Flavonoids from Selaginella doederleinii Hieron and Their Antioxidant and Antiproliferative Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experimental Reagents and Instruments
2.3. Extraction and Separation
2.4. Determination of the Total Flavonoid Content (TFC)
2.5. In Vitro Antioxidant Assays
2.5.1. DPPH (2,2-diphenyl-1-picrylhydrazyl) Assay
2.5.2. Ferric Reducing Antioxidant Power (FRAP) Assay
2.6. Antiproliferative Activity
2.7. Statistical Analysis
3. Results and Discussion
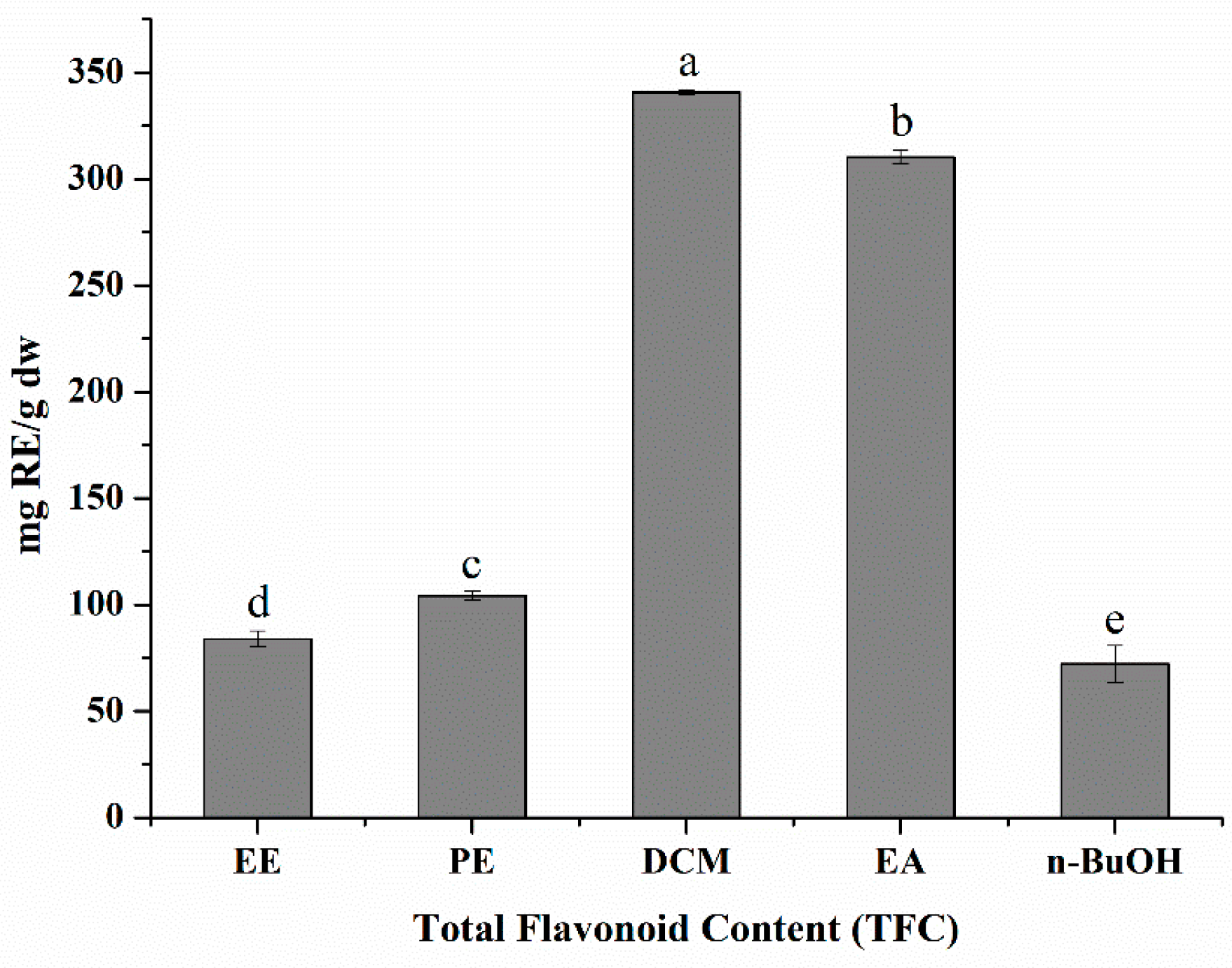
3.1. Total Flavonoid Content
3.2. In-Vitro Antioxidant Potential of S. doederleinii Extracts
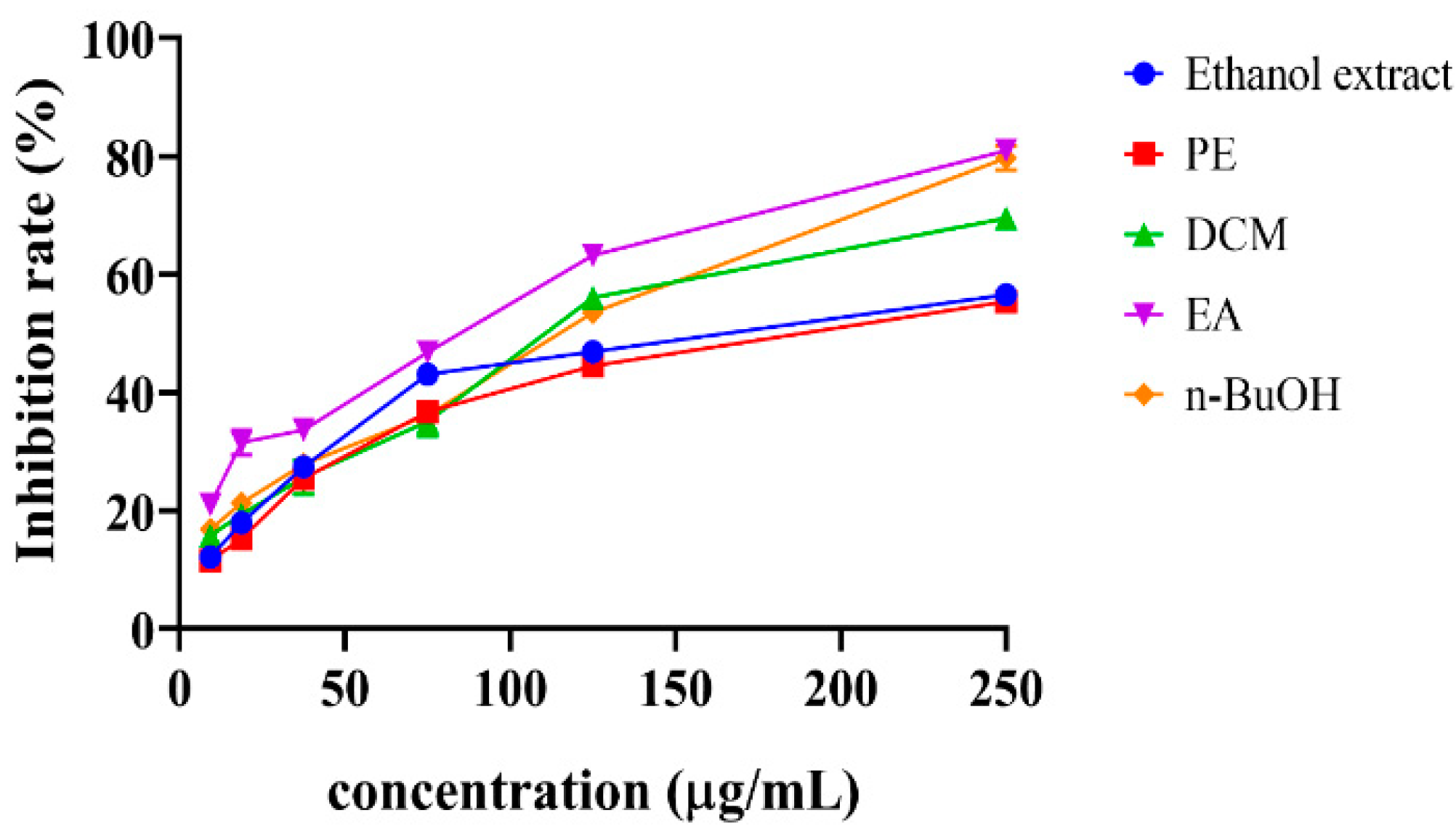
3.3. Antiproliferative Activity of S. doederleinii Extracts
3.4. Isolation and Structure Elucidation
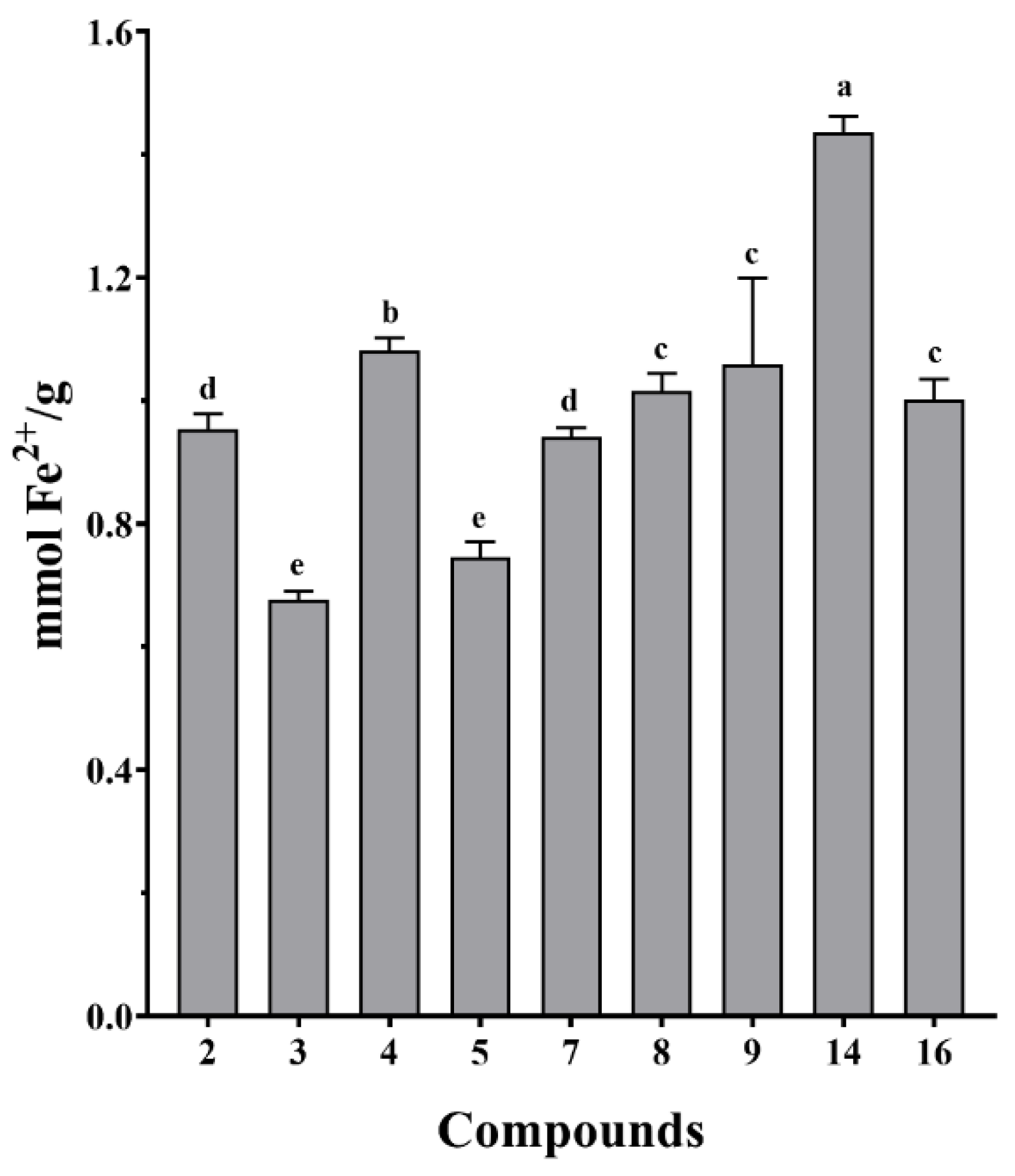
3.5. Antioxidant Activities of Isolated Compounds from S. doederleinii
3.6. Antiproliferation Activity of Compounds Isolated from S. doederleinii
3.7. Structure–Activity Relationship of S. doederleinii Phytochemicals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saunders, F.R.; Wallace, H.M. On the natural chemoprevention of cancer. Plant Physiol. Biochem. 2010, 48, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Caddeo, C.; Gabriele, M.; Nácher, A.; Fernàndez-Busquets, X.; Valenti, D.; Maria Fadda, A.; Pucci, L.; Manconi, M. Resveratrol and artemisinin eudragit-coated liposomes: A strategy to tackle intestinal tumors. Int. J. Pharm. 2021, 592, 120083. [Google Scholar] [CrossRef]
- Attia, M.; Essa, E.A.; Zaki, R.M.; Elkordy, A.A. An Overview of the Antioxidant Effects of Ascorbic Acid and Alpha Lipoic Acid (in Liposomal Forms) as Adjuvant in Cancer Treatment. Antioxidants 2020, 9, 359. [Google Scholar] [CrossRef]
- George, S.; Abrahamse, H. Redox Potential of Antioxidants in Cancer Progression and Prevention. Antioxidants 2020, 9, 156. [Google Scholar] [CrossRef]
- Suraweera, L.T.; Rupasinghe, H.P.V.; Dellaire, G.; Xu, Z. Regulation of Nrf2/ARE Pathway by Dietary Flavonoids: A Friend or Foe for Cancer Management? Antioxidants 2020, 9, 973. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, E.J.; Den Hartogh, D.J.; Azizi, K.; Tsiani, E. Anticancer Properties of Carnosol: A Summary of In Vitro and In Vivo Evidence. Antioxidants 2020, 9, 961. [Google Scholar] [CrossRef]
