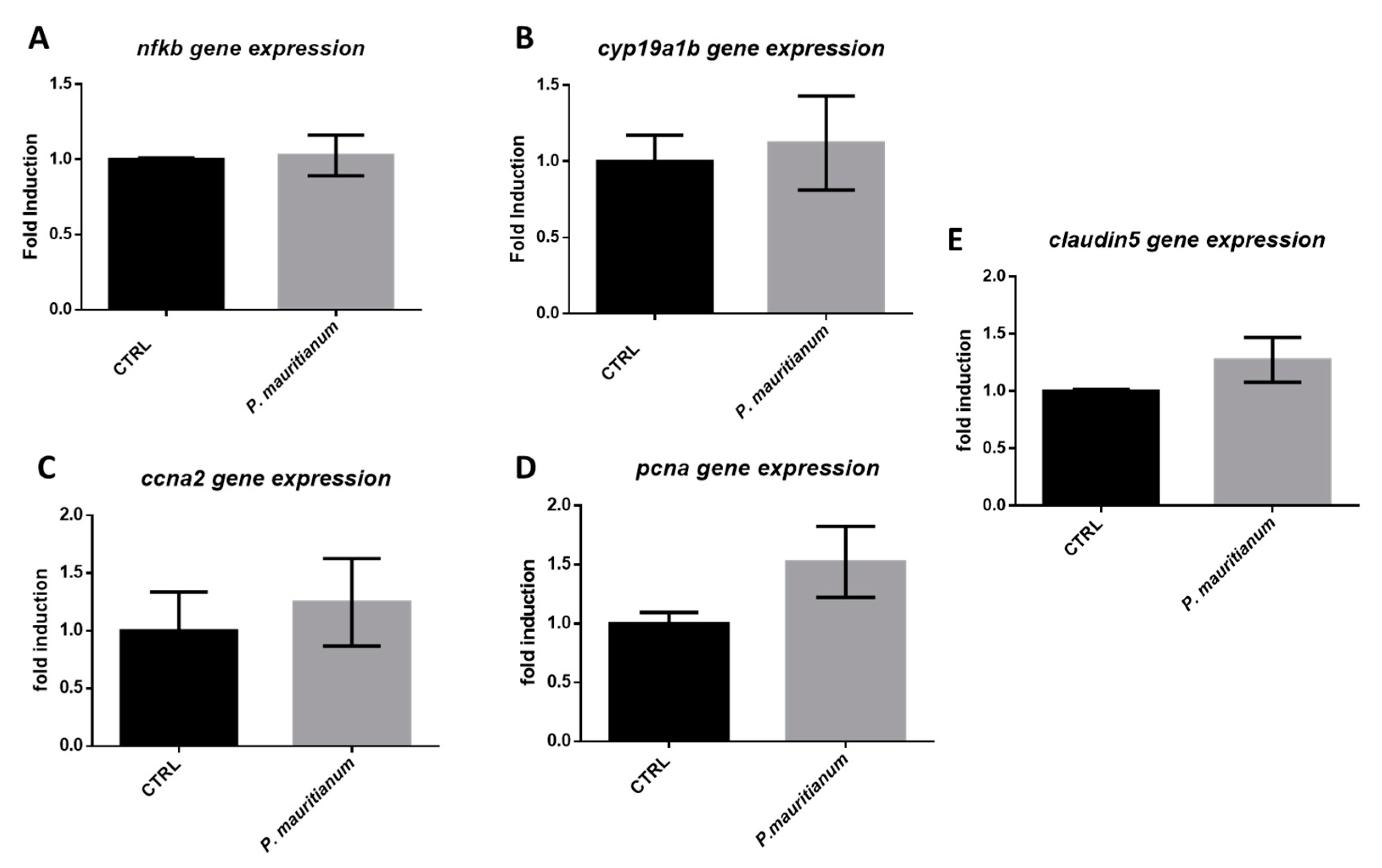
3.7. Aqueous Extract of P. mauritianum Has No Impact on Brain Homeostasis under Normal Chow Feeding Conditions
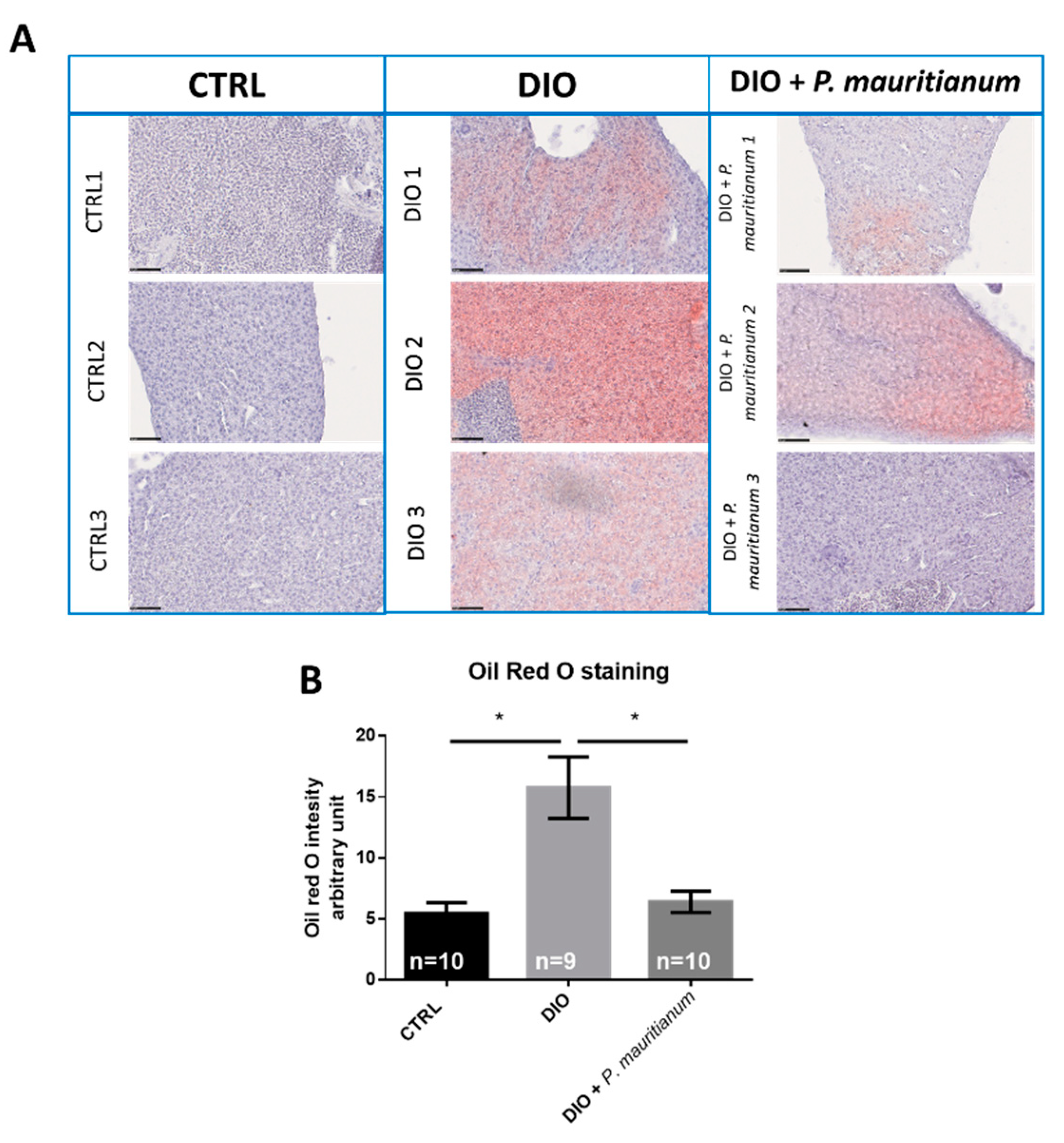
3.8. Therapeutic Effects of Aqueous Extract of P. mauritianum on Weight Gain
4. Discussion
4.1. Composition of Aqueous Extract of Polyphenols
4.2. Toxicity of the Aqueous Extract of P. mauritianum
4.3. Effects of the Aqueous Extract of P. mauritianum on Metabolic Parameters
4.4. Aqueous Extract of P. mauritianum: Polyphenol Content, Lipid Absorption/Metabolism and Microbiota
4.5. Aqueous Extract of P. mauritianum and Brain Homeostasis
4.6. Suggested Mechanisms of Action for Aqueous Extract of P. mauritianum
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Obesity and Overweight Fact Sheet No 311; World Health Organisation: Geneva, Switzerland, 2021. [Google Scholar]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose tissue and insulin resistance in obese. Biomed. Pharm. 2021, 137, 111315. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Despres, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Azhar, A.; Hassan, N.; Tapolyai, M.; Molnar, M.Z. Obesity, Chronic Kidney Disease, and Kidney Transplantation: An Evolving Relationship. Semin. Nephrol. 2021, 41, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metab. Clin. Exp. 2019, 92, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Barnes, A.S. The epidemic of obesity and diabetes: Trends and treatments. Tex. Heart Inst. J. 2011, 38, 142–144. [Google Scholar]
- Convit, A. Obesity is associated with structural and functional brain abnormalities: Where do we go from here? Psychosom. Med. 2012, 74, 673–674. [Google Scholar] [CrossRef] [Green Version]
- Carmo-Silva, S.; Cavadas, C. Hypothalamic Dysfunction in Obesity and Metabolic Disorders. Adv. Neurobiol. 2017, 19, 73–116. [Google Scholar]
- Prickett, C.; Brennan, L.; Stolwyk, R. Examining the relationship between obesity and cognitive function: A systematic literature review. Obes. Res. Clin. Pact. 2015, 9, 93–113. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.; Hsuchou, H.; Kastin, A.J.; Wang, Y.; Yu, C.; Pan, W. Diet-Induced obesity suppresses expression of many proteins at the blood-brain barrier. J. Cereb. Blood Flow Metab. 2014, 34, 43–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobesky, J.L.; Barrientos, R.M.; De May, H.S.; Thompson, B.M.; Weber, M.D.; Watkins, L.R.; Maier, S.F. High-Fat diet consumption disrupts memory and primes elevations in hippocampal IL-1beta, an effect that can be prevented with dietary reversal or IL-1 receptor antagonism. Brain Behav. Immun. 2014, 42, 22–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogrodnik, M.; Zhu, Y.; Langhi, L.G.P.; Tchkonia, T.; Kruger, P.; Fielder, E.; Victorelli, S.; Ruswhandi, R.A.; Giorgadze, N.; Pirtskhalava, T.; et al. Obesity-Induced Cellular Senescence Drives Anxiety and Impairs Neurogenesis. Cell Metab. 2019, 29, 1061–1077.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, A.B.; Cole, J.W.; McArdle, P.F.; Cheng, Y.C.; Ryan, K.A.; Sparks, M.J.; Mitchell, B.D.; Kittner, S.J. Obesity increases risk of ischemic stroke in young adults. Stroke 2015, 46, 1690–1692. [Google Scholar] [CrossRef] [Green Version]
