Application of Citrus By-Products in the Production of Active Food Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents and Raw Materials
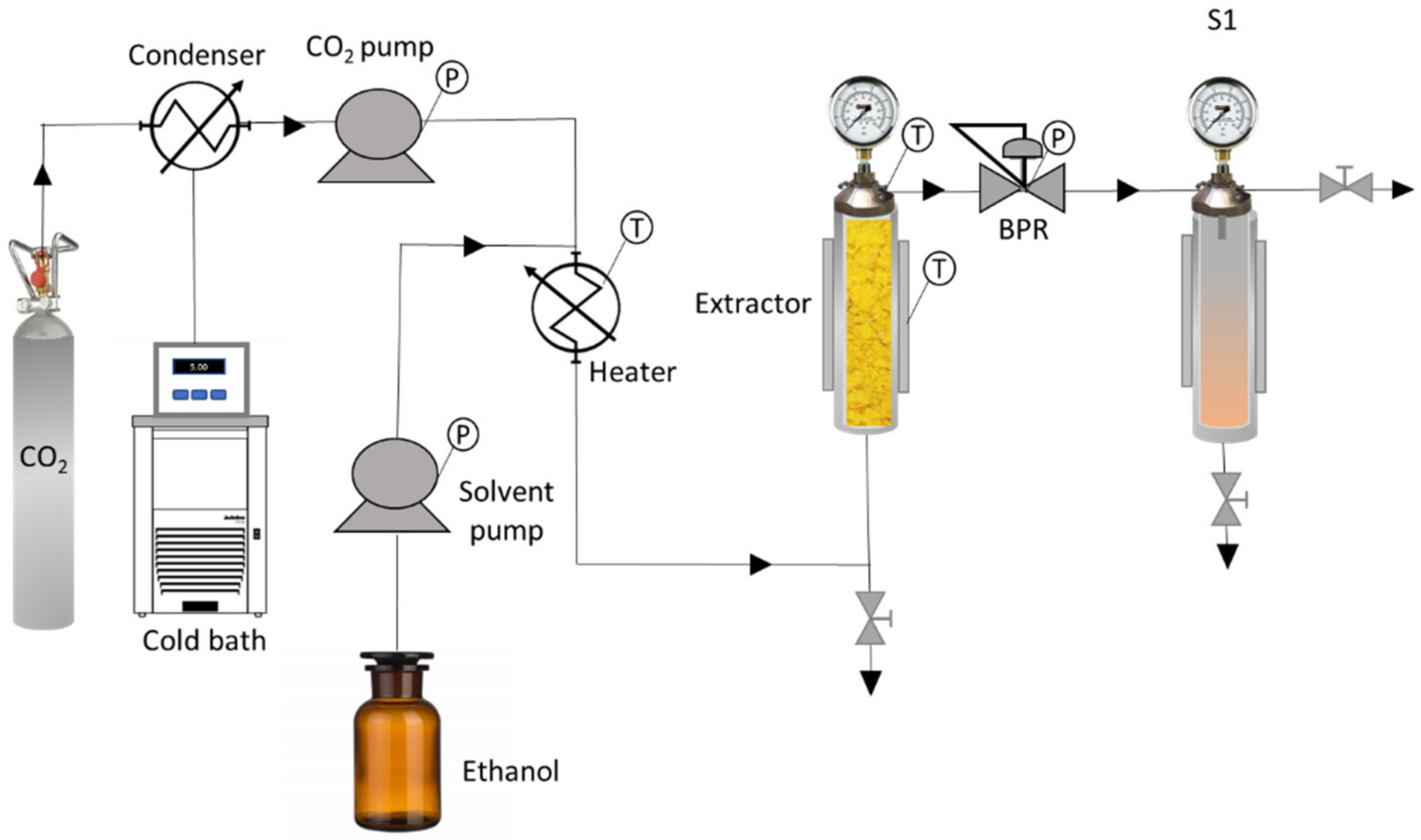
2.2. Extraction and Fractionation at High Pressure
2.3. Supercritical Impregnation at High Pressure
2.4. Bioactivity of the Extracts and of the Impregnated PP
2.4.1. Determining the Antioxidant Activity by DPPH Assays
2.4.2. Antimicrobial Activity
2.5. Analysis of Phenolic Composition
2.6. Scanning Electron Microscopy (SEM)
2.7. Statistical Analysis of the Results
3. Results
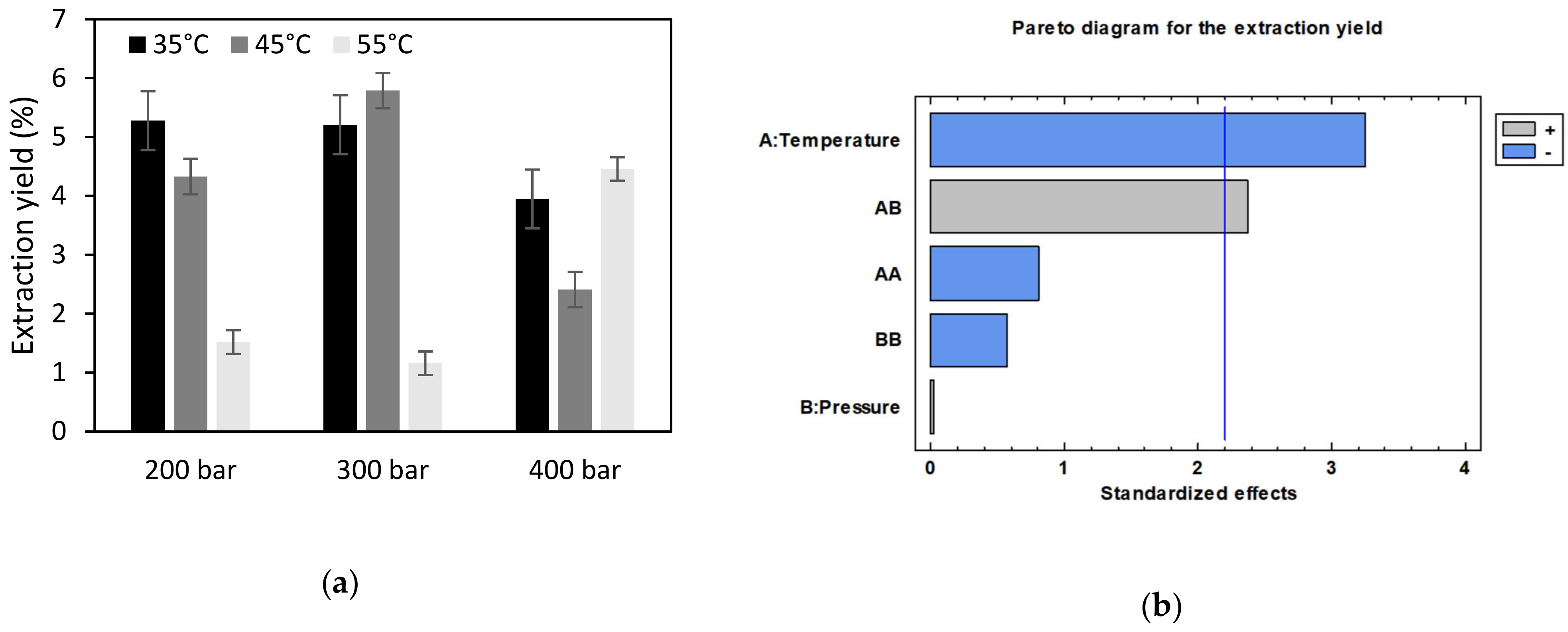
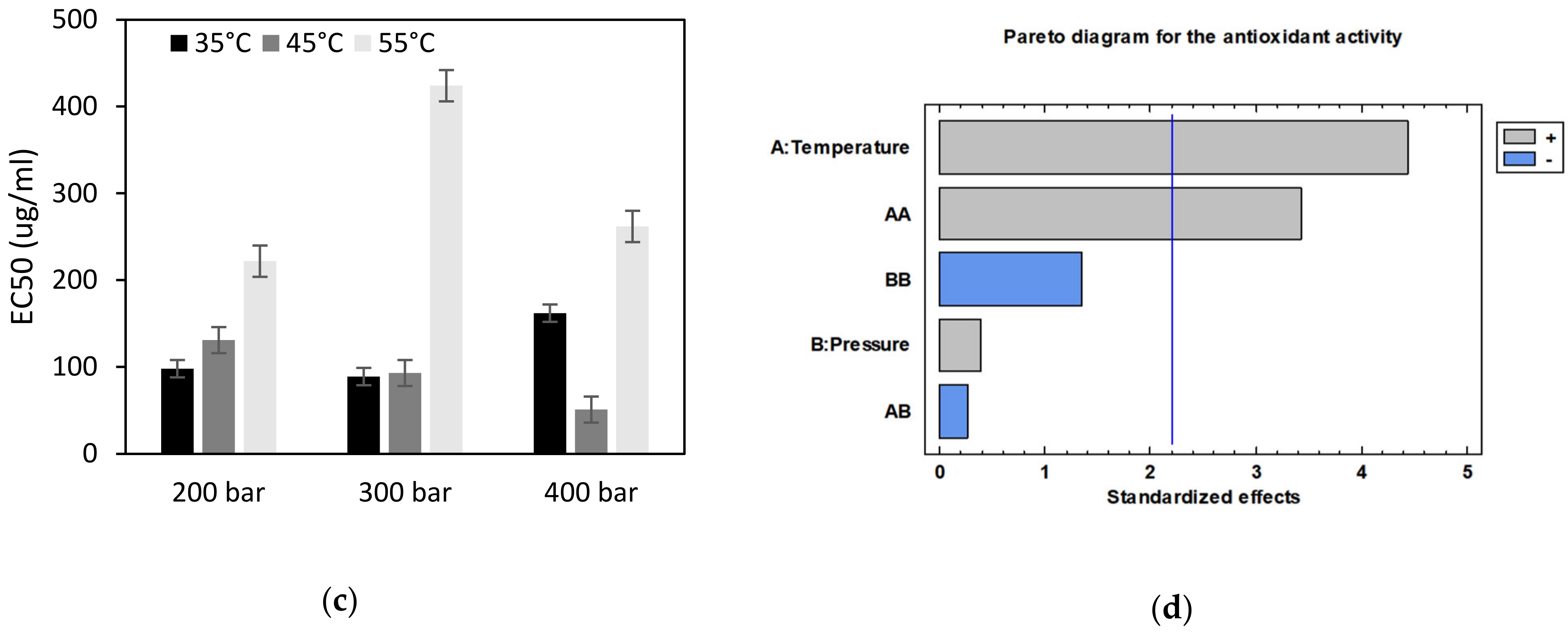
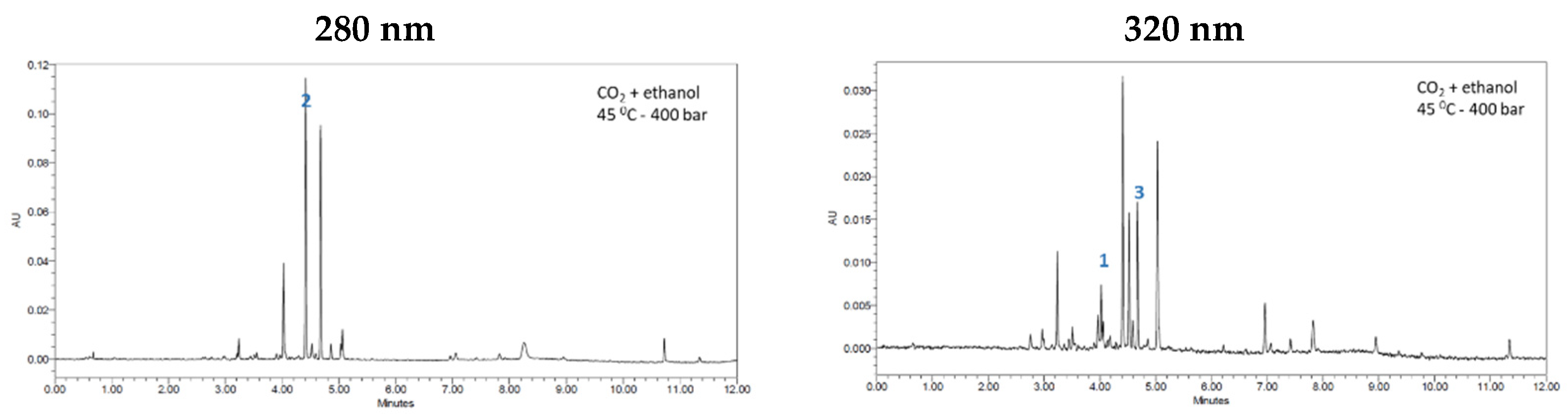
