Flooding Tolerance in Sweet Potato (Ipomoea batatas (L.) Lam) Is Mediated by Reactive Oxygen Species and Nitric Oxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
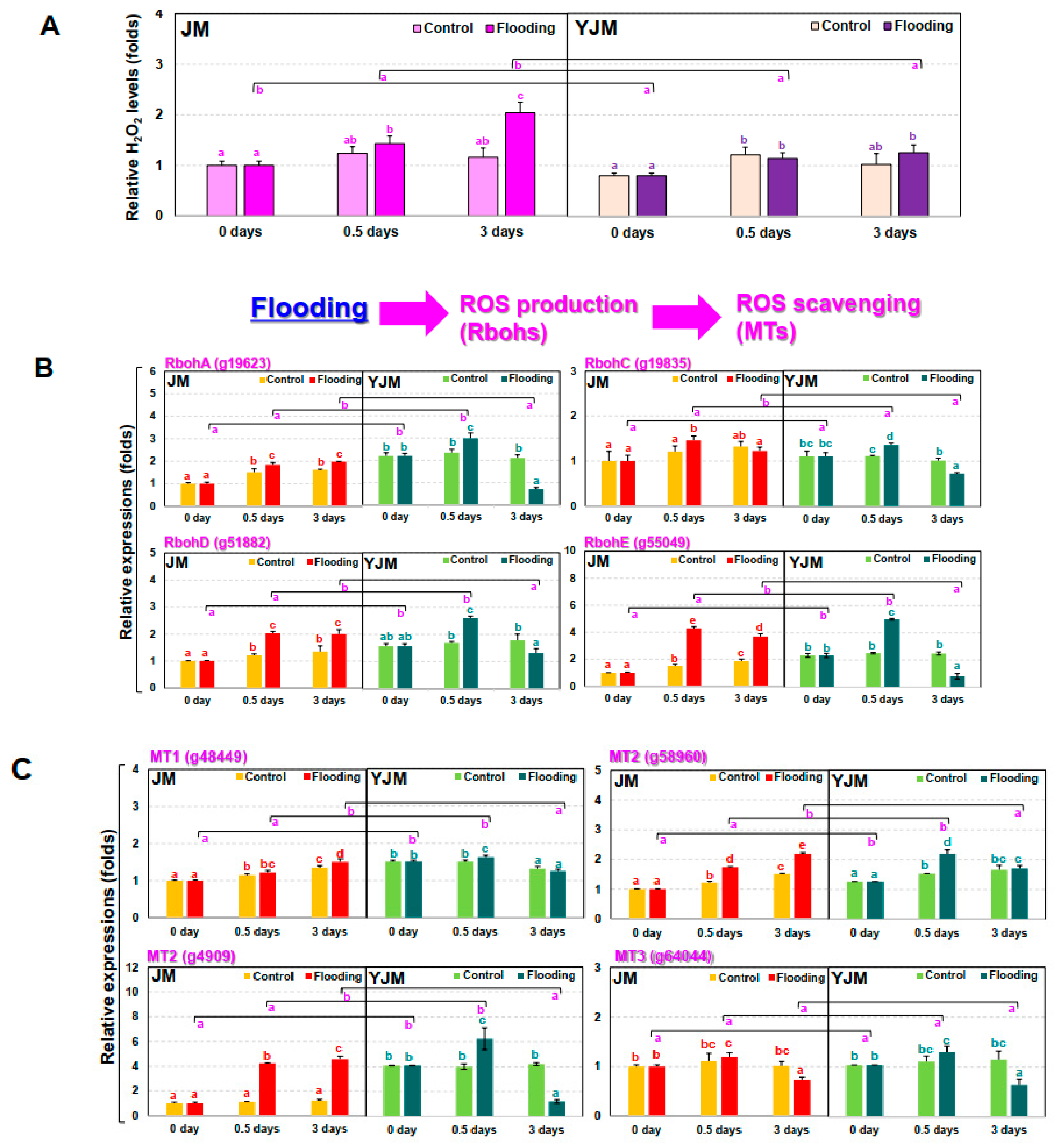
2.2. Analysis of H2O2 Levels
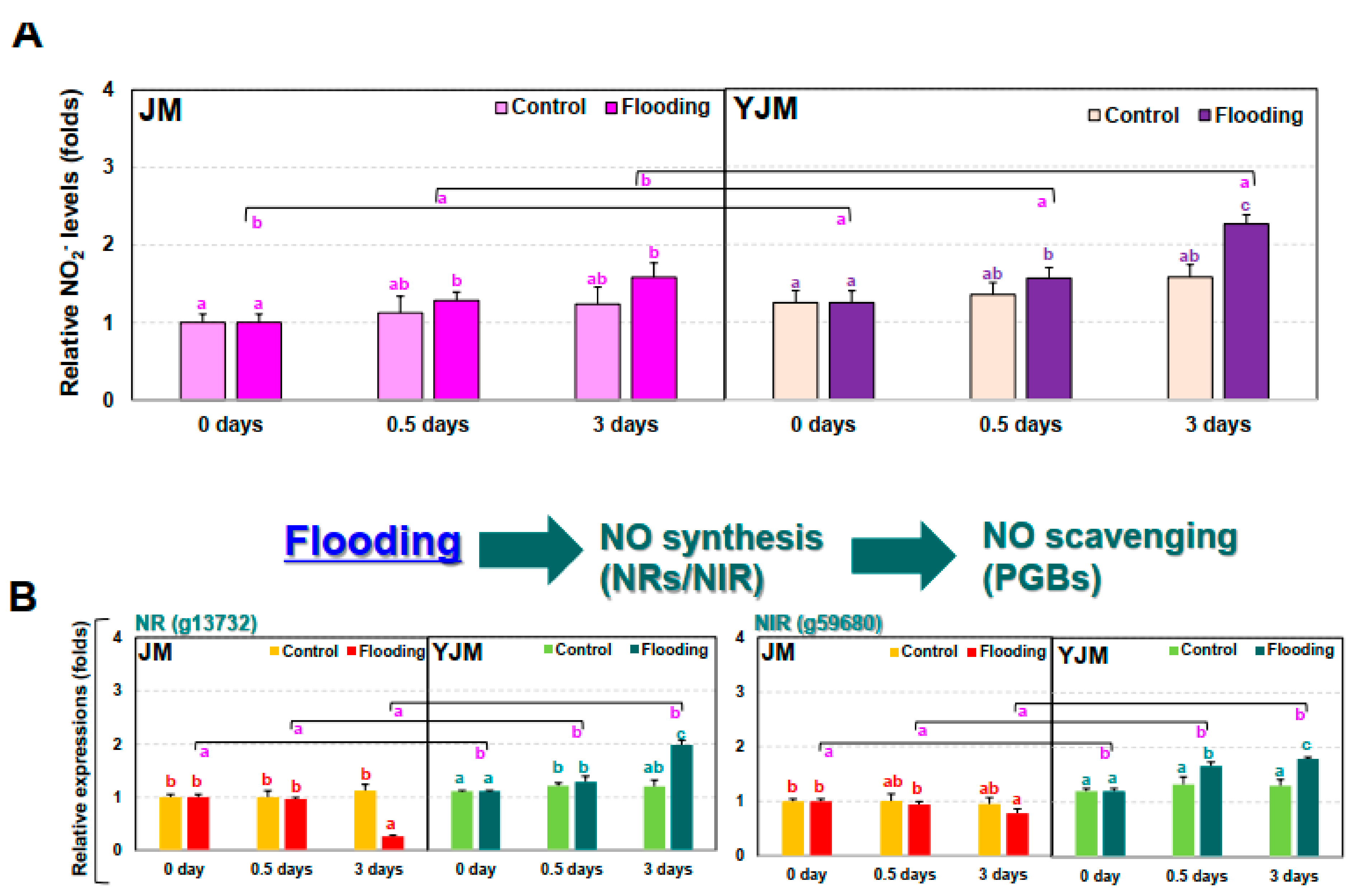
2.3. Analysis of Nitrite Content
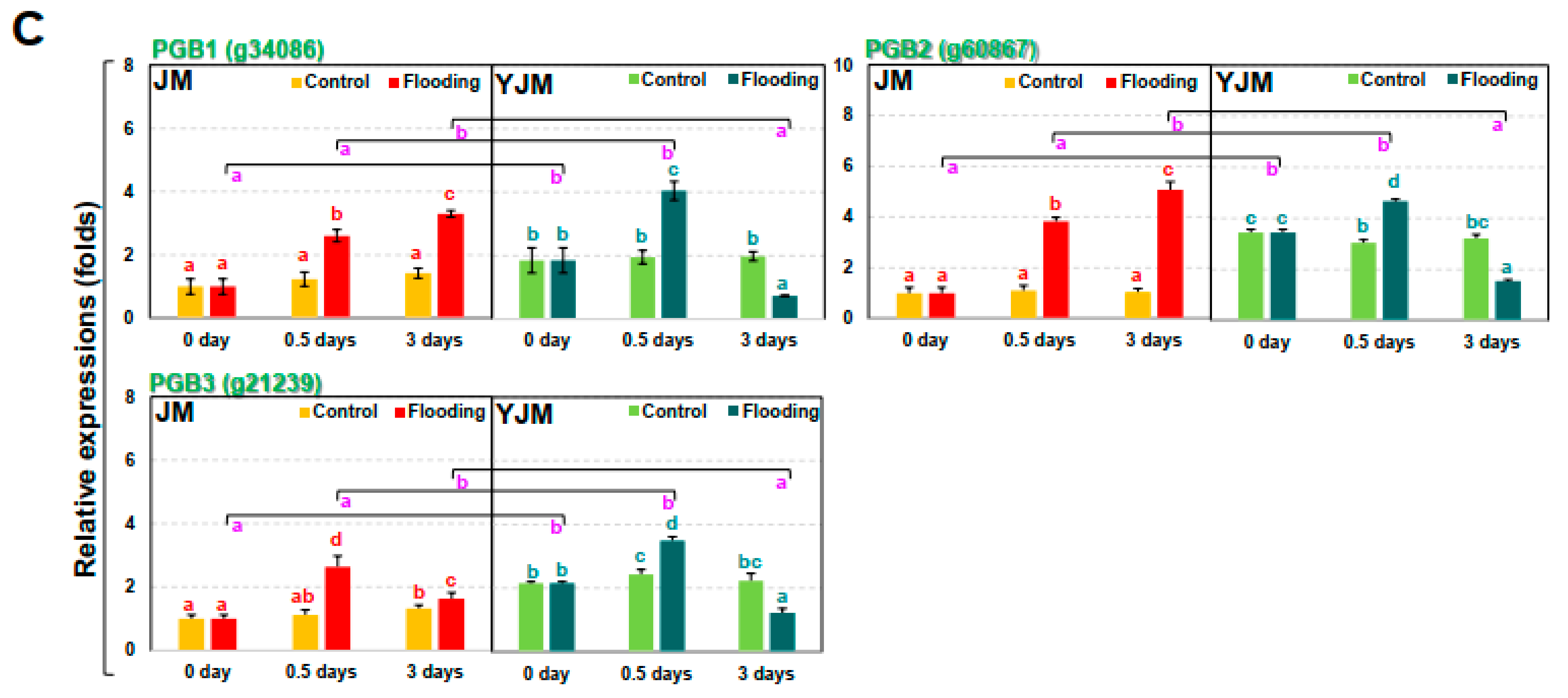
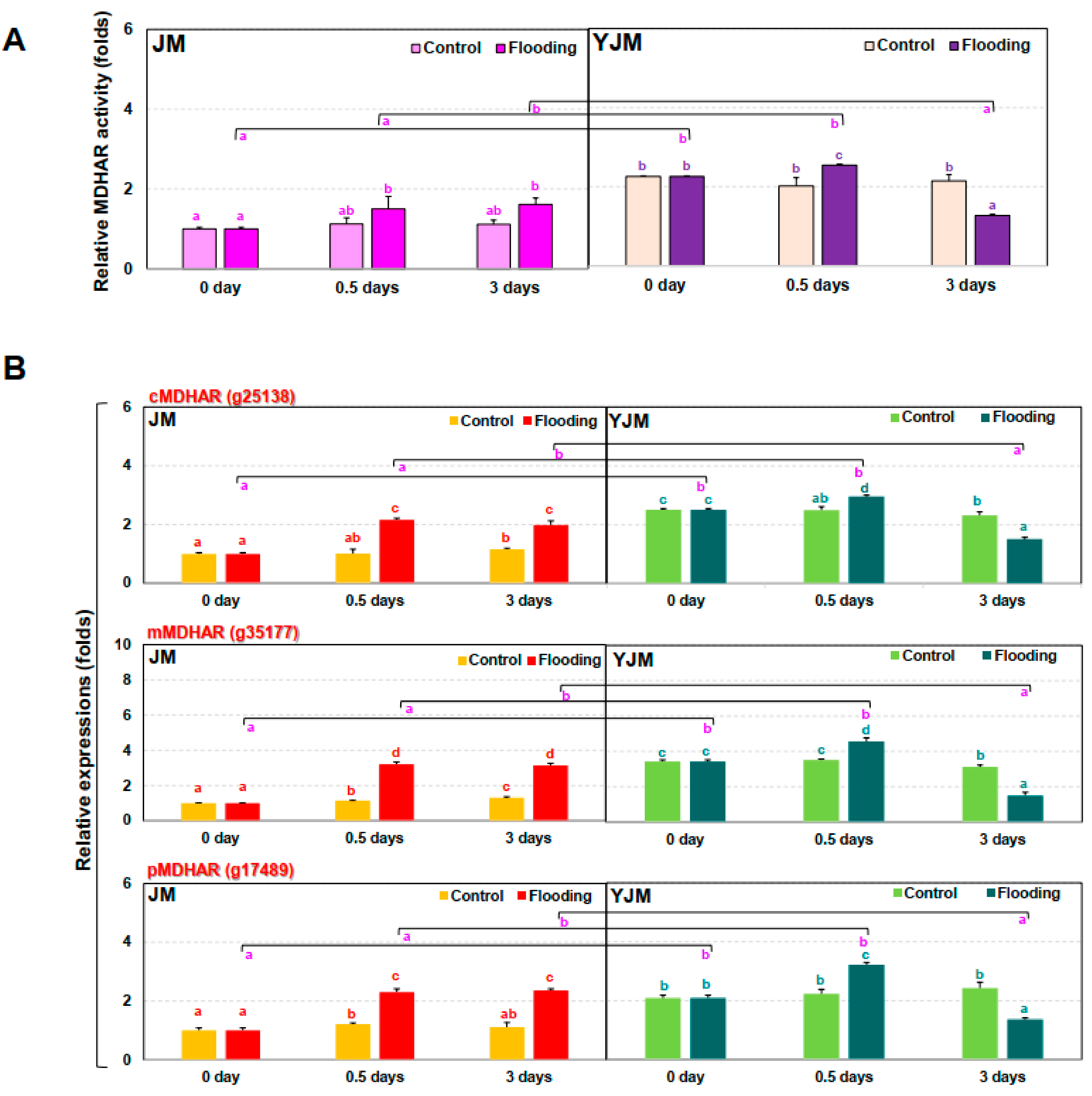
2.4. RNA Isolation and Analysis of Gene Expression
2.5. Monodehydroascorbate Reductase Assay
2.6. Statistical Analyses
3. Results
3.1. Expression of ERFVII during Early Flooding Treatment
3.2. The Responses of ROS during Early Flooding Treatment
3.3. The Responses of NO during Early Flooding Treatment
3.4. The Responses of MDHAR during Early Flooding Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | 1-aminocyclopropane-1 carboxylate |
| ADH | alcohol dehydrogenase |
| ANOVA | analysis of variance |
| AP2/ERF | Apetala2/ethylene response factor |
| AsA | ascorbic acid |
| DPI | diphenyleneiodonium |
| ET | ethylene |
| ETR | ET receptor |
| Hb | hemoglobin |
| JM | Jeonmi |
| LOES | low-O2 escape syndrome |
| LOQS | low-O2 quiescence syndrome |
| LSD | least significant difference |
| MAPK | mitogen-activated protein kinase |
| MDHA | monodehydroascorbate |
| MDHAR | monodehydroascorbate reductase |
| MT | metallothionein |
| NED | N-(1-naphthyl) ethylenediamine |
| NIR | nitrite reductase |
| NO | nitric oxide |
| NR | nitrate reductase |
| PGB | phytoglobin |
| RBOH | respiratory burst oxidase homolog |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| TF | transcription factor |
| YJM | Yeonjami |
References