- Zhuang, X.-C.; Chen, G.-L.; Liu, Y.; Zhang, Y.-L.; Guo, M.-Q. New Lignanamides with Antioxidant and Anti-Inflammatory Activities Screened Out and Identified from Warburgia ugandensis Combining Affinity Ultrafiltration LC-MS with SOD and XOD Enzymes. Antioxidants 2021, 10, 370. [Google Scholar] [CrossRef]
- Ullah, R.; Khan, M.; Shah, S.A.; Saeed, K.; Kim, M.O. Natural Antioxidant Anthocyanins—A Hidden Therapeutic Candidate in Metabolic Disorders with Major Focus in Neurodegeneration. Nutrients 2019, 11, 1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moosavi, M.A.; Haghi, A.; Rahmati, M.; Taniguchi, H.; Mocan, A.; Echeverría, J.; Gupta, V.K.; Tzvetkov, N.T.; Atanasov, A.G. Phytochemicals as potent modulators of autophagy for cancer therapy. Cancer Lett. 2018, 424, 46–69. [Google Scholar] [CrossRef] [PubMed]
- Tuorkey, M.J. Cancer Therapy with Phytochemicals: Present and Future Perspectives. Biomed. Environ. Sci. 2015, 28, 808–819. [Google Scholar] [CrossRef]
- Al-Ishaq, R.K.; Overy, A.J.; Büsselberg, D. Phytochemicals and Gastrointestinal Cancer: Cellular Mechanisms and Effects to Change Cancer Progression. Biomolecules 2020, 10, 105. [Google Scholar] [CrossRef] [Green Version]
- Si, H.; Liu, D. Dietary antiaging phytochemicals and mechanisms associated with prolonged survival. J. Nutr. Biochem. 2014, 25, 581–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.K.; Li, X.F.; Zhang, J.Y.; Lei, J.; Li, W.W.; Wang, G. Analysis of the Volatile Components in Selaginella doederleinii by Headspace Solid Phase Microextraction-Gas Chromatography-Mass Spectrometry. Molecules 2020, 25, 115. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Wang, X.; Wang, G.; Shi, P.; Lin, S.; Xu, D.; Chen, B.; Liu, A.; Huang, L.; Lin, X.; et al. Ethyl Acetate Extract of Selaginella doederleinii Hieron Induces Cell Autophagic Death and Apoptosis in Colorectal Cancer via PI3K-Akt-mTOR and AMPKα-Signaling Pathways. Front. Pharmacol. 2020, 11, 565090. [Google Scholar] [CrossRef]
- Lee, N.Y.; Min, H.Y.; Lee, J.; Nam, J.W.; Lee, Y.J.; Han, A.R.; Wiryawan, A.; Suprapto, W.; Lee, S.K.; Seo, E.K. Identification of a New Cytotoxic Biflavanone from Selaginella doederleinii. Chem. Pharm. Bull. 2008, 56, 1360–1361. [Google Scholar] [CrossRef] [Green Version]
