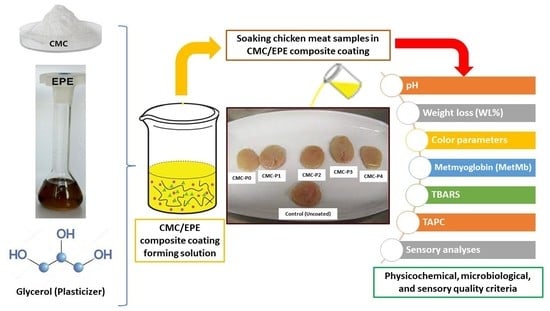
Impact of a Carboxymethyl Cellulose Coating Incorporated with an Ethanolic Propolis Extract on the Quality Criteria of Chicken Breast Meat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemicals and Reagents
2.3. Preparation of Ethanolic Propolis Extract (EPE)
2.4. Preparation of the Coating Films
2.5. Chicken Breast Meat Coating Process
2.6. Physicochemical Analyses
2.6.1. Analysis of pH Value
2.6.2. Weight Loss (WL%)
2.6.3. Color Assessment
2.6.4. Metmyoglobin (MetMb) Content Determination
2.6.5. Assessment of Lipid Oxidation (Thiobarbituric Acid Reactive Substances, TBARS)
2.7. Microbiological Analysis
2.8. Sensory Evaluation
2.9. Statistical Analysis
3. Results and Discussion
3.1. Test Probabilities for Physicochemical, Microbiological, and Sensory Criteria of Chicken Meat Samples Generally and Depending on Storage Time and the Coating Treatment—Multi-Aspect Variance Analysis Including Interactions