- Bonds, J.A.; Shetti, A.; Stephen, T.K.L.; Bonini, M.G.; Minshall, R.D.; Lazarov, O. Deficits in hippocampal neurogenesis in obesity-dependent and -independent type-2 diabetes mellitus mouse models. Sci. Rep. 2020, 10, 16368. [Google Scholar] [CrossRef]
- Muller, T.D.; Bluher, M.; Tschop, M.H.; DiMarchi, R.D. Anti-Obesity drug discovery: Advances and challenges. Nat. Rev. Drug Discov. 2022, 21, 201–223. [Google Scholar] [CrossRef]
- Zhang, Q.; Delessa, C.T.; Augustin, R.; Bakhti, M.; Collden, G.; Drucker, D.J.; Feuchtinger, A.; Caceres, C.G.; Grandl, G.; Harger, A.; et al. The glucose-dependent insulinotropic polypeptide (GIP) regulates body weight and food intake via CNS-GIPR signaling. Cell Metab. 2021, 33, 833–844.e5. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Farzaei, M.H.; Rahimi, R. Medicinal plants and their natural components as future drugs for the treatment of burn wounds: An integrative review. Arch. Derm. Res. 2014, 306, 601–617. [Google Scholar] [CrossRef]
- Aplamedom. Les Plantes Médicinales de la Réunion; Aplamedom: Saint Denis, France, 2021. [Google Scholar]
- Smadja, J.; Marodon, C. Le Grand Livre des Plantes Médicinales de l’ile de La Réunion: Inscrites à la Pharmacopée Française; Orphie Editions: Montgaillard, France, 2016. [Google Scholar]
- Poullain, C.; Girard-Valenciennes, E.; Smadja, J. Plants from reunion island: Evaluation of their free radical scavenging and antioxidant activities. J. Ethnopharmacol. 2004, 95, 19–26. [Google Scholar] [CrossRef]
- Aplamedom. Plantes Médicinales de la Réunion Inscrites à la Pharmacopée Française; Aplamedom: Saint Denis, France, 2015. [Google Scholar]
- Checkouri, E.; Reignier, F.; Robert-Da Silva, C.; Meilhac, O. Evaluation of Polyphenol Content and Antioxidant Capacity of Aqueous Extracts from Eight Medicinal Plants from Reunion Island: Protection against Oxidative Stress in Red Blood Cells and Preadipocytes. Antioxidants 2020, 9, 959. [Google Scholar] [CrossRef] [PubMed]
- Checkouri, E.; Ramin-Mangata, S.; Diotel, N.; Viranaicken, W.; Marodon, C.; Reignier, F.; Robert-Da Silva, C.; Meilhac, O. Protective Effects of Medicinal Plant Decoctions on Macrophages in the Context of Atherosclerosis. Nutrients 2021, 13, 280. [Google Scholar] [CrossRef] [PubMed]
- Veeren, B.; Bringart, M.; Turpin, C.; Rondeau, P.; Planesse, C.; Ait-Arsa, I.; Gimie, F.; Marodon, C.; Meilhac, O.; Gonthier, M.P.; et al. Caffeic Acid, One of the Major Phenolic Acids of the Medicinal Plant Antirhea borbonica, Reduces Renal Tubulointerstitial Fibrosis. Biomedicines 2021, 9, 358. [Google Scholar] [CrossRef]
- Taile, J.; Patche, J.; Veeren, B.; Gonthier, M.P. Hyperglycemic Condition Causes Pro-Inflammatory and Permeability Alterations Associated with Monocyte Recruitment and Deregulated NFkappaB/PPARgamma Pathways on Cerebral Endothelial Cells: Evidence for Polyphenols Uptake and Protective Effect. Int. J. Mol. Sci. 2021, 22, 1385. [Google Scholar] [CrossRef] [PubMed]