- Zou, Z.; Xu, P.; Zhang, G.; Cheng, F.; Chen, K.; Li, J.; Zhu, W.; Cao, D.; Xu, K.; Tan, G. Selagintriflavonoids with BACE1 inhibitory activity from the fern Selaginella doederleinii. Phytochemistry 2017, 134, 114–121. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Wang, L. Identification of Biflavones in Ethyl Acetate Fraction from Ethanol Extract of Selaginella doederleinii Hieron. Adv. Mater. Res. 2012, 550–553, 1862–1865. [Google Scholar] [CrossRef]
- Lin, R.C.; Skaltsounis, A.-L.; Seguin, E.; Tillequin, F.; Koch, M. Phenolic Constituents of Selaginella doederleinii. Planta Med. 1994, 60, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.-Y.; Lee, S.-g.; Lee, M. Biflavonoids Isolated from Selaginella tamariscina and Their Anti-Inflammatory Activities via ERK 1/2 Signaling. Molecules 2018, 23, 926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, K.-Y.; Lin, J.-L.; Chen, J.-S. Severe Reversible Bone Marrow Suppression Induced by Selaginella doederleinii. J. Toxicol. Clin. Toxicol. 2001, 39, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Peng, H.; Ji, Z.; Zhao, S.; Zhang, Y.; Wu, J.; Fan, J.; Liao, J. Reactive oxygen species-mediated mitochondrial dysfunction is involved in apoptosis in human nasopharyngeal carcinoma CNE cells induced by Selaginella doederleinii extract. J. Ethnopharmacol. 2011, 138, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, M.; Li, Y.; Sui, Y.; Yao, H.; Huang, L.; Lin, X. Preparative Isolation of six Anti-Tumour Biflavonoids from Selaginella doederleinii Hieron by High-Speed Counter-Current Chromatography. Phytochem. Anal. 2014, 25, 127–133. [Google Scholar] [CrossRef]
- Chao, L.R.; Seguin, E.; Skaltsounis, A.L.; Tillequin, F.; Koch, M. Synthesis of the glycoalkaloids of Selaginella doederleinii and structure revision of one of them. J. Nat. Prod. 1990, 53, 882–893. [Google Scholar] [CrossRef]
- Zou, Z.X.; Tan, G.-S.; Zhang, G.-G.; Yu, X.; Xu, P.-S.; Xu, K.-P. New cytotoxic apigenin derivatives from Selaginella doederleinii. Chin. Chem. Lett. 2017, 28, 931–934. [Google Scholar] [CrossRef]