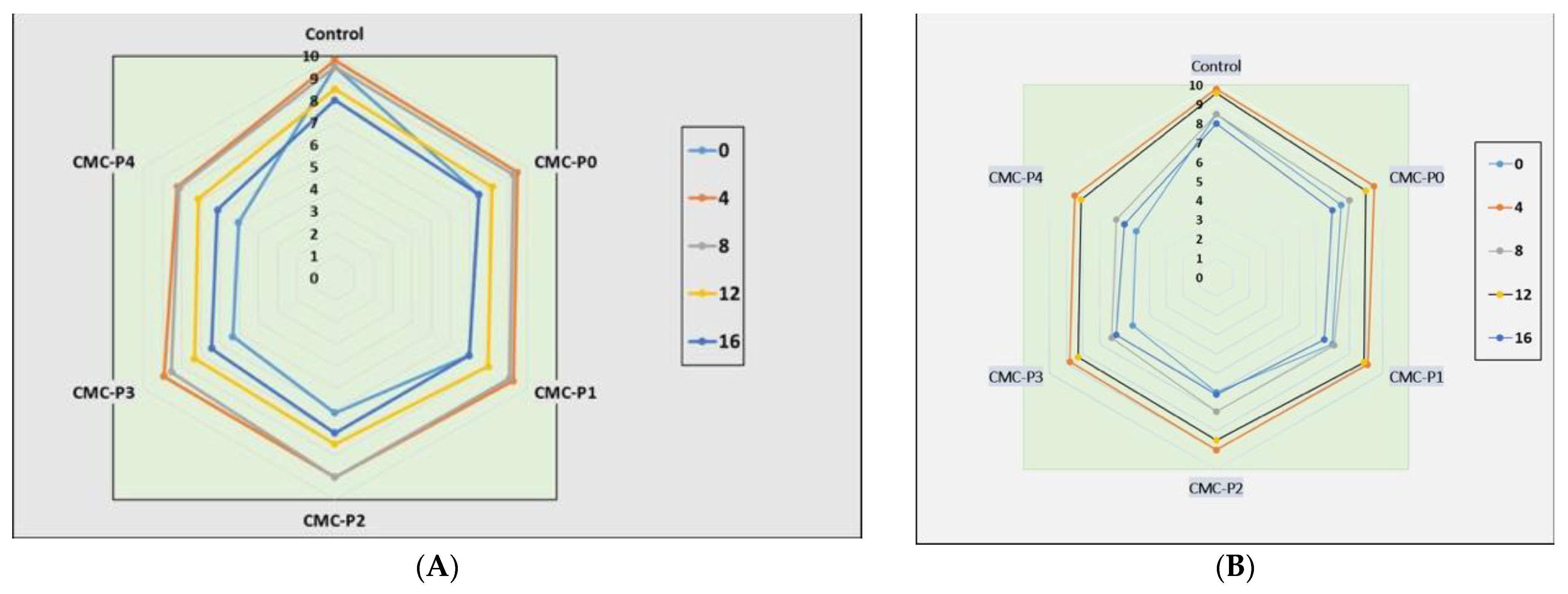
3.2. Physicochemical Analyses of the Chicken Breast Meat Samples
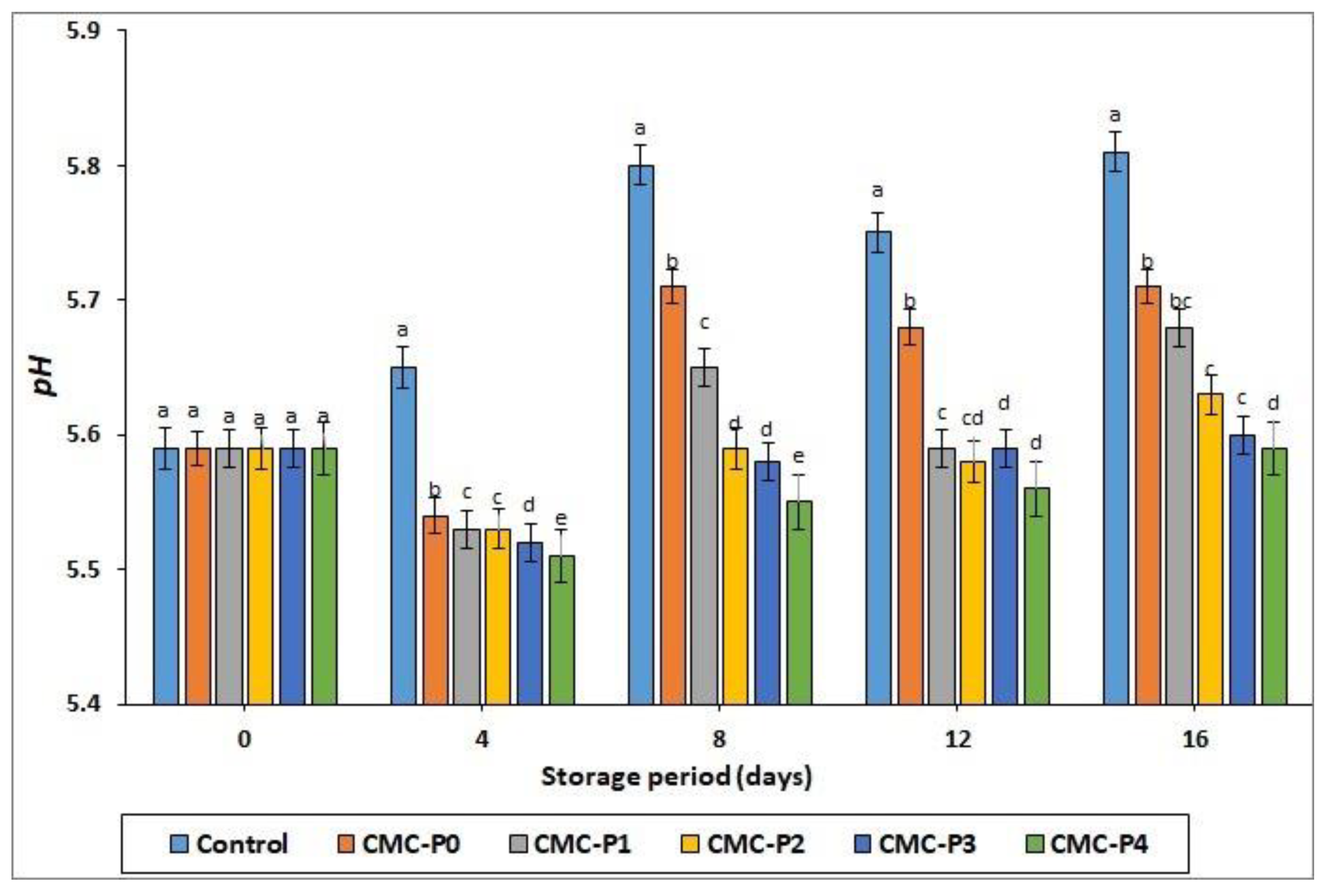
3.2.1. pH Values
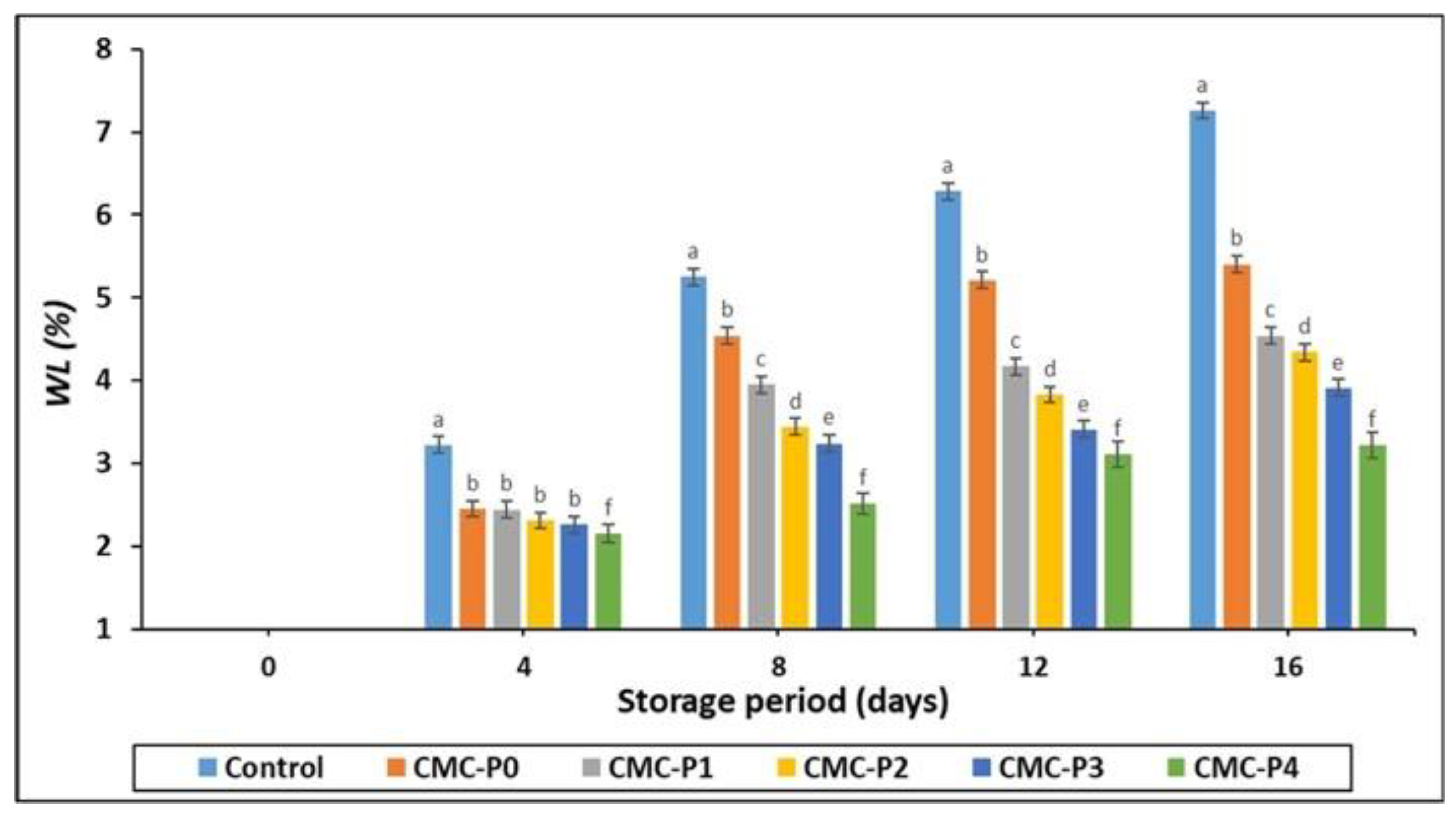
3.2.2. Weight Loss (WL%)
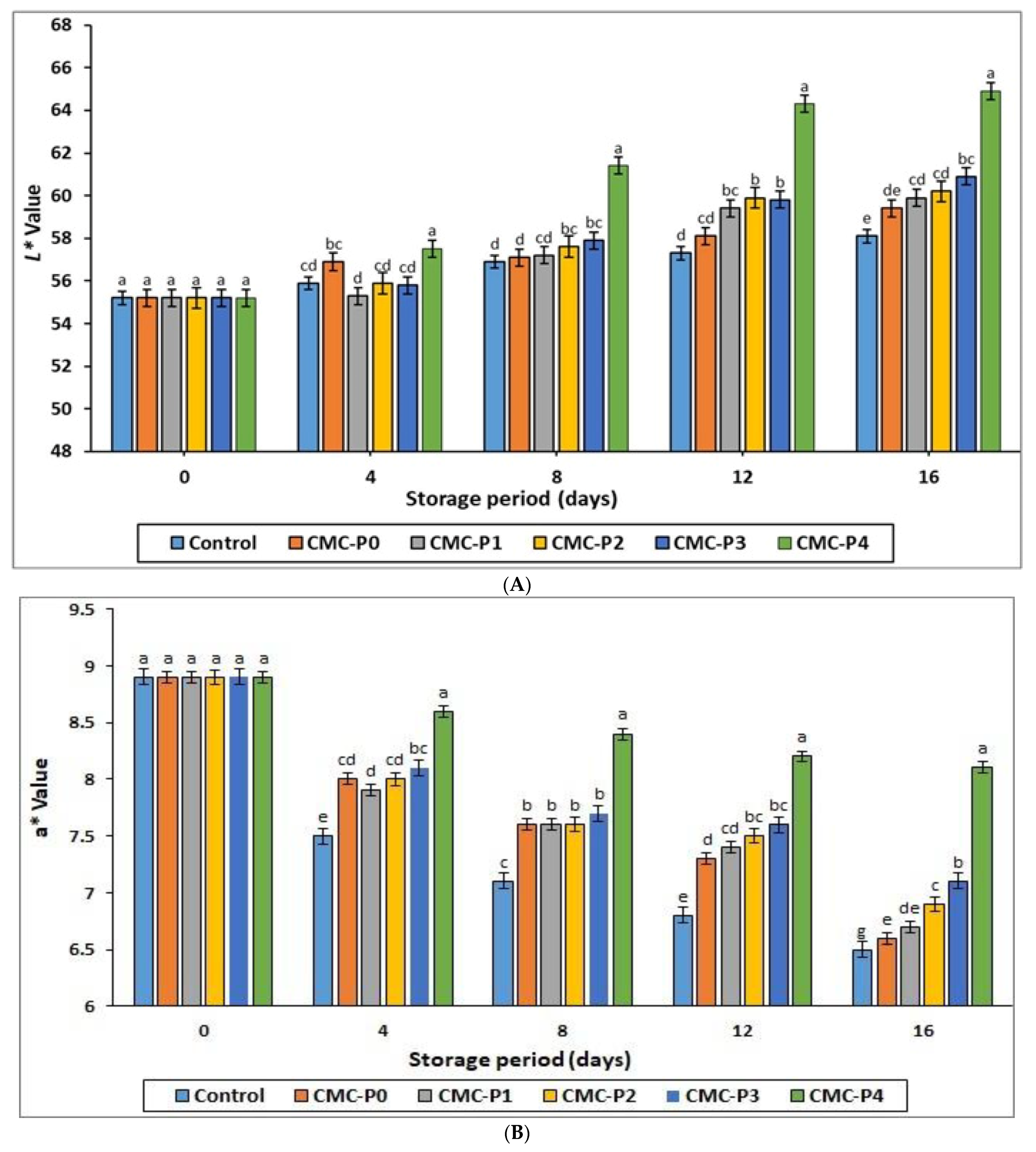
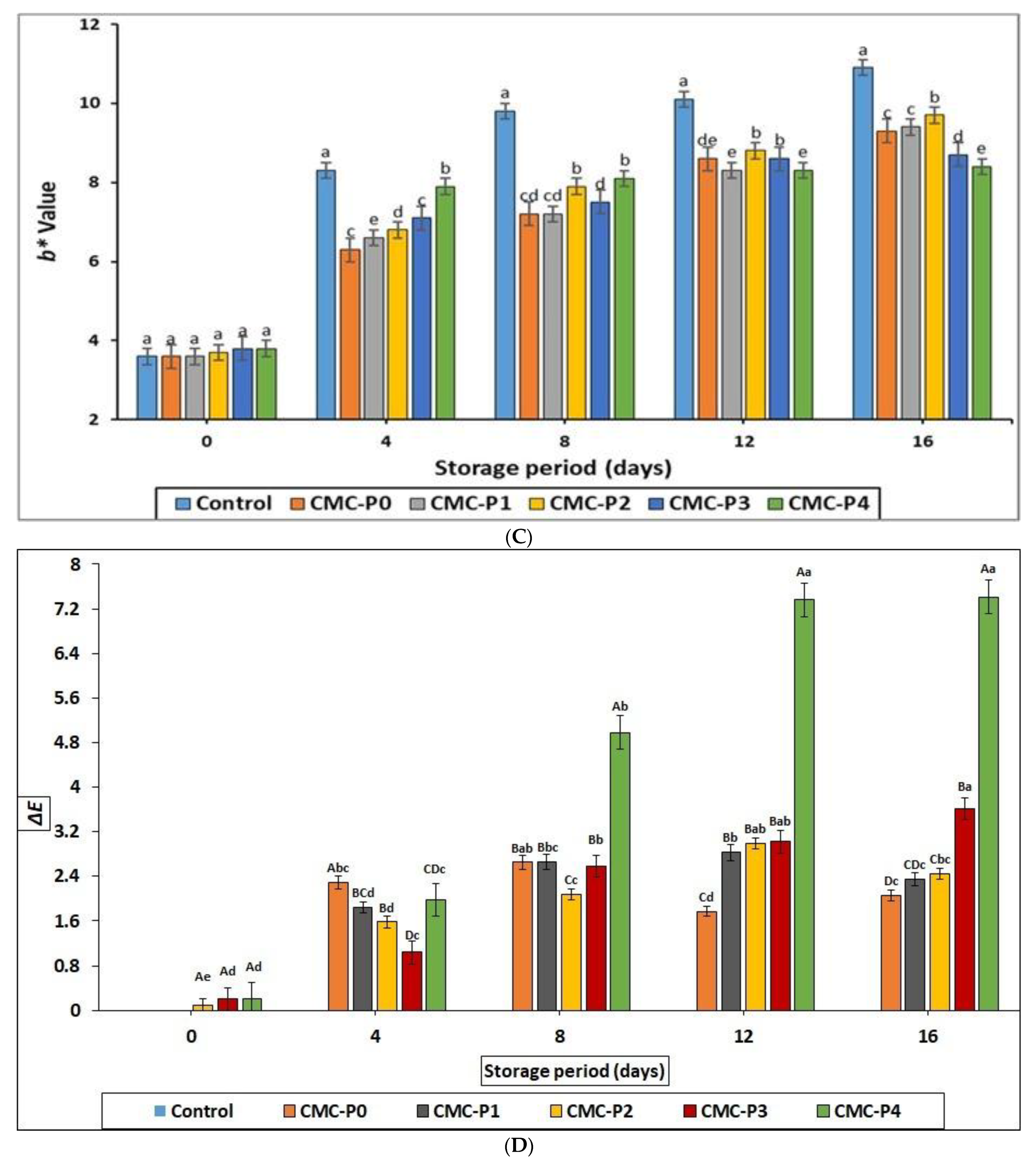
3.2.3. Color Assessment
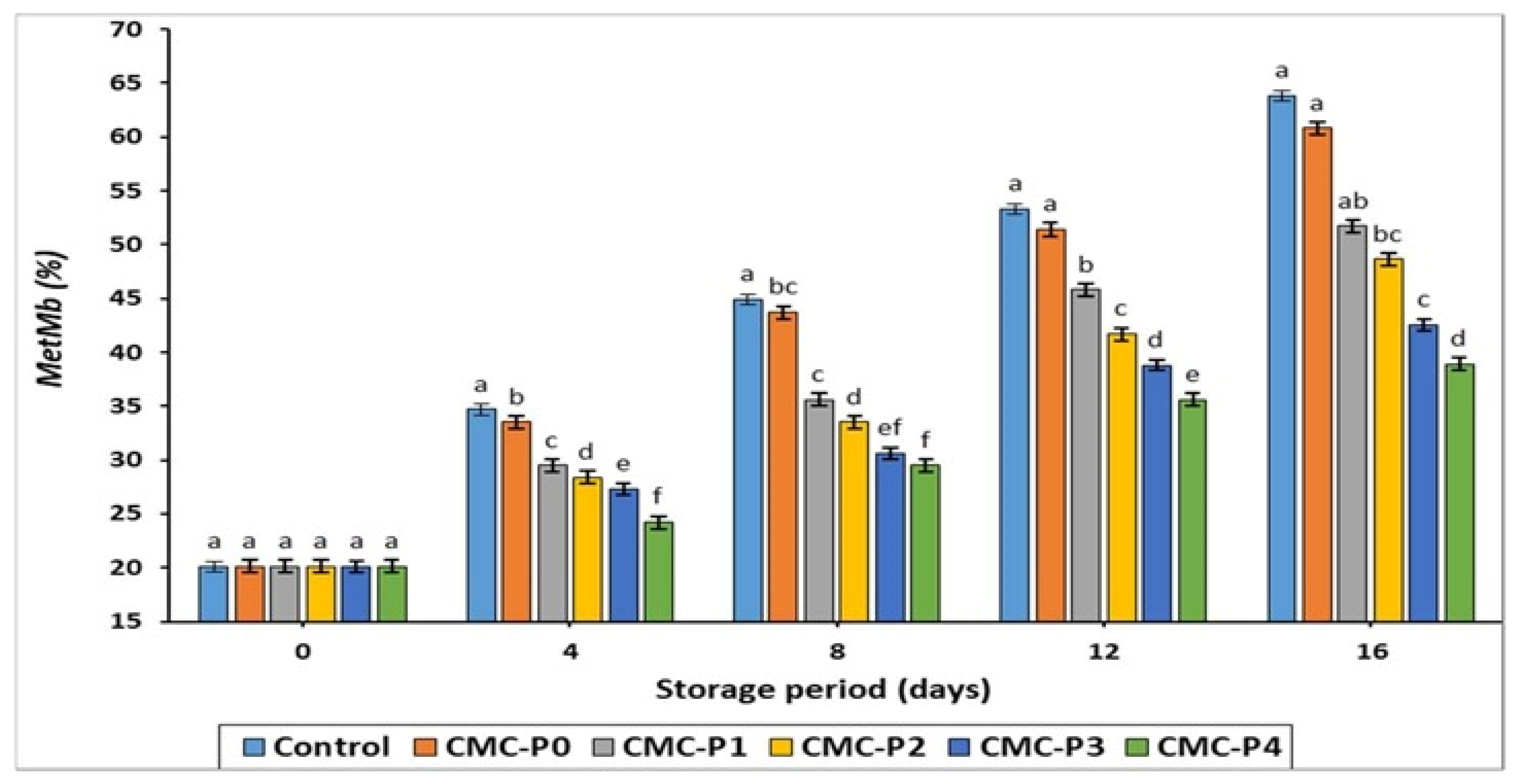
3.2.4. Metmyoglobin (MetMb) Content
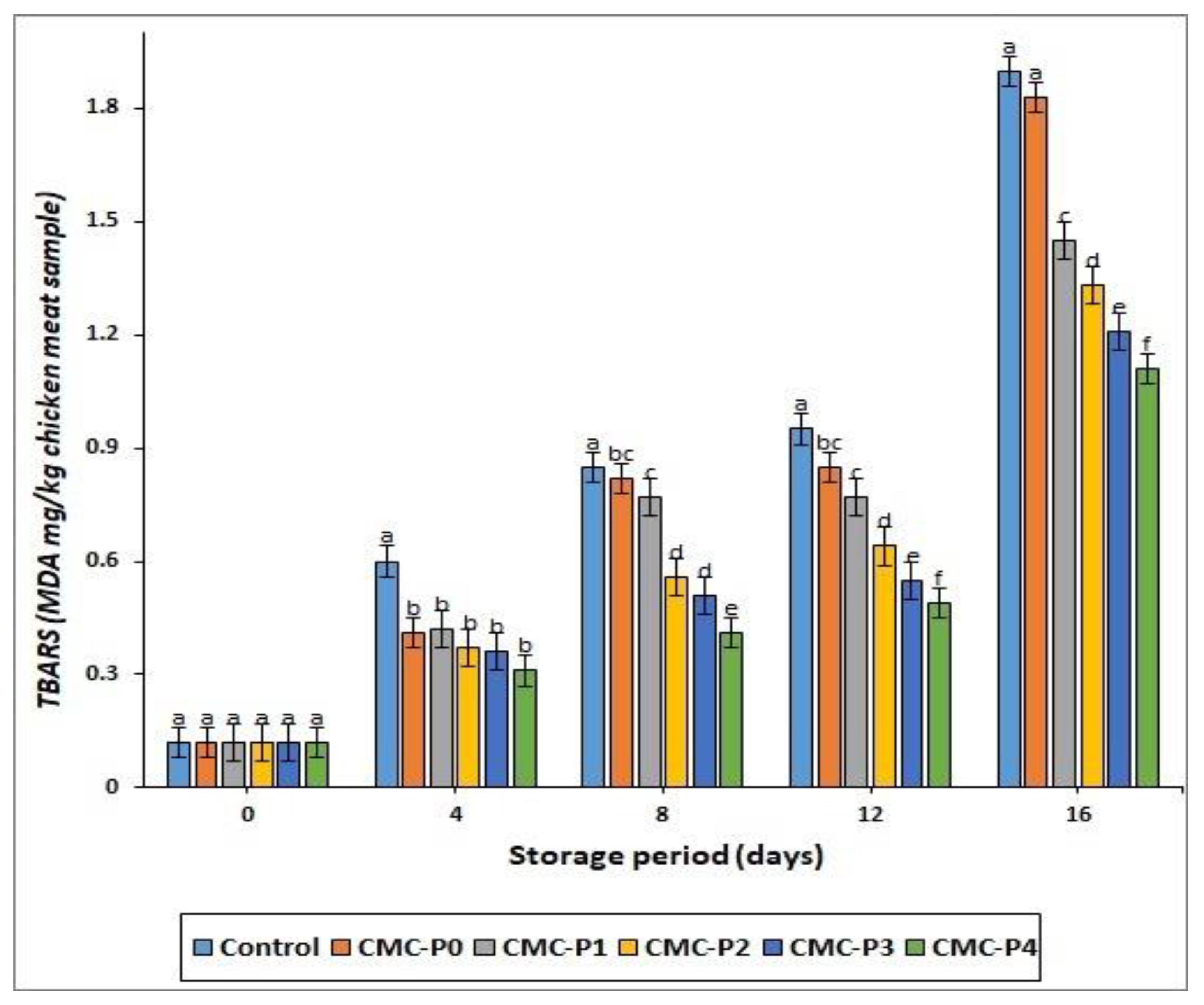
3.2.5. Lipid Oxidation