- Ghaddar, B.; Veeren, B.; Rondeau, P.; Bringart, M.; Lefebvre d’Hellencourt, C.; Meilhac, O.; Bascands, J.L.; Diotel, N. Impaired brain homeostasis and neurogenesis in diet-induced overweight zebrafish: A preventive role from A. borbonica extract. Sci. Rep. 2020, 10, 14496. [Google Scholar] [CrossRef]
- Arcambal, A.; Taile, J.; Couret, D.; Planesse, C.; Veeren, B.; Diotel, N.; Gauvin-Bialecki, A.; Meilhac, O.; Gonthier, M.P. Protective Effects of Antioxidant Polyphenols against Hyperglycemia-Mediated Alterations in Cerebral Endothelial Cells and a Mouse Stroke Model. Mol. Nutr. Food Res. 2020, 64, e1900779. [Google Scholar] [CrossRef]
- Gence, L.; Fernezelian, D.; Bringart, M.; Veeren, B.; Christophe, A.; Brion, F.; Meilhac, O.; Bascands, J.L.; Diotel, N. Hypericum lanceolatum Lam. Medicinal Plant: Potential Toxicity and Therapeutic Effects Based on a Q2 Zebrafish Model. Front. Pharmacol. 2022, 13, 832928. [Google Scholar] [CrossRef]
- Mahomoodally, M.F.; Korumtollee, H.N.; Chady, Z.Z. Psiloxylon mauritianum (Bouton ex Hook.f.) Baillon (Myrtaceae): A promising traditional medicinal plant from the Mascarene Islands. J. Intercult. Ethnopharmacol. 2014, 3, 192–195. [Google Scholar] [CrossRef]
- Mootoosamy, A.; Fawzi Mahomoodally, M. Ethnomedicinal application of native remedies used against diabetes and related complications in Mauritius. J. Ethnopharmacol. 2014, 151, 413–444. [Google Scholar] [CrossRef]
- Rangasamy, O.; Mahomoodally, F.M.; Gurib-Fakim, A.; Quetin-Leclercq, J. Two anti-staphylococcal triterpenoid acids isolated from Psiloxylon mauritianum (Bouton ex Hook.f.) Baillon, an endemic traditional medicinal plant of Mauritius. S. Afr. J. Bot. 2014, 93, 198–203. [Google Scholar] [CrossRef] [Green Version]
- Benchoula, K.; Khatib, A.; Jaffar, A.; Ahmed, Q.U.; Sulaiman, W.; Wahab, R.A.; El-Seedi, H.R. The promise of zebrafish as a model of metabolic syndrome. Exp. Anim. 2019, 68, 407–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zang, L.; Shimada, Y.; Nishimura, N. Development of a Novel Zebrafish Model for Type 2 Diabetes Mellitus. Sci. Rep. 2017, 7, 1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zang, L.; Maddison, L.A.; Chen, W. Zebrafish as a Model for Obesity and Diabetes. Front. Cell Dev. Biol. 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capiotti, K.M.; Antonioli, R., Jr.; Kist, L.W.; Bogo, M.R.; Bonan, C.D.; Da Silva, R.S. Persistent impaired glucose metabolism in a zebrafish hyperglycemia model. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2014, 171, 58–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montalbano, G.; Mania, M.; Guerrera, M.C.; Laura, R.; Abbate, F.; Levanti, M.; Maugeri, A.; Germana, A.; Navarra, M. Effects of a Flavonoid-Rich Extract from Citrus sinensis Juice on a Diet-Induced Obese Zebrafish. Int. J. Mol. Sci. 2019, 20, 5116. [Google Scholar] [CrossRef] [Green Version]
- Montalbano, G.; Maugeri, A.; Guerrera, M.C.; Miceli, N.; Navarra, M.; Barreca, D.; Cirmi, S.; Germana, A. A White Grape Juice Extract Reduces Fat Accumulation through the Modulation of Ghrelin and Leptin Expression in an In Vivo Model of Overfed Zebrafish. Molecules 2021, 26, 1119. [Google Scholar] [CrossRef]