- Hirabayashi, Y.; Mahendran, R.; Koirala, S.; Konoshima, L.; Yamazaki, D.; Watanabe, S.; Kim, H.; Kanae, S. Global flood risk under climate change. Nat. Clim. Change 2013, 3, 816–821. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Voesenek, L.A.C.J. Flooding stress: Acclimations and genetic diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasidharan, R.; Voesenek, L.A.C.J. Ethylene-mediated acclimations to flooding stress. Plant Physiol. 2015, 169, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Parlanti, S.; Kudahettige, N.P.; Lombardi, L.; Mensuali-Sodi, A.; Alpi, A.; Perata, P.; Pucciariello, C. Distinct mechanisms for aerenchyma formation in leaf sheaths of rice genotypes displaying a quiescence or escape strategy for flooding tolerance. Ann. Bot. 2011, 107, 1335–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steffens, B.; Geske, T.; Sauter, M. Aerenchyma formation in the rice stem and its promotion by H2O2. New Phytol. 2011, 190, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Randlett, M.D.; Findell, J.L.; Schaller, E.G. Localization of the ethylene receptor ETR1 to the endoplasmic reticulum in Arabidopsis. J. Biol. Chem. 2002, 277, 19861–19866. [Google Scholar] [CrossRef] [Green Version]
- Jung, K.H.; Seo, Y.S.; Walia, H.; Cao, P.; Fukao, T.; Canlas, P.E.; Amonpant, F.; Bailey-Serres, J.; Ronald, P.C. The submergence tolerance regulator Sub1A mediates stress-responsive expression of AP2/ERF transcription factors. Plant Physiol. 2010, 152, 1674–1692. [Google Scholar] [CrossRef] [Green Version]
- Steffens, B.; Steffen-Heins, A.; Sauter, M. Reactive oxygen species mediate growth and death in submerged plants. Front. Plant Sci. 2013, 4, 179. [Google Scholar] [CrossRef] [Green Version]