- Sui, Y.; Yao, H.; Li, S.; Jin, L.; Shi, P.; Li, Z.; Wang, G.; Lin, S.; Wu, Y.; Li, Y.; et al. Delicaflavone induces autophagic cell death in lung cancer via Akt/mTOR/p70S6K signaling pathway. J. Mol. Med. 2017, 95, 311–322. [Google Scholar] [CrossRef]
- Lin, L.C.; Kuo, Y.C.; Chou, C.J. Cytotoxic Biflavonoids from Selaginella delicatula. J. Nat. Prod. 2000, 63, 627–630. [Google Scholar] [CrossRef]
- Lian, R.; Li, J.; Ma, H.; Zhang, G.; Guo, X.; Li, X.; Yang, J. Effect of ethanol extract of Selaginella doederleinii Hieron on the proliferation of nasopharyngeal carcinoma CNE-1 and C666-1 cells. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 490–493. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Qian, Y.; Tian, Y.J.; Yuan, S.M.; Wei, W.; Wang, G. Optimization of Ionic Liquid-Assisted Extraction of Biflavonoids from Selaginella doederleinii and Evaluation of Its Antioxidant and Antitumor Activity. Molecules 2017, 22, 586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Yao, S.; Zhang, X.X.; Song, H. Rapid Screening and Structural Characterization of Antioxidants from the Extract of Selaginella doederleinii Hieron with DPPH-UPLC-Q-TOF/MS Method. Int. J. Anal. Chem. 2015, 2015, 849769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, Y.; Chang, S.K.; Gu, Y.; Qian, S.Y. Antioxidant activity and phenolic compositions of lentil (Lens culinaris var. Morton) extract and its fractions. J. Agric. Food Chem. 2011, 59, 2268–2276. [Google Scholar] [CrossRef] [Green Version]
- Ru, Q.M.; Wang, L.J.; Li, W.M.; Wang, J.L.; Ding, Y.T. In Vitro Antioxidant Properties of Flavonoids and Polysaccharides Extract from Tobacco (Nicotiana tabacum L.) Leaves. Molecules 2012, 17, 11281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Zhang, Y.; Muema, F.W.; Kimutai, F.; Chen, G.; Guo, M. Phenolic Compounds from Carissa spinarum Are Characterized by Their Antioxidant, Anti-Inflammatory and Hepatoprotective Activities. Antioxidants 2021, 10, 652. [Google Scholar] [CrossRef] [PubMed]
- Mutungi, M.M.; Muema, F.W.; Kimutai, F.; Xu, Y.-B.; Zhang, H.; Chen, G.L.; Guo, M.Q. Antioxidant and Antiproliferative Potentials of Ficus glumosa and Its Bioactive Polyphenol Metabolites. Pharmaceuticals 2021, 14, 266. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-B.; Chen, G.L.; Guo, M.Q. Antioxidant and Anti-Inflammatory Activities of the Crude Extracts of Moringa oleifera from Kenya and Their Correlations with Flavonoids. Antioxidants 2019, 8, 296. [Google Scholar] [CrossRef] [Green Version]