- Dorsemans, A.C.; Lefebvre d’Hellencourt, C.; Ait-Arsa, I.; Jestin, E.; Meilhac, O.; Diotel, N. Acute and Chronic Models of Hyperglycemia in Zebrafish: A Method to Assess the Impact of Hyperglycemia on Neurogenesis and the Biodistribution of Radiolabeled Molecules. J. Vis. Exp. 2017, 124, 55203. [Google Scholar] [CrossRef]
- O’Brien, P.D.; Hinder, L.M.; Callaghan, B.C.; Feldman, E.L. Neurological consequences of obesity. Lancet Neurol. 2017, 16, 465–477. [Google Scholar] [CrossRef]
- Milic, S.; Lulic, D.; Stimac, D. Non-Alcoholic fatty liver disease and obesity: Biochemical, metabolic and clinical presentations. World J. Gastroenterol. 2014, 20, 9330–9337. [Google Scholar]
- Brockman, D.A.; Chen, X.; Gallaher, D.D. High-Viscosity dietary fibers reduce adiposity and decrease hepatic steatosis in rats fed a high-fat diet. J. Nutr. 2014, 144, 1415–1422. [Google Scholar] [CrossRef] [Green Version]
- Calligaris, S.D.; Lecanda, M.; Solis, F.; Ezquer, M.; Gutierrez, J.; Brandan, E.; Leiva, A.; Sobrevia, L.; Conget, P. Mice long-term high-fat diet feeding recapitulates human cardiovascular alterations: An animal model to study the early phases of diabetic cardiomyopathy. PLoS ONE 2013, 8, e60931. [Google Scholar]
- Montalbano, G.; Mania, M.; Guerrera, M.C.; Abbate, F.; Laura, R.; Navarra, M.; Vega, J.A.; Ciriaco, E.; Germana, A. Morphological differences in adipose tissue and changes in BDNF/Trkb expression in brain and gut of a diet induced obese zebrafish model. Ann. Anat. 2016, 204, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, K.; Schuster, S.; Meusel, A.; Garten, A.; Riemer, T.; Schleinitz, D.; Kiess, W.; Korner, A. Short-Term overfeeding of zebrafish with normal or high-fat diet as a model for the development of metabolically healthy versus unhealthy obesity. BMC Physiol. 2017, 17, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosacak, M.I.; Papadimitriou, C.; Kizil, C. Regeneration, Plasticity, and Induced Molecular Programs in Adult Zebrafish Brain. Biomed. Res. Int. 2015, 2015, 769763. [Google Scholar] [CrossRef] [Green Version]
- Diotel, N.; Lubke, L.; Strahle, U.; Rastegar, S. Common and Distinct Features of Adult Neurogenesis and Regeneration in the Telencephalon of Zebrafish and Mammals. Front. Neurosci. 2020, 14, 568930. [Google Scholar] [CrossRef]
- März, M.; Schmidt, R.; Rastegar, S.; Strahle, U. Regenerative response following stab injury in the adult zebrafish telencephalon. Dev. Dyn. 2011, 240, 2221–2231. [Google Scholar] [CrossRef]
- Labusch, M.; Mancini, L.; Morizet, D.; Bally-Cuif, L. Conserved and Divergent Features of Adult Neurogenesis in Zebrafish. Front. Cell Dev. Biol. 2020, 8, 525. [Google Scholar] [CrossRef]
- Capiotti, K.M.; De Moraes, D.A.; Menezes, F.P.; Kist, L.W.; Bogo, M.R.; Da Silva, R.S. Hyperglycemia induces memory impairment linked to increased acetylcholinesterase activity in zebrafish (Danio rerio). Behav. Brain Res. 2014, 274, 319–325. [Google Scholar] [CrossRef]