- Torres, M.A.; Onouchi, H.; Hamada, S.; Machida, C.; Hammond-Kosack, K.E.; Jones, J.D. Six Arabidopsis thaliana homologues of the human respiratory burst oxidase (gp91phox). Plant J. 1998, 14, 365–370. [Google Scholar] [CrossRef] [Green Version]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Manjunatha, G.; Gupta, K.J.; Lokesh, V.; Mur, L.A.; Neelwarne, B. Nitric oxide counters ethylene effects on ripening fruits. Plant Signal. Behav. 2012, 7, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Farnese, F.S.; Menezes-Silva, P.E.; Gusman, G.S.; Oliveira, J.A. When bad guys become good ones: The key role of reactive oxygen species and nitric oxide in the plant responses to abiotic stress. Front. Plant Sci. 2016, 7, 471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Planchet, E.; Jagadis Gupta, K.; Sonoda, M.; Kaiser, W.M. Nitric oxide emission from tobacco leaves and cell suspensions: Rate limiting factors and evidence for the involvement of mitochondrial electron transport. Plant J. 2005, 41, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Chamizo-Ampudia, A.; Sanz-Luque, E.; Llamas, A.; Galvan, A.; Fernandez, E. Nitrate reductase regulates plant nitric oxide homeostasis. Trends Plant Sci. 2017, 22, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, J.R.; Monte, D.C.; Durzan, D. Nitric oxide and ethylene emission in Arabidopsis thaliana. Physiol. Mol. Biol. Plant 2000, 6, 117–127. [Google Scholar]
- Copolovici, L.; Niinemets, U. Flooding induced emissions of volatile signalling compounds in three tree species with differing waterlogging tolerance. Plant Cell Environ. 2010, 33, 1582–1594. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, X.; Dai, J.; Luo, Z.; Li, Z.; Lu, H.; Xu, S.; Tang, W.; Zhang, D.; Li, W.; et al. Global gene expression in cotton (Gossypium hirsutum L.) leaves to waterlogging stress. PLoS ONE 2017, 12, e0185075. [Google Scholar] [CrossRef]