- Hemlata; Meena, P.R.; Singh, A.P.; Tejavath, K.K. Biosynthesis of Silver Nanoparticles Using Cucumis prophetarum Aqueous Leaf Extract and Their Antibacterial and Antiproliferative Activity Against Cancer Cell Lines. ACS Omega 2020, 5, 5520–5528. [Google Scholar] [CrossRef] [Green Version]
- Sivaraman, A.; Johnson, M.; Parimelazhagan, T.; Irudayaraj, V. Evaluation of antioxidant potential of ethanolic extracts of selected species of Selaginella. NIScPR Online Period. Repos. 2013, 4, 238–244. [Google Scholar]
- Siddhuraju, P.; Becker, K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J. Agric. Food Chem. 2003, 51, 2144–2155. [Google Scholar] [CrossRef]
- Song, G.; Yao, S.; Cheng, L.; Luo, Y.F.; Song, H. Antioxidant and anticancer effection of the volatile oil from various habitats of Selaginella doederleinii Hieron. Technol. Health Care 2015, 23 (Suppl. S1), S21–S27. [Google Scholar]
- Wang, J.Z.; Li, J.; Zhao, P.; Ma, W.T.; Feng, X.H.; Chen, K.L. Antitumor Activities of Ethyl Acetate Extracts from Selaginella doederleinii Hieron In Vitro and In Vivo and Its Possible Mechanism. Evid. Based. Complement. Alternat. Med. 2015, 2015, 865714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, W.; Finch, A.; Ollis, W.; Robinson, K. The structures of the naturally occurring biflavonyls. J. Chem. Soc. (Resumed) 1963, 208, 1477–1490. [Google Scholar] [CrossRef]
- Bedir, E.; Tatli, I.I.; Khan, R.A.; Zhao, J.; Takamatsu, S.; Walker, L.A.; Goldman, P.; Khan, I.A. Biologically Active Secondary Metabolites from Ginkgo biloba. J. Agric. Food Chem. 2002, 50, 3150–3155. [Google Scholar] [CrossRef] [PubMed]
- He, C.W.; Wei, J.H.; Zeng, L.Y.; Deng, J.G. Triterpenoids and Flavonoids from Cassava Leaves. Chem. Nat. Compd. 2020, 56, 331–333. [Google Scholar] [CrossRef]
- Das, B.; Mahender, G.; Koteswara Rao, Y.; Prabhakar, A.; Jagadeesh, B. Biflavonoids from Cycas beddomei. Chem. Pharm. Bull. 2005, 53, 135–136. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.J.; Duh, C.Y.; Chen, J.F. New cytotoxic biflavonoids from Selaginella delicatula. Planta Med. 2005, 71, 659–665. [Google Scholar] [CrossRef]