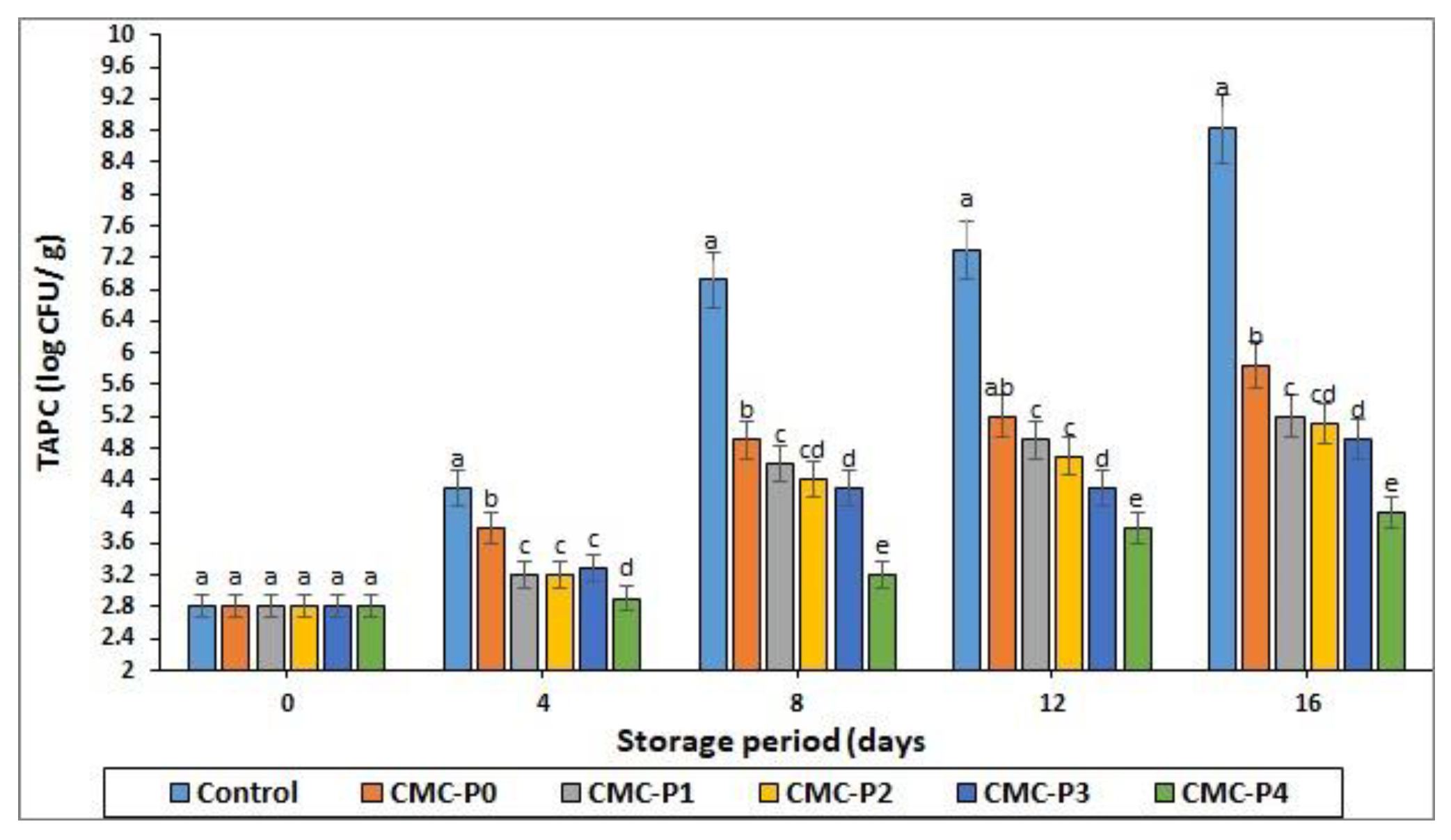
3.3. Total Aerobic Plate Count (TAPC)
3.4. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hertanto, B.S.; Nurmalasari, C.D.A.; Nuhriawangsa, A.M.P.; Cahyadi, M.; Kartikasari, L.R. The physical and microbiological quality of chicken meat in the different type of enterprise poultry slaughterhouse: A case study in Karanganyar District. IOP Conf. Ser. Earth Environ. Sci. 2012, 102, 012051. [Google Scholar] [CrossRef]
- Boz, M.A.; Oz, F.; Yamak, U.S.; Sarica, M.; Cilavdaroglu, E. The carcass traits, carcass nutrient composition, amino acid, fatty acid, and cholesterol contents of local Turkish goose varieties reared in an extensive production system. Poult. Sci. 2019, 98, 3067–3080. [Google Scholar] [CrossRef] [PubMed]
- Link, R. Is Chicken Healthy? Nutrition, Benefits, and Tips. 20 October 2020. Available online: https://www.healthline.com/nutrition/is-chicken-good-for-you (accessed on 12 June 2022).