- Mugnai, S.; Azzarello, E.; Baluška, F.; Mancuso, S. Local root apex hypoxia induces NO-mediated hypoxic acclimation of the entire root. Plant Cell Physiol. 2012, 53, 912–920. [Google Scholar] [CrossRef] [Green Version]
- Gupta, K.J.; Igamberdiev, A.U. Reactive nitrogen species in mitochondria and their implications in plant energy status and hypoxic stress tolerance. Front. Plant Sci. 2016, 7, 369. [Google Scholar] [CrossRef] [Green Version]
- Park, S.U.; Lee, C.J.; Kim, S.E.; Lim, Y.H.; Lee, H.U.; Nam, S.S.; Kim, H.S.; Kwak, S.S. Selection of flooding stress tolerant sweetpotato cultivars based on biochemical and phenotypic characterization. Plant Physiol. Biochem. 2020, 155, 243–251. [Google Scholar] [CrossRef]
- Park, S.U.; Kim, Y.H.; Lee, C.J.; Kim, S.E.; Lim, Y.H.; Yoon, U.H.; Kim, H.S.; Kwak, S.S. Comparative transcriptome profiling of two sweetpotato cultivars with contrasting flooding stress tolerance levels. Plant Biotechnol. Rep. 2020, 14, 743–756. [Google Scholar] [CrossRef]
- Bindschedler, L.V.; Minibayeva, F.; Gardner, S.L.; Gerrish, C.; Davies, D.R.; Bolwell, G.P. Early signaling events in the apoplastic oxidative burst in suspension cultured French bean cells involved cAMP and Ca2+. New Phytol. 2001, 151, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Park, S.C.; Kim, Y.H.; Ji, C.Y.; Park, S.; Jeong, J.C.; Lee, H.S.; Kwak, S.S. Stable internal reference genes for the normalization of real-time PCR in different sweetpotato cultivars subjected to abiotic stress conditions. PLoS ONE 2012, 7, e51502. [Google Scholar] [CrossRef] [PubMed]