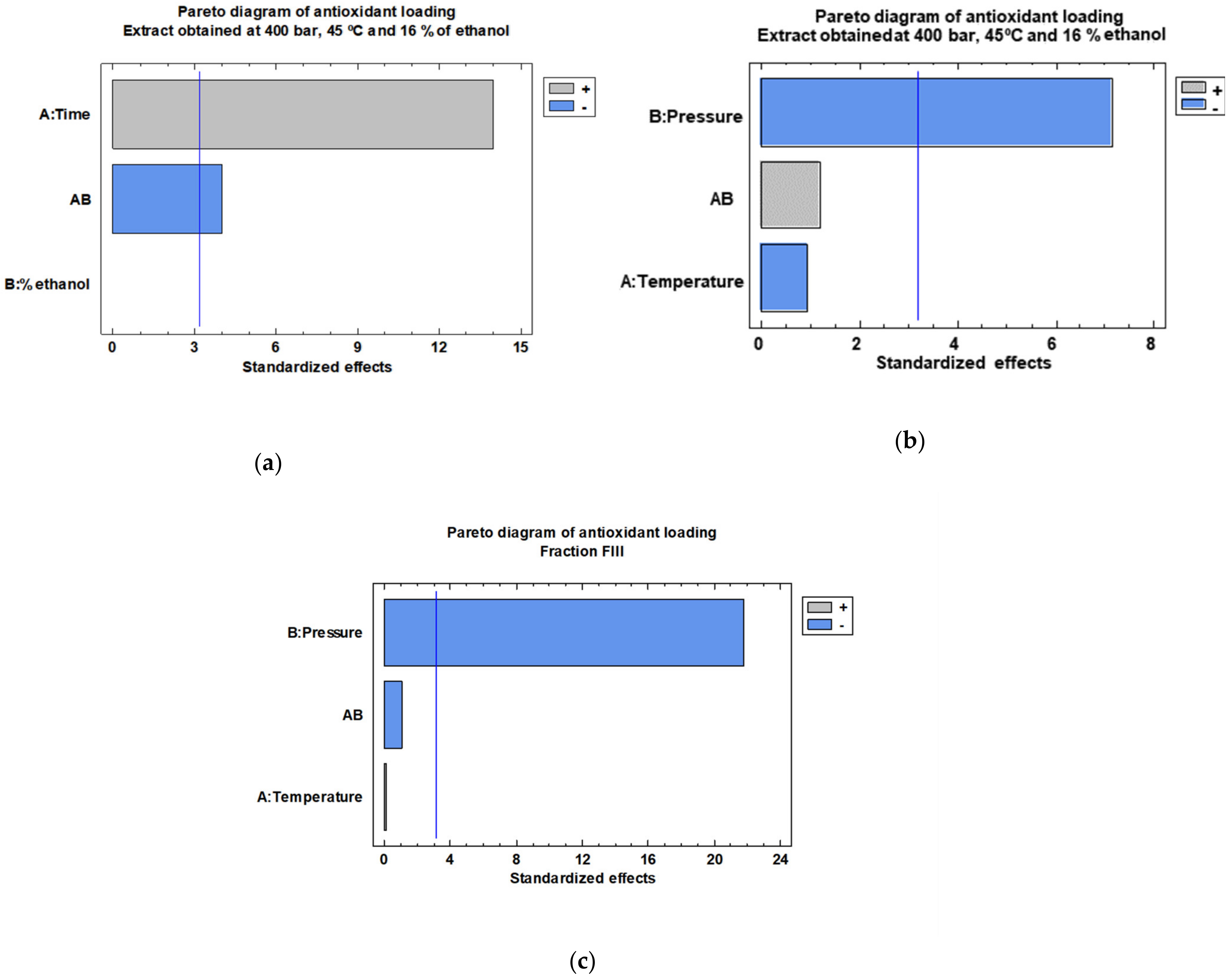
3.1. Extraction of Bioactive Compounds from Orange Peels
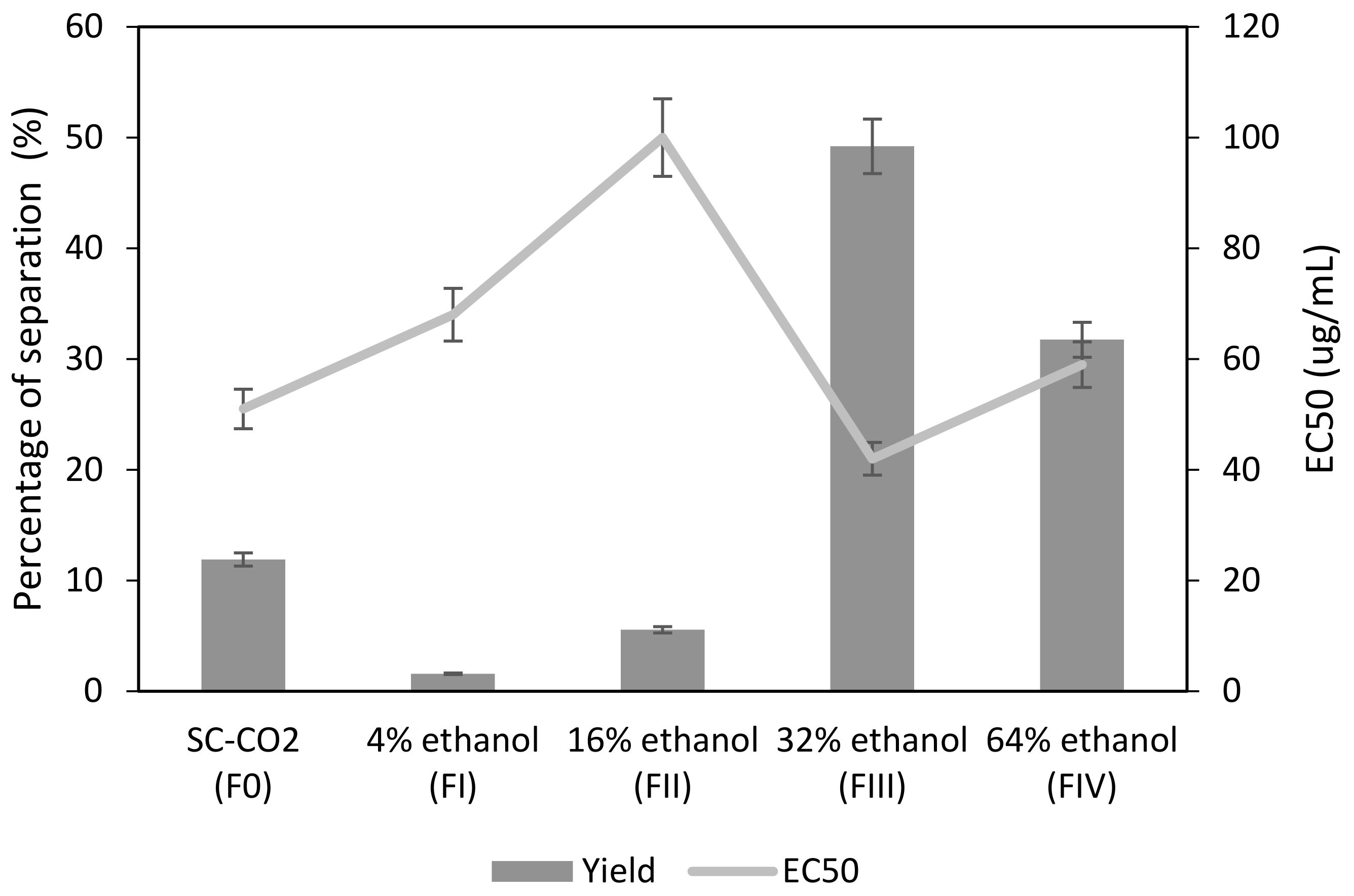
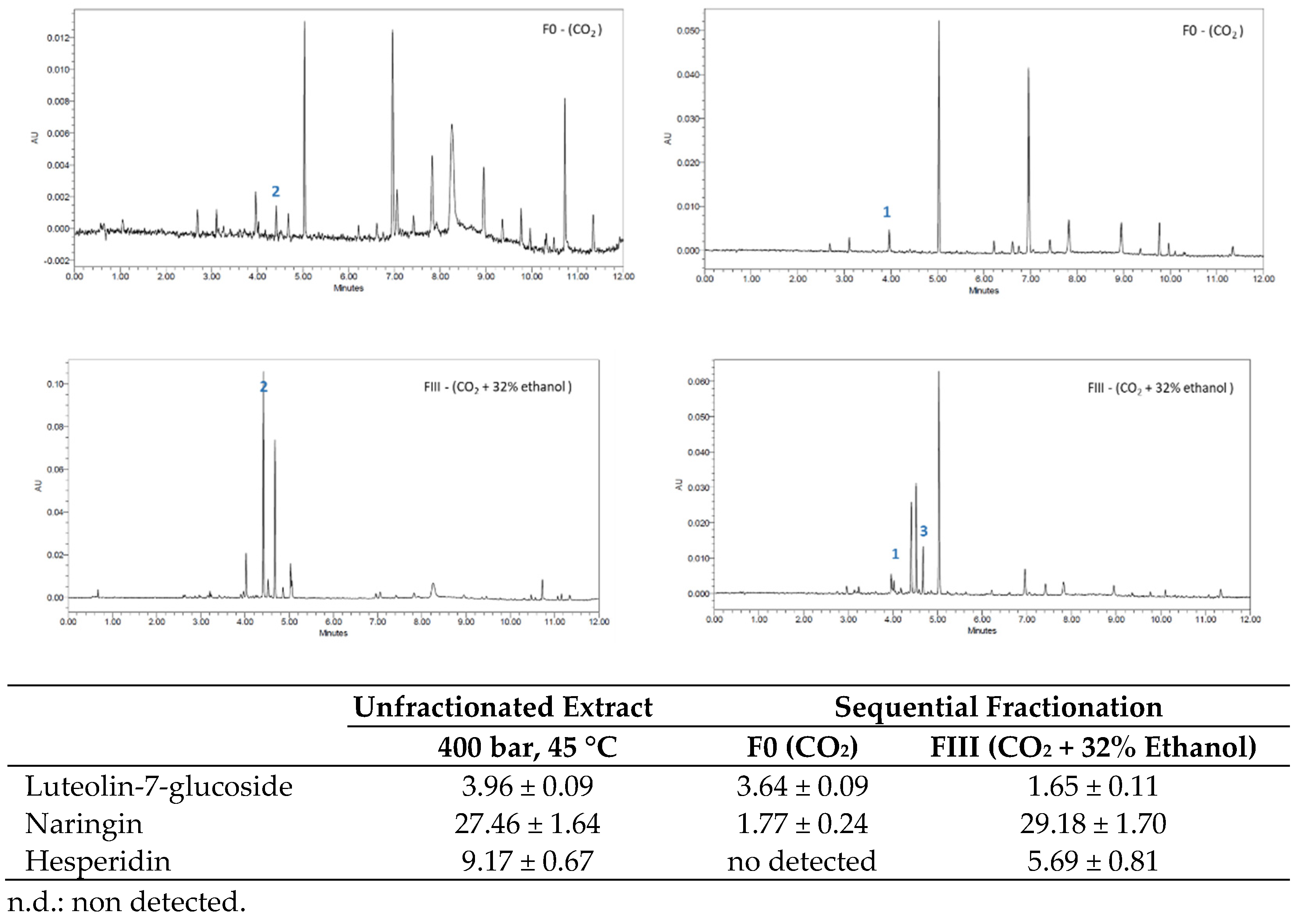
3.2. Sequential Fractionation of the Orange Peel Extract
3.3. Impregnation of the Orange Peel Extract and the Purified Fraction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Satari, B.; Karimi, K. Citrus processing wastes: Environmental impacts, recent advances, and future perspectives in total valorization. Resour. Conserv. Recycl. 2018, 129, 153–167. [Google Scholar] [CrossRef]
- Paggiola, G.; Van Stempvoort, S.; Bustamante, J.; Vega Barbero, J.M.; Hunt, A.J.; Clark, J.H. Can bio-based chemicals meet