- Frey, M. Chicken Breast Nutrition Facts and Health Benefits. Updated on 24 March 2021. Available online: https://www.verywellfit.com/how-many-calories-in-chicken-breast-3495665#citation-8 (accessed on 12 June 2022).
- U.S. Department of Agriculture (USDA). Chicken, Broiler or Fryers, Breast, Skinless, Boneless, Meat Only, Cooked, Grilled. SR Legacy, Released in April 2018, Is the Final Release of This Data Type and Will Not Be Updated. For More Recent Data, Users Should Search Other Data Types in FoodData Central. Published: 4/1/2019. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/171534/nutrients (accessed on 12 June 2022).
- Argyri, A.A.; Panagou, E.Z.; Nychas, G.-J.E. Advances in vacuum and modified atmosphere packaging of poultry products. In Advances in Meat, Poultry and Seafood Packaging; Kerry, J.P., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 205–247. [Google Scholar] [CrossRef]
- Matiacevich, S.; Acevedo, N.; López, D. Characterization of edible active coating based on alginate–thyme oil–propionic acid for the preservation of fresh chicken breast fillets. J. Food Process. Preserv. 2015, 39, 2792–2801. [Google Scholar] [CrossRef]
- Beach, C. New Salmonella Outbreak Traced to Raw, Frozen, Breaded Chicken Products. 2 June 2021. Available online: https://www.foodsafetynews.com/2021/06/new-salmonella-outbreak-traced-to-raw-frozen-breaded-chicken-products/ (accessed on 14 December 2021).
- Wessels, K.; Rip, D.; Gouws, P. Salmonella in chicken meat: Consumption, outbreaks, characteristics, current control methods and the potential of bacteriophage use. Foods 2021, 10, 1742. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.B.; da Silva, M.V.; Lannes, S.C.D.S. Lipid oxidation in meat: Mechanisms and protective factors—A review. Food Sci. Technol. 2018, 38, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Khaledian, Y.; Pajohi-Alamoti, M.; Bazargani-Gilani, B. Development of cellulose nanofibers coating incorporated with ginger essential oil and citric acid to extend the shelf life of ready-to-cook barbecue chicken. J. Food Process. Preserv. 2019, 43, e14114. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Nieto, G.; Pateiro, M.; Lorenzo, J.M. Phenolic compounds obtained from Olea europaea by-products and their use to improve the quality and shelf life of meat and meat products—A Review. Antioxidants 2020, 9, 1061. [Google Scholar] [CrossRef]
- Wagh, V.D. Propolis: A wonder bees product and its pharmacological potentials. Adv. Pharmacol. Sci. 2013, 2013, 308249. [Google Scholar] [CrossRef] [Green Version]
- Nader, R.A.; Mackieh, R.; Wehbe, R.; El Obeid, D.; Sabatier, J.M.; Fajloun, Z. Beehive products as antibacterial gents: A review. Antibiotics 2021, 10, 717. [Google Scholar] [CrossRef]
- Borges, A.; Saavedra, M.J.; Simões, M. Insights on antimicrobial resistance, biofilms and the use of phytochemicals as new antimicrobial agents. Curr. Med. Chem. 2015, 22, 2590–2614. [Google Scholar] [CrossRef] [Green Version]
- Vats, S. Ingredients extraction by physicochemical methods in food. A volume in handbook of food bioengineering. In Methods for Extractions of Value-Added Nutraceuticals from Lignocellulosic Wastes and Their Health Application; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Oxford, UK, 2017; Volume 4, pp. 1–64. [Google Scholar] [CrossRef]
- Kadar, N.N.M.A.; Ahmad, F.; Teoh, S.L.; Yahaya, M.F. Caffeic acid on metabolic syndrome: A review. Molecules 2021, 26, 5490. [Google Scholar] [CrossRef] [PubMed]
- Pobiega, K.; Kraśniewska, K.; Gniewosz, M. Application of propolis in antimicrobial and antioxidative protection of food quality—A review. Trends Food Sci. Technol. 2019, 83, 53–62. [Google Scholar] [CrossRef]
- Przybyłek, I.; Karpiński, T.M. Antibacterial properties of propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef] [Green Version]
- Şentürk, E.T.; Yildiz, A.Ö.; Olgun, O.; Mutlu, M. Effects of dietary supplementation of propolis on performance, egg quality and blood parameters of layer quails. J. Hell. Vet. Med. Soc. 2021, 72, 2953–2960. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Application of edible coating with essential oil in food preservation. Crit. Rev. Food Sci. Nutr. 2019, 59, 2467–2480. [Google Scholar] [CrossRef] [PubMed]
- Chawla, R.; Sivakumar, S.; Kaur, H. Antimicrobial edible films in food packaging: Current scenario and recent nanotechnological advancements—A review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100024. [Google Scholar] [CrossRef]
- Moreno, O.; Atarés, L.; Chiralt, A.; Cruz-Romero, M.C.; Kerry, J. Starch-gelatin antimicrobial packaging materials to extend the shelf life of chicken breast fillets. LWT 2018, 97, 483–490. [Google Scholar] [CrossRef]
- Go, E.-J.; Song, K.B. Antioxidant properties of rye starch films containing rosehip extract and their application in packaging of chicken breast. Starch-Stärke 2019, 71, 1900116. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, B.; Li, C.; Xu, Y.; Luo, Y.; Liang, D.; Huang, C. Comprehensive review of polysaccharide-based materials in edible packaging: A sustainable approach. Foods 2021, 10, 1845. [Google Scholar] [CrossRef]
- Lavanya, D.; Kulkarni, P.K.; Dixit, M.; Raavi, P.K.; Krishna, L.N.V. Sources of cellulose and their applications—A review. Int. J. Drug Form. Res. 2011, 2, 19–38. Available online: https://www.truthinadvertising.org/wp-content/uploads/2021/05/Sources-of-cellulose-and-their-applications-2011-article.pdf (accessed on 14 December 2021).