- Truffault, V.; Riqueau, G.; Garchery, C.; Gautier, H.; Stevens, R. Is monodehydroascorbate reductase activity in leaf tissue critical for the maintenance of yield in tomato? J. Plant Physiol. 2018, 222, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Sasidharan, R.; Hartman, S.; Liu, Z.; Martopawiro Sajeev, N.; van Veen, H.; Yeung, E.; Voeseneka, L.A.C.J. Signal dynamics and interactions during flooding stress. Plant Physiol. 2018, 176, 1106–1117. [Google Scholar] [CrossRef] [Green Version]
- Kolbert, Z.; Feigl, G.; Freschi, L.; Poor, P. Gasotransmitters in action: Nitric oxide-ethylene crosstalk during plant growth and abiotic stress responses. Antioxidants 2019, 8, 167. [Google Scholar] [CrossRef] [Green Version]
- Giuntoli, B.; Perata, P. Group VII ethylene response factors in arabidopsis: Regulation and physiological roles. Plant Physiol. 2018, 176, 1143–1155. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Hargrove, M.S. Nitric oxide in plants: The roles of ascorbate and hemoglobin. PLoS ONE 2013, 8, e82611. [Google Scholar] [CrossRef] [Green Version]
- Igamberdiev, A.U.; Bykova, N.V.; Hill, R.D. Nitric oxide scavenging by barley hemoglobin is facilitated by a monodehydroascorbate reductase-mediated ascorbate reduction of methemoglobin. Planta 2006, 223, 1033–1040. [Google Scholar] [CrossRef]
- Voesenek, L.A.C.V.; Bailey-Serres, J. Flood adaptive traits and processes: An overview. New Phytol. 2015, 206, 57–73. [Google Scholar] [CrossRef]
- Gasch, P.; Fundinger, M.; Müller, J.T.; Lee, T.; Bailey-Serres, J.; Mustroph, A. Redundant ERF-VII transcription factors bind an evolutionarily conserved cis-motif to regulate hypoxia-responsive gene expression in Arabidopsis. Plant Cell 2016, 28, 160–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bui, L.T.; Giuntoli, B.; Kosmacz, M.; Parlanti, S.; Licausi, F. Constitutively expressed ERF-VII transcription factors redundantly