demand? Global and regional case-study around citrus waste-derived limonene as a solvent for cleaning applications. Biofuels Bioprod. Biorefin. 2016, 10, 686–698. [Google Scholar] [CrossRef]
- Zema, D.A.; Calabrò, P.S.; Folino, A.; Tamburino, V.; Zappia, G.; Zimbone, S.M. Valorisation of citrus processing waste: A review. Waste Manag. 2018, 80, 252–273. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Mahato, N.; Moo, D.; Cho, H.; Yong, D.; Lee, R. Converting citrus wastes into value-added products: Economic and environmently friendly approaches. Nutrition 2017, 34, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, S.; Kaul, R.; Sofi, S.A.; Bashir, N.; Nazir, F.; Nayik, G.A. Citrus peel as a source of functional ingredient: A review. J. Saudi Soc. Agric. Sci. 2018, 17, 351–358. [Google Scholar] [CrossRef] [Green Version]
- Putnik, P.; Bursać Kovačević, D.; Režek Jambrak, A.; Barba, F.J.; Cravotto, G.; Binello, A.; Lorenzo, J.M.; Shpigelman, A. Innovative “green” and novel strategies for the extraction of bioactive added value compounds from citrus wastes—A review. Molecules 2017, 22, 680. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, S.S.; Khodaiyan, F.; Yarmand, M.S. Aqueous extraction of pectin from sour orange peel and its preliminary physicochemical properties. Int. J. Biol. Macromol. 2016, 82, 920–926. [Google Scholar] [CrossRef]