- Azeredo, H.M.C.; Waldron, K.W. Crosslinking in polysaccharide and protein films and coatings for food contact—A review. Trends Food Sci. Technol. 2016, 52, 109–122. [Google Scholar] [CrossRef]
- Irigoiti, Y.; Yamul, D.K.; Navarro, A.S. Co-crystallized sucrose with propolis extract as a food ingredient: Powder characterization and antioxidant stability. LWT Food Sci. Technol. 2021, 143, 111164. [Google Scholar] [CrossRef]
- Wieczyńska, A.; Weżgowiec, J.; Więckiewicz, W.; Czarny, A.; Kulbacka, J.; Nowakowska, D.; Gancarz, R.; Wilk, K.A. Antimicrobial activity, cytotoxicity and total phenolic content of different extracts of propolis from the West Pomeranian region in Poland. Acta Pol. Pharm. Drug Res. 2017, 74, 715–722. Available online: www.ptfarm.pl/pub/File/Acta_Poloniae/2017/2/715.pdf (accessed on 14 December 2021).
- Dashipour, A.; Razavilar, V.; Hosseini, H.; Shojaee-Aliabadi, S.; German, J.B.; Ghanati, K.; Khakpour, M.; Khaksar, R. Antioxidant and antimicrobial carboxymethyl cellulose films containing Zataria multiflora essential oil. Int. J. Biol. Macromol. 2015, 72, 606–613. [Google Scholar] [CrossRef]
- Feldsine, P.; Abeyta, C.; Andrews, W.H. AOAC International methods committee guidelines for validation of qualitative and quantitative food microbiological official methods of analysis. J. AOAC Int. 2002, 85, 1187–1200. [Google Scholar] [CrossRef] [Green Version]
- Campañone, L.A.; Roche, L.A.; Salvadori, V.O.; Mascheroni, R.H. Monitoring of weight losses in meat products during freezing and frozen storage. Food Sci. Technol. Int. 2002, 8, 229–238. [Google Scholar] [CrossRef]
- Kandil, A.A.; Aly-Aldin, M.M.; Allam, A.Y. Quality characteristics of processed low-fat beef sausage as affected by chickpea protein isolates prolonged cold storage. J. Food Dairy Sci. 2020, 11, 363–368. [Google Scholar] [CrossRef]
- Garavito, J.; Moncayo-Martínez, D.; Castellanos, D.A. Evaluation of antimicrobial coatings on preservation and shelf life of fresh chicken breast fillets under cold storage. Foods 2020, 9, 1203. [Google Scholar] [CrossRef]
- Lindahl, G.; Lundström, K.; Tornberg, E. Contribution of pigment content, myoglobin forms and internal reflectance to the colour of pork loin and ham from pure breed pigs. Meat Sci. 2001, 59, 141–151. [Google Scholar] [CrossRef]
- Tang, J.; Faustman, C.; Hoagland, T.A. Krzywicki revisited: Equations for spectrophotometric determination of myoglobin redox forms in aqueous meat extracts. J. Food Sci. 2004, 69, C717–C720. [Google Scholar] [CrossRef]
- Peiretti, P.G.; Medana, C.; Visentin, S.; Giancotti, V.; Zunino, V.; Meineri, G. Determination of carnosine, anserine, homocarnosine, pentosidine and thiobarbituric acid reactive substances contents in meat from different animal species. Food Chem. 2011, 126, 1939–1947. [Google Scholar] [CrossRef] [PubMed]
- Horváth, K.; Seregély, Z.; Andrássy, É.; Dalmadi, I.; Farkas, J. A preliminary study using near infrared spectroscopy to evaluate freshness and detect spoilage in sliced pork meat. Acta Aliment. 2008, 37, 93–102. [Google Scholar] [CrossRef]
- Chew, S.F.; Ip, Y.K. Excretory nitrogen metabolism and defence against ammonia toxicity in air-breathing fishes. J. Fish Biol. 2014, 84, 603–638. [Google Scholar] [CrossRef] [PubMed]
- Toldrá, F. The storage and preservation of meat: III—Meat processing. In Lawrie´s Meat Science. A volume in Woodhead Publishing Series in Food Science, Technology and Nutrition, 8th ed.; Toldrá, F., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 265–296. [Google Scholar] [CrossRef]
- Singh, P.K.; Shrivastava, N.; Ojha, B.K. Enzymes in the meat industry. In Enzymes in Food Biotechnology. Production, Applications, and Future Prospects; Kuddus, M., Ed.; Academic Press: Oxford, UK, 2019; pp. 111–128. [Google Scholar] [CrossRef]
- Bodini, R.B.; Sobral, P.J.A.; Favaro-Trindade, C.S.; Carvalho, R.A. Properties of gelatin-based films with added ethanol–propolis extract. LWT-Food Sci. Technol. 2013, 51, 104–110. [Google Scholar] [CrossRef]
- Ulloa, P.A.; Vidal, J.; Lopéz de Dicastillo, C.; Rodriguez, F.; Guarda, A.; Cruz, R.M.S.; Galotto, M.J. Development of poly(lactic acid) films with propolis as a source of active compounds: Biodegradability, physical, and functional properties. J. Appl. Polym. Sci. 2019, 136, 47090. [Google Scholar] [CrossRef]
- Yong, H.; Liu, J. Active packaging films and edible coatings based on polyphenol-rich propolis extract: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2106–2145. [Google Scholar] [CrossRef] [PubMed]
- Heinz, G.; Hautzinger, P. Meat Processing Technology for Small to Medium Scale Producers; RAP Publication 2007/20; FAO: Bangkok, Thailand, 2007; Available online: https://www.fao.org/3/ai407e/ai407e.pdf (accessed on 14 December 2021).