- Stankiewicz, A.J.; Mortazavi, F.; Kharchenko, P.V.; McGowan, E.M.; Kharchenko, V.; Zhdanova, I.V. Cell Kinetics in the Adult Neurogenic Niche and Impact of Diet-Induced Accelerated Aging. J. Neurosci. 2019, 39, 2810–2822. [Google Scholar] [CrossRef]
- Ghaddar, B.; Bringart, M.; Lefebvre d’Hellencourt, C.; Meilhac, O.; Diotel, N. Deleterious Effects of Overfeeding on Brain Homeostasis and Plasticity in Adult Zebrafish. Zebrafish 2021, 18, 190–206. [Google Scholar] [CrossRef]
- Dorsemans, A.C.; Soule, S.; Weger, M.; Bourdon, E.; Lefebvre d’Hellencourt, C.; Meilhac, O.; Diotel, N. Impaired constitutive and regenerative neurogenesis in adult hyperglycemic zebrafish. J. Comp. Neurol. 2017, 525, 442–458. [Google Scholar] [CrossRef] [PubMed]
- Ghaddar, B.; Diotel, N. Zebrafish: A New Promise to Study the Impact of Metabolic Disorders on the Brain. Int. J. Mol. Sci. 2022, 23, 5372. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.Y.; Renquist, B.J. High Throughput Danio Rerio Energy Expenditure Assay. J. Vis. Exp. 2016, 107, e53297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OECD. OECD Guidelines for the Testing of Chemicals; Test No. 236: Fish Embryo Acute Toxicity (FET) Test; OECD: Paris, France, 2013. [Google Scholar]
- Diotel, N.; Vaillant, C.; Kah, O.; Pellegrini, E. Mapping of brain lipid binding protein (Blbp) in the brain of adult zebrafish, co-expression with aromatase B and links with proliferation. Gene Expr. Patterns 2016, 20, 42–54. [Google Scholar] [CrossRef]
- Kaya, M.; Ahishali, B. Assessment of permeability in barrier type of endothelium in brain using tracers: Evans blue, sodium fluorescein, and horseradish peroxidase. Methods Mol. Biol. 2011, 763, 369–382. [Google Scholar]
- Wullimann, M.F.; Reichert, B.R. Neuroanatomy of the Zebrafish Brain; Springer: Cham, Switzerland, 1996. [Google Scholar]
- Pellegrini, E.; Mouriec, K.; Anglade, I.; Menuet, A.; Le Page, Y.; Gueguen, M.M.; Marmignon, M.H.; Brion, F.; Pakdel, F.; Kah, O. Identification of aromatase-positive radial glial cells as progenitor cells in the ventricular layer of the forebrain in zebrafish. J. Comp. Neurol. 2007, 501, 150–167. [Google Scholar] [CrossRef]
- Sorres, J.; Andre, A.; Elslande, E.V.; Stien, D.; Eparvier, V. Potent and Non-Cytotoxic Antibacterial Compounds Against Methicillin-Resistant Staphylococcus aureus Isolated from Psiloxylon mauritianum, A Medicinal Plant from Reunion Island. Molecules 2020, 25, 3565. [Google Scholar] [CrossRef]
- Clain, E.; Haddad, J.G.; Koishi, A.C.; Sinigaglia, L.; Rachidi, W.; Despres, P.; Duarte Dos Santos, C.N.; Guiraud, P.; Jouvenet, N.; El Kalamouni, C. The Polyphenol-Rich Extract from Psiloxylon mauritianum, an Endemic Medicinal Plant from Reunion Island, Inhibits the Early Stages of Dengue and Zika Virus Infection. Int. J. Mol. Sci. 2019, 20, 1860. [Google Scholar] [CrossRef] [Green Version]
- Veeren, B.; Ghaddar, B.; Bringart, M.; Khazaal, S.; Gonthier, M.P.; Meilhac, O.; Diotel, N.; Bascands, J.L. Phenolic Profile of Herbal Infusion and Polyphenol-Rich Extract from Leaves of the Medicinal Plant Antirhea borbonica: Toxicity Assay Determination in Zebrafish Embryos and Larvae. Molecules 2020, 25, 4482. [Google Scholar] [CrossRef]