- Skopp, G.; Schwenker, G. Biflavonoide aus Schinus terebinthifolius Raddi (Anacardiaceae)/Biflavonoids from Schinus terebinthifolius Raddi (Anacardiaceae). Z. Naturforsch. B. 1986, 41, 1479–1482. [Google Scholar] [CrossRef]
- Zheng, J.X.; Wang, N.L.; Liu, H.W.; Chen, H.F.; Li, M.M.; Wu, L.Y.; Fan, M.; Yao, X.S. Four new biflavonoids from Selaginella uncinata and their anti-anoxic effect. J. Asian Nat. Prod. Res. 2008, 10, 945–952. [Google Scholar] [CrossRef]
- Tan, C.X.; Schrader, K.K.; Khan, I.A.; Rimando, A.M. Activities of Wogonin Analogs and Other Flavones against Flavobacterium columnare. Chem. Biodivers. 2015, 12, 259–272. [Google Scholar] [CrossRef]
- Yao, H.; Yuan, Z.; Wei, G.; Chen, C.; Duan, J.; Li, Y.; Wang, Y.; Zhang, C.; Liu, Y. Thevetiaflavone from Wikstroemia indica ameliorates PC12 cells injury induced by OGD/R via improving ROS-mediated mitochondrial dysfunction. Mol. Med. Rep. 2017, 16, 9197–9202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boukaabache, R.; Boubekri, N.; Boumaza, O.; Mekkiou, R.; Seghiri, R.; Sarri, D.; Zama, D.; Benayache, F.; Benayache, S. Phytochemical study of ethyl acetate extract and antioxidant activity of Genista quadriflora Munby (Fabaceae). Der. Pharm. Lett. 2013, 5, 56–59. [Google Scholar]
- Das, B.; Mahender, G.; Rao, Y.K.; Thirupathi, P. A new biflavonoid from Cycas beddomei. Indian J. Chem. 2006, 450, 1933–1935. [Google Scholar]
- Fan, X.; Xu, J.; Lin, X.; Chen, K. Study on biflavonoids from Selaginella uncinata (Desv.) Spring. Chin. Pharm. J. 2009, 44, 15–19. [Google Scholar]
- Setyawan, A.D. Natural products from genus Selaginella (Selaginellaceae). Nusant. Biosci. 2011, 3, 44–58. [Google Scholar] [CrossRef]
- Karak, P. Biological activities of flavonoids: An overview. Int. J. Pharm. Sci. Res. 2019, 10, 1567–1574. [Google Scholar]
- Orčić, D.Z.; Mimica-Dukić, N.M.; Francišković, M.M.; Petrović, S.S.; Jovin, E.Đ. Antioxidant activity relationship of phenolic compounds in Hypericum perforatum L. Chem. Cent. J. 2011, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Goossens, J.-F.; Goossens, L.; Bailly, C. Hinokiflavone and Related C–O–C-Type Biflavonoids as Anti-cancer Compounds: Properties and Mechanism of Action. Nat. Prod. Bioprospect. 2021, 11, 365–377. [Google Scholar] [CrossRef]