- Ruelas-Chacon, X.; Aguilar-González, A.; Reyes-Vega, M.D.L.L.; Peralta-Rodríguez, R.D.; Corona-Flores, J.; Rebolloso-Padilla, O.N.; Aguilera-Carbo, A.F. Bioactive protecting coating of guar gum with thyme oil to extend shelf life of tilapia (Oreoschromis niloticus) fillets. Polymers 2020, 12, 3019. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.; Kamkar, A.; Misaghi, A.; Zunabovic-Pichler, M.; Fatehi, S. Nanocomposite films with CMC, okra mucilage, and ZnO nanoparticles: Extending the shelf-life of chicken breast meat. Food Packag. Shelf Life 2019, 21, 100330. [Google Scholar] [CrossRef]
- Ruan, C.; Zhang, Y.; Sun, Y.; Gao, X.; Xiong, G.; Liang, J. Effect of sodium alginate and carboxymethyl cellulose edible coating with epigallocatechin gallate on quality and shelf life of fresh pork. Int. J. Biol. Macromol. 2019, 141, 178–184. [Google Scholar] [CrossRef]
- Ebadi, Z.; Khodanazary, A.; Hosseini, S.M.; Zanguei, N. The shelf life extension of refrigerated Nemipterus japonicas fillets by chitosan coating incorporated with propolis extract. Int. J. Aquat. Res. 2021, 2, 23–42. Available online: http://injoar.com/files/site1/user_files_695706/admin-A-10-1-17-3689aeb.pdf (accessed on 14 December 2021).
- Santos, M.S.; Estevinho, M.L.M.F.; de Carvalho, C.A.L.; Magalhães-Guedes, K.T.; Schwan, R.F.; Almeida, R.C.D.C. Propolis as natural additive: A systematic review. Afr. J. Biotechnol. 2018, 17, 1282–1291. [Google Scholar] [CrossRef] [Green Version]
- Jo, H.Y.; Tango, C.H.; Oh, D.H. Influence of different organic materials on chlorine concentration and sanitization of slightly acidic electrolyzed water. LWT-Food Sci. Technol. 2018, 92, 187–194. [Google Scholar] [CrossRef]
- Wang, S.K.; Fu, L.M.; Chen, G.W.; Xiao, H.M.; Pan, D.; Shi, R.F.; Yang, L.G.; Sun, G.J. Multisite survey of bacterial contamination in ready-to-eat meat products throughout the cooking and selling processes in urban supermarket, Nanjing, China. Food Sci. Nutr. 2020, 8, 2427–2435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezaei, F.; Shahbazi, Y. Shelf-life extension and quality attributes of sauced silver carp fillet: A comparison among direct addition, edible coating and biodegradable film. LWT-Food Sci. Technol. 2018, 87, 122–133. [Google Scholar] [CrossRef]
- Almuhayawi, M.S. Propolis as a novel antibacterial agent. Saudi J. Biol. Sci. 2020, 27, 3079–3086. [Google Scholar] [CrossRef] [PubMed]
- Shavisi, N.; Khanjari, A.; Basti, A.A.; Misaghi, A.; Shahbazi, Y. Effect of PLA films containing propolis ethanolic extract, cellulose nanoparticle and Ziziphora clinopodioides essential oil on chemical, microbial and sensory properties of minced beef. Meat Sci. 2017, 124, 95–104. [Google Scholar] [CrossRef]
- Pastor, C.; Sánchez-González, L.; Marcilla, A.; Chiralt, A.; Cháfer, M.; González-Martínez, C. Quality and safety of table grapes coated with hydroxypropylmethylcellulose edible coatings containing propolis extract. Postharvest Biol. Technol. 2011, 60, 64–70. [Google Scholar] [CrossRef]
- Palou, L.; Ali, A.; Fallik, E.; Romanazzi, G. GRAS, plant- and animal-derived compounds as alternatives to conventional fungicides for the control of postharvest diseases of fresh horticultural produce. Postharvest Biol. Technol. 2016, 122, 41–52. [Google Scholar] [CrossRef]

| Criteria | Effect | Interaction T × ST | |
|---|---|---|---|
| Treatment (T) | Storage Time (ST) | ||
| pH | XXX 1 | XXX | XXX |
| Weight loss (%) | XXX | XXX | XXX |
| L* | XXX | XXX | XXX |
| a* | XX | XXX | XXX |
| b* | NS 2 | XXX | XXX |
| ∆E 3 | X | XXX | XXX |
| TBARS | XX | XXX | XXX |
| MetMb | NS | XXX | XXX |
| TAPC | NS | XXX | XXX |
| Color | XXX | X | XXX |
| Odor | XXX | XXX | XXX |
| Overall acceptance | XXX | XX | XXX |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Sheikha, A.F.; Allam, A.Y.; ElObeid, T.; Basiouny, E.A.; Abdelaal, A.A.; Amarowicz, R.; Oz, E.; Proestos, C.; Karrar, E.; Oz, F. Impact of a Carboxymethyl Cellulose Coating Incorporated with an Ethanolic Propolis Extract on the Quality Criteria of Chicken Breast Meat. Antioxidants 2022, 11, 1191. https://doi.org/10.3390/antiox11061191
El Sheikha AF, Allam AY, ElObeid T, Basiouny EA, Abdelaal AA, Amarowicz R, Oz E, Proestos C, Karrar E, Oz F. Impact of a Carboxymethyl Cellulose Coating Incorporated with an Ethanolic Propolis Extract on the Quality Criteria of Chicken Breast Meat. Antioxidants. 2022; 11(6):1191. https://doi.org/10.3390/antiox11061191
Chicago/Turabian StyleEl Sheikha, Aly Farag, Ayman Younes Allam, Tahra ElObeid, Elham Abdelrahman Basiouny, Ahmad Abdelkaway Abdelaal, Ryszard Amarowicz, Emel Oz, Charalampos Proestos, Emad Karrar, and Fatih Oz. 2022. "Impact of a Carboxymethyl Cellulose Coating Incorporated with an Ethanolic Propolis Extract on the Quality Criteria of Chicken Breast Meat" Antioxidants 11, no. 6: 1191. https://doi.org/10.3390/antiox11061191