- Sun, W.; Xu, G.; Guo, X.; Luo, G.; Wu, L.; Hou, Y.; Guo, X.; Zhou, J.; Xu, T.; Qin, L.; et al. Protective effects of asiatic acid in a spontaneous type 2 diabetic mouse model. Mol. Med. Rep. 2017, 16, 1333–1339. [Google Scholar] [CrossRef] [Green Version]
- Uddandrao, V.V.S.; Rameshreddy, P.; Brahmanaidu, P.; Ponnusamy, P.; Balakrishnan, S.; Ramavat, R.N.; Swapna, K.; Pothani, S.; Nemani, H.; Meriga, B.; et al. Antiobesity efficacy of asiatic acid: Down-Regulation of adipogenic and inflammatory processes in high fat diet induced obese rats. Arch. Physiol. Biochem. 2020, 126, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Her, G.M.; Yeh, Y.H.; Wu, J.L. 435-bp liver regulatory sequence in the liver fatty acid binding protein (L-FABP) gene is sufficient to modulate liver regional expression in transgenic zebrafish. Dev. Dyn. 2003, 227, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Bonkovsky, H.L.; de Lemos, A.; Burczynski, F.J. Recent insights into the biological functions of liver fatty acid binding protein 1. J. Lipid Res. 2015, 56, 2238–2247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Zhu, H.; Yan, Y.; Lv, P.; Wu, W. Toxicity Evaluation and Biomarker Selection with Validated Reference Gene in Embryonic Zebrafish Exposed to Mitoxantrone. Int. J. Mol. Sci. 2018, 19, 3516. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.K.; Liu, R.Z.; Thisse, C.; Thisse, B.; Denovan-Wright, E.M.; Wright, J.M. Hierarchical subfunctionalization of fabp1a, fabp1b and fabp10 tissue-specific expression may account for retention of these duplicated genes in the zebrafish (Danio rerio) genome. FEBS J. 2006, 273, 3216–3229. [Google Scholar] [CrossRef]
- Ma, J.; Zheng, Y.; Tang, W.; Yan, W.; Nie, H.; Fang, J.; Liu, G. Dietary polyphenols in lipid metabolism: A role of gut microbiome. Anim. Nutr. 2020, 6, 404–409. [Google Scholar] [CrossRef]
- Bian, Y.; Lei, J.; Zhong, J.; Wang, B.; Wan, Y.; Li, J.; Liao, C.; He, Y.; Liu, Z.; Ito, K.; et al. Kaempferol reduces obesity, prevents intestinal inflammation, and modulates gut microbiota in high-fat diet mice. J. Nutr. Biochem. 2022, 99, 108840. [Google Scholar] [CrossRef]
- Zhao, L.; Zhu, X.; Xia, M.; Li, J.; Guo, A.Y.; Zhu, Y.; Yang, X. Quercetin Ameliorates Gut Microbiota Dysbiosis That Drives Hypothalamic Damage and Hepatic Lipogenesis in Monosodium Glutamate-Induced Abdominal Obesity. Front. Nutr. 2021, 8, 671353. [Google Scholar] [CrossRef]
- Etxeberria, U.; Arias, N.; Boque, N.; Macarulla, M.T.; Portillo, M.P.; Martinez, J.A.; Milagro, F.I. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J. Nutr. Biochem. 2015, 26, 651–660. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, L.; Liao, P.; Xiao, Z.; Zhang, F.; Sindaye, D.; Xin, Z.; Tan, C.; Deng, J.; Yin, Y.; et al. Impact of Gallic Acid on Gut Health: Focus on the Gut Microbiome, Immune Response, and Mechanisms of Action. Front. Immunol. 2020, 11, 580208. [Google Scholar] [CrossRef]