- Kothandan, G.; Gadhe, C.G.; Madhavan, T.; Choi, C.H.; Cho, S.J. Docking and 3D-QSAR (quantitative structure activity relationship) studies of flavones, the potent inhibitors of p-glycoprotein targeting the nucleotide binding domain. Eur. J. Med. Chem. 2011, 46, 4078–4088. [Google Scholar] [CrossRef]
- Dao, P.T.A.; Le Quan, T.; Mai, N.T.T. Antioxidant constituents from the stem of Tetrastigma erusbescense Planch (Vitaceae). Nat. Prod. Sci. 2014, 20, 22–28. [Google Scholar]
- López-Posadas, R.; Ballester, I.; Abadía-Molina, A.C.; Suárez, M.D.; Zarzuelo, A.; Martínez-Augustin, O.; Sánchez de Medina, F. Effect of flavonoids on rat splenocytes, a structure–activity relationship study. Biochem. Pharmacol. 2008, 76, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Chen, H. The methoxyflavones in Citrus reticulata Blanco cv. ponkan and their antiproliferative activity against cancer cells. Food Chem. 2010, 119, 567–572. [Google Scholar]




| Sample | DPPH | FRAP |
|---|---|---|
| IC50 (µg/mL) | mmol Fe2+/g | |
| Ethanol extract | 187.5 ± 1.3 a | 1.1 ± 0.0 c |
| PE | 176.5 ± 0.8 b | 0.9 ± 0.1 c |
| DCM | 110.6 ± 1.3 d | 2.6 ± 0.1 b |
| EA | 82.1 ± 1.1 e | 1.7 ± 0.0 bc |
| n-BuOH | 115.2 ± 1.4 c | 1.6 ± 0.0 bc |
| Vitamin C | 5.8 ± 1.9 f | 7.8 ± 1.2 a |
| BHT | 5.9 ± 1.6 f | Nt |
| Fraction | IC50 (µg/mL) | ||
|---|---|---|---|
| HT-29 | HeLa | A549 | |
| DCM | 145.4 ± 3.0 | 92.5 ± 0.6 | 55.9 ± 12.6 |
| EA | 55.6 ± 1.3 | 69.2 ± 1.3 | 112.7 ± 6.7 |
| 1 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|
| H/C | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC |
| 2 | 164.6 | 165.7 | 163.5 | 163.4 | ||||
| 3 | 6.68 1H, s | 103.8 | 6.68 1H, s | 103.9 | 6.67 1H, s | 103.9 | 6.71 1H, s | 103.9 |
| 4 | 184.5 | 184.3 | 179.4 | 179.4 | ||||
| 5 | 163.1 | 162.5 | 164.6 | |||||
| 6 | 6.60 1H, s | 96.4 | 6.40 1H, s | 100.1 | 6.38 1H, s | 99.9 | 6.63 1H, s | 96.4 |
| 7 | 163.3 | 166.7 | 164.4 | 164.4 | ||||
| 8 | 105.6 | 105.4 | 104.6 | 105.7 | ||||
| 9 | 155.5 | 156.5 | 156.8 | 155.5 | ||||
| 10 | 103.8 | 104.3 | 104.9 | 106.4 | ||||