activate the core anaerobic response in Arabidopsis thaliana. Plant Sci. 2015, 236, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Giuntoli, B.; Shukla, V.; Maggiorelli, F.; Giorgi, F.M.; Lombardi, L.; Perata, P.; Licausi, F. Age-dependent regulation of ERF-VII transcription factor activity in Arabidopsis thaliana. Plant Cell Environ. 2017, 40, 2333–2346. [Google Scholar] [CrossRef] [PubMed]
- Papdi, C.; Pérez-Salamó, I.; Joseph, M.P.; Giuntoli, B.; Bögre, L.; Koncz, C.; Szabados, L. The low oxygen, oxidative and osmotic stress responses synergistically act through the ethylene response factor VII genes RAP2.12, RAP2.2 and RAP2.3. Plant J. 2015, 82, 772–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pucciariello, C.; Parlanti, S.; Banti, V.; Novi, G.; Perata, P. Reactive oxygen species-driven transcription in Arabidopsis under oxygen deprivation. Plant Physiol. 2012, 159, 184–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Sun, L.; Ma, L.; Hao, F.S. Both AtrbohD and AtrbohF are essential for mediating responses to oxygen deficiency in Arabidopsis. Plant Cell Rep. 2017, 36, 947–957. [Google Scholar] [CrossRef]
- Sun, L.; Ma, L.; He, S.; Hao, F. AtrbohD functions downstream of ROP2 and positively regulates waterlogging response in Arabidopsis. Plant Signal Behav. 2018, 13, e1513300. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, T.; Rajhi, I.; Nakazono, M. Lysigenous aerenchyma formation in maize root is confined to cortical cells by regulation of genes related to generation and scavenging of reactive oxygen species. Plant Signal. Behav. 2011, 6, 759–761. [Google Scholar] [CrossRef] [Green Version]
- Umbreen, S.; Lubega, J.; Cui, B.; Pan, Q.; Jiang, J.; Loake, G.J. Specificity in nitric oxide signalling. J. Exp. Bot. 2018, 69, 3439–3448. [Google Scholar] [CrossRef]