- Bak, E.J.; Kim, J.; Jang, S.; Woo, G.H.; Yoon, H.G.; Yoo, Y.J.; Cha, J.H. Gallic acid improves glucose tolerance and triglyceride concentration in diet-induced obesity mice. Scand. J. Clin. Lab. Investig. 2013, 73, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Sugama, A.; Sumi, K.; Shimizu, K.; Kishimoto, Y.; Kondo, K.; Iida, K. Gallic acid regulates adipocyte hypertrophy and suppresses inflammatory gene expression induced by the paracrine interaction between adipocytes and macrophages in vitro and in vivo. Nutr. Res. 2020, 73, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Al-Goblan, A.S.; Al-Alfi, M.A.; Khan, M.Z. Mechanism linking diabetes mellitus and obesity. Diabetes Metab. Syndr. Obes. 2014, 7, 587–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richard, J.; Lingvay, I. Hepatic steatosis and Type 2 diabetes: Current and future treatment considerations. Expert Rev. Cardiovasc. Ther. 2011, 9, 321–328. [Google Scholar] [CrossRef]
- Totten, M.S.; Pierce, D.M.; Erikson, K.M. Diet-Induced Obesity Disrupts Trace Element Homeostasis and Gene Expression in the Olfactory Bulb. Nutrients 2020, 12, 3909. [Google Scholar] [CrossRef]
- Meguro, S.; Hosoi, S.; Hasumura, T. High-fat diet impairs cognitive function of zebrafish. Sci. Rep. 2019, 9, 17063. [Google Scholar] [CrossRef]
- Stankiewicz, A.J.; McGowan, E.M.; Yu, L.; Zhdanova, I.V. Impaired Sleep, Circadian Rhythms and Neurogenesis in Diet-Induced Premature Aging. Int. J. Mol. Sci. 2017, 18, 2243. [Google Scholar] [CrossRef] [Green Version]
- Diaz, A.; Munoz-Arenas, G.; Caporal-Hernandez, K.; Vazquez-Roque, R.; Lopez-Lopez, G.; Kozina, A.; Espinosa, B.; Flores, G.; Trevino, S.; Guevara, J. Gallic acid improves recognition memory and decreases oxidative-inflammatory damage in the rat hippocampus with metabolic syndrome. Synapse 2020, 75, e22186. [Google Scholar] [CrossRef]
- Seo, M.J.; Lee, Y.J.; Hwang, J.H.; Kim, K.J.; Lee, B.Y. The inhibitory effects of quercetin on obesity and obesity-induced inflammation by regulation of MAPK signaling. J. Nutr. Biochem. 2015, 26, 1308–1316. [Google Scholar] [CrossRef]
- Lee, Y.J.; Choi, H.S.; Seo, M.J.; Jeon, H.J.; Kim, K.J.; Lee, B.Y. Kaempferol suppresses lipid accumulation by inhibiting early adipogenesis in 3T3-L1 cells and zebrafish. Food Funct. 2015, 6, 2824–2833. [Google Scholar] [CrossRef]
- Kliewer, S.A.; Sundseth, S.S.; Jones, S.A.; Brown, P.J.; Wisely, G.B.; Koble, C.S.; Devchand, P.; Wahli, W.; Willson, T.M.; Lenhard, J.M.; et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc. Natl. Acad. Sci. USA 1997, 94, 4318–4323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paraiso, A.F.; Sousa, J.N.; Andrade, J.M.O.; Mangabeira, E.S.; Lelis, D.F.; de Paula, A.M.B.; Martins, A.; Lima, W.J.N.; Guimaraes, A.L.S.; Melo, G.A.; et al. Oral gallic acid improves metabolic profile by modulating SIRT1 expression in obese mice brown adipose tissue: A molecular and bioinformatic approach. Life Sci. 2019, 237, 116914. [Google Scholar] [CrossRef] [PubMed]




| Gene | Forward Primer | Reverse Primer | Gene Accesion Number |
|---|---|---|---|
| ef1α | AGCAGCAGCTGAGGAGTGAT | CCGCATTTGTAGATCAGATGG | ENSDARG00000039502 |
| erg | CAGATGCTCCGTGTGAAAGA | TGCGGTTCAGATGAAGACAG | ENSDARG00000077304 |