| 1′ | 124.3 | 124.6 | 124.7 | 124.3 | ||||
| 2′/6′ | 7.56 2H, d, J = 8.9 Hz | 129.1 | 7.64 2H, d, J = 8.9 Hz | 129.1 | 7.54 2H, d, J = 8.9 Hz | 129.0 | 7.56 2H, d, J = 8.9 Hz | 129.0 |
| 3′/5′ | 6.93 2H, d, J = 8.9 Hz | 115.5 | 6.94 2H, d, J = 8.9 Hz | 115.5 | 6.93 2H, d, J = 8.9 Hz | 115.5 | 6.94 2H, d, J = 8.9 Hz | 115.5 |
| 4′ | 164.4 | 164.3 | 164.4 | 165.0 | ||||
| 1″ | 122.0 | 119.7 | 122.6 | 122.5 | ||||
| 2″ | 163.1 | 163.3 | 163.3 | 163.3 | ||||
| 3″ | 7.20 1H, d, J = 8.7 Hz | 111.4 | 7.08 1H, d, J = 8.5 Hz | 116.7 | 7.24 1H, d, J = 8.8 Hz | 111.8 | 7.28 1H, d, J = 8.8 Hz | 111.7 |
| 4″ | 8.13 1H, dd, J = 8.7, 2.2 Hz | 132.7 | 8.01 1H, dd, J = 8.4, 2.3 Hz | 130.9 | 8.16 1H, dd, J = 8.7, 2.4 Hz | 131.9 | 8.17 1H, dd, J = 8.7, 2.3 Hz | 131.1 |
| 5″ | 126.2 | 130.1 | 130.9 | 130.9 | ||||
| 6″ | 7.93 1H, d, J = 2.2 Hz | 135.5 | 7.99 1H, d, J = 2.2 Hz | 135.7 | 7.99 1H, d, J = 2.3 Hz | 134.8 | 7.96 1H, d, J = 2.3 Hz | 134.6 |
| 7″ | 165.9 | 199.6 | 196.5 | 199.5 | ||||
| 8″ | 2.57 3H, s | 26.9 | 2.57 3H, s | 26.9 | 2.57 3H, s | 28.1 | ||
| OMe-7 | 3.82 3H, s | 56.0 | 3.84 3H, s | 56.9 | ||||
| OMe-4′ | 3.86 3H, s | 56.8 | 3.84 3H, s | 56.0 | 3.82 3H, s | 56.5 | 3.87 3H, s | 56.5 |
| OMe-2″ | 3.77 3H, s | 56.3 | 3.82 3H, s | 56.0 | 3.82 3H, S | 56.0 | ||
| Compound No. | IC50 (µM) | ||
|---|---|---|---|
| HT-29 | HeLa | A549 | |
| 8 | 56.9 ± 3.4 | 43.5 ± 4.2 | 24.3 ± 1.2 |
| 9 | 44.7 ± 3.2 | >100 | >100 |
| 16 | 27.9 ± 1.0 | 35.5 ± 4.2 | 20.7 ± 3.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muema, F.W.; Liu, Y.; Zhang, Y.; Chen, G.; Guo, M. Flavonoids from Selaginella doederleinii Hieron and Their Antioxidant and Antiproliferative Activities. Antioxidants 2022, 11, 1189. https://doi.org/10.3390/antiox11061189
Muema FW, Liu Y, Zhang Y, Chen G, Guo M. Flavonoids from Selaginella doederleinii Hieron and Their Antioxidant and Antiproliferative Activities. Antioxidants. 2022; 11(6):1189. https://doi.org/10.3390/antiox11061189
Chicago/Turabian StyleMuema, Felix Wambua, Ye Liu, Yongli Zhang, Guilin Chen, and Mingquan Guo. 2022. "Flavonoids from Selaginella doederleinii Hieron and Their Antioxidant and Antiproliferative Activities" Antioxidants 11, no. 6: 1189. https://doi.org/10.3390/antiox11061189