- Gibbs, D.J.; Md Isa, N.; Movahedi, M.; Lozano-Juste, J.; Mendiondo, G.M.; Berckhan, S.; Marín-dela Rosa, N.; Vicente Conde, J.; Sousa Correia, C.; Pearce, S.P.; et al. Nitric oxide sensing in plants is mediated by proteolytic control of group VII ERF transcription factors. Mol. Cell 2014, 53, 369–379. [Google Scholar] [CrossRef] [Green Version]
- Hartman, S.; Liu, Z.; van Veen, H.; Vicente, J.; Reinen, E.; Martopawiro, S.; Zhang, H.; van Dongen, N.; Bosman, F.; Bassel, G.W.; et al. Ethylene-mediated nitric oxide depletion pre-adapts plants to hypoxia stress. Nat. Commun. 2019, 10, 4020. [Google Scholar] [CrossRef] [Green Version]
- Hebelstrup, K.H.; van Zanten, M.; Mandon, J.; Voesenek, L.A.; Harren, F.J.; Cristescu, S.M.; Møller, I.M.; Mur, L.A. Haemoglobin modulates NO emission and hyponasty under hypoxia-related stress in Arabidopsis thaliana. J. Exp. Bot. 2012, 63, 5581–5591. [Google Scholar] [CrossRef] [PubMed]
- Mira, M.M.; Hill, R.D.; Stasolla, C. Phytoglobins improve hypoxic root growth by alleviating apical meristem cell death. Plant Physiol. 2016, 172, 2044–2056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohwaki, Y.; Kawagishi-Kobayashi, M.; Wakasa, K.; Fujihara, S.; Yoneyama, T. Induction of class-1 non-symbiotic hemoglobin genes by nitrate, nitrite and nitric oxide in cultured rice cells. Plant Cell Physiol. 2005, 46, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.L.; Zhong, N.Q.; Wang, H.Y.; Chen, A.P.; Jian, G.L.; Xia, G.X. Ectopic expression of the cotton non-symbiotic hemoglobin gene GhHbd1 triggers defense responses and increases disease tolerance in Arabidopsis. Plant Cell Physiol. 2006, 47, 1058–1068. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Long, J.; He, X.; Yan, J.; Chen, X.; Tan, Y.; Li, K.; Chen, L.; Xu, H. Overexpression of spinach non-symbiotic hemoglobin in Arabidopsis resulted in decreased NO content and lowered nitrate and other abiotic stresses tolerance. Sci. Rep. 2016, 6, 26400. [Google Scholar] [CrossRef] [Green Version]
- Licausi, F.; Kosmacz, M.; Weits, D.A.; Giuntoli, B.; Giorgi, F.M.; Voesenek, L.A.C.J.; Perata, P.; Van Dongen, J.T. Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature 2011, 479, 419–422. [Google Scholar] [CrossRef]
- Dhungana, S.K.; Kim, H.S.; Kang, B.K.; Seo, J.H.; Kim, H.T.; Oh, J.H.; Shin, S.O.; Baek, I.Y. Analysis of differentially expressed genes in soybean leaf tissue of tolerant and susceptible cultivars under flooding stress revealed by RNA sequencing. J. Crop Sci. Biotechnol. 2021, 24, 83–91. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-U.; Lee, C.-J.; Park, S.-C.; Nam, K.J.; Lee, K.-L.; Kwak, S.-S.; Kim, H.S.; Kim, Y.-H. Flooding Tolerance in Sweet Potato (Ipomoea batatas (L.) Lam) Is Mediated by Reactive Oxygen Species and Nitric Oxide. Antioxidants 2022, 11, 878. https://doi.org/10.3390/antiox11050878
Park S-U, Lee C-J, Park S-C, Nam KJ, Lee K-L, Kwak S-S, Kim HS, Kim Y-H. Flooding Tolerance in Sweet Potato (Ipomoea batatas (L.) Lam) Is Mediated by Reactive Oxygen Species and Nitric Oxide. Antioxidants. 2022; 11(5):878. https://doi.org/10.3390/antiox11050878
Chicago/Turabian StylePark, Sul-U, Chan-Ju Lee, Sung-Chul Park, Ki Jung Nam, Kang-Lok Lee, Sang-Soo Kwak, Ho Soo Kim, and Yun-Hee Kim. 2022. "Flooding Tolerance in Sweet Potato (Ipomoea batatas (L.) Lam) Is Mediated by Reactive Oxygen Species and Nitric Oxide" Antioxidants 11, no. 5: 878. https://doi.org/10.3390/antiox11050878