| fabp10a | CCAGTGACAGAAATCCAGCA | GTTCTGCAGACCAGCTTTCC | ENSDARG00000038439 |
| gclc | AAAATGTCCGGAACTGATCG | AACGTTTCCATTTTCGTTGC | ENSDARG00000013095 |
| tnfα | GCGCTTTTCTGAATCCTACG | TGCCCAGTCTGTCTCCTTCT | ENSDARG00000009511 |
| claudin 5 | TCCTGGGTCTGATCCTGTG | CTCGATGAAGGCGGTGAC | ENSDARG00000043716 |
| ccna2 | AAAGCAGCTAACAACAGGACAGT | GGTTTACACGCAATTATCTGTGG | ENSDARG00000011094 |
| cyp19a1b | TCGGCACGGCGTGCAACTAC | CATACCTATGCATTGCAGACC | ENSDARG00000098360 |
| pcna | GGACAGAGGAGTGGCTTTGG | CTCACAGACCAGCAACGTCG | ENSDARG00000054155 |
| ctgf | CTCCCCAAGTAACCGTCGTA | TCCACCAAACACACAAGTGG | ENSDARG00000042934 |
| Peak Number | RT (min) | Compound | [M-H]- | MS/MS Fragments | Concentration P. mauritianum Infusion (ng/mL) |
|---|---|---|---|---|---|
| 1 | 1.3 | Gallic acid | 169.0137 | 125.0236 | 172.3 ± 7.5 |
| 2 | 2.1 | Protocatechuic acid | 153.019 | 109.0287 | 5.6 ± 0.4 |
| 3 | 3.6 | Caffeoylquinic acid | 353.0886 | 191.0558 | 32.3 ± 2.1 |
| 4 | 3.8 | Caffeic acid | 179.0346 | 135.0445 | 1.6 ± 0.1 |
| 5 | 4.1 | Caffeoylquinic acid | 353.0886 | 191.0558 | 8.9 ± 1.1 |
| 6 | 4.3 | Coumaroylquinic acid | 337.0936 | 191.0558, 173.0452, 93.0336 | nq |
| 7 | 4.5 | Quercetin hexoside | 463.0882 | 300.0282 | 0.3 ± 0.0 |
| 8 | 5.1 | Quercetin hexoside | 463.0882 | 300.0282 | 18.2± 0.6 |
| 9 | 5.1 | Quercetin-O-(acetyl-hexoside) | 505.099 | 300.0282 | 2.5 ± 0.0 |
| 10 | 5.5 | Kaempferol hexoside | 447.0940 | 284.0332 | 3.7 ± 0.1 |
| 11 | 5.6 | Kaempferol hexoside | 447.0940 | 284.0332 | 32.9 ± 0.1 |
| 12 | 6.5 | Quercetin-O-(acetyl-hexoside) | 505.099 | 300.0282 | 8.3 ± 0.5 |
| 13 | 6.6 | kaempferol-O-(acetyl-hexoside) | 489.1040 | 284.0333, 429.0836, 447.095 | 20.9 ± 0.0 |
| 14 | 7.4 | Kaempferol-O-(acetyl-rhamnoside) | 473.1090 | 284.0333, 413.0884 | 3.4 ± 0.0 |
| 15 | 7.5 | Quercetin | 301.0360 | 151.0029, 178.9981, 121.0285 | 2.0 ± 0.5 |
| 16 | 7.6 | Kaempferol-O-(acetyl-rhamnoside) | 473.1090 | 284.0333, 413.0884 | 2.0 ± 0.0 |
| 17 | 8.3 | Kaempferol | 285.0411 | 151.0032 | 2.1 ± 0.0 |
| 18 | 8.4 | Asiatic acid | 487,344 | _ | nq |
| 19 | 8.6 | Corosolic acid | 471,3488 | _ | nq |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghaddar, B.; Gence, L.; Veeren, B.; Bringart, M.; Bascands, J.-L.; Meilhac, O.; Diotel, N. Aqueous Extract of Psiloxylon mauritianum, Rich in Gallic Acid, Prevents Obesity and Associated Deleterious Effects in Zebrafish. Antioxidants 2022, 11, 1309. https://doi.org/10.3390/antiox11071309
Ghaddar B, Gence L, Veeren B, Bringart M, Bascands J-L, Meilhac O, Diotel N. Aqueous Extract of Psiloxylon mauritianum, Rich in Gallic Acid, Prevents Obesity and Associated Deleterious Effects in Zebrafish. Antioxidants. 2022; 11(7):1309. https://doi.org/10.3390/antiox11071309
Chicago/Turabian StyleGhaddar, Batoul, Laura Gence, Bryan Veeren, Matthieu Bringart, Jean-Loup Bascands, Olivier Meilhac, and Nicolas Diotel. 2022. "Aqueous Extract of Psiloxylon mauritianum, Rich in Gallic Acid, Prevents Obesity and Associated Deleterious Effects in Zebrafish" Antioxidants 11, no. 7: 1309. https://doi.org/10.3390/antiox11071309