- Mahato, N.; Sharma, K.; Sinha, M.; Cho, M.H. Citrus waste derived nutra-/pharmaceuticals for health benefits: Current trends and future perspectives. J. Funct. Foods 2018, 40, 307–316. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Du, W.X.; de Jesús Avena-Bustillos, R.; Soares, N.D.F.F.; McHugh, T.H. Edible films from pectin: Physical-mechanical and antimicrobial properties—A review. Food Hydrocoll. 2014, 35, 287–296. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, D.; Chen, J.; Ye, X.; Yu, D. Effects of different factors of ultrasound treatment on the extraction yield of the all-trans-β-carotene from citrus peels. Ultrason. Sonochem. 2011, 18, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Montero-Calderon, A.; Cortes, C.; Zulueta, A.; Frigola, A.; Esteve, M.J. Green solvents and Ultrasound-Assisted Extraction of bioactive orange (Citrus sinensis) peel compounds. Sci. Rep. 2019, 9, 16120. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.; Dahmoune, F.; Moussi, K.; Remini, H.; Dairi, S.; Aoun, O.; Khodir, M. Comparison of microwave, ultrasound, and accelerated-assisted solvent extraction for recovery of polyphenols from Citrus sinensis peels. Food Chem. 2015, 187, 507–516. [Google Scholar] [CrossRef]
- Dahmoune, F.; Boulekbache, L.; Moussi, K.; Aoun, O.; Spigno, G.; Madani, K. Valorization of Citrus limon residues for the recovery of antioxidants: Evaluation and optimization of microwave and ultrasound application to solvent extraction. Ind. Crops Prod. 2013, 50, 77–87. [Google Scholar] [CrossRef]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Sharmila, G.; Nikitha, V.S.; Ilaiyarasi, S.; Dhivya, K.; Rajasekar, V.; Kumar, N.M.; Muthukumaran, K.; Muthukumaran, C. Ultrasound assisted extraction of total phenolics from Cassia auriculata leaves and evaluation of its antioxidant activities. Ind. Crops Prod. 2016, 84, 13–21. [Google Scholar] [CrossRef]
- Del Valle, J.M.; Calderón, D.; Núñez, G.A. Pressure drop may negatively impact supercritical CO2 extraction of citrus peel essential oils in an industrial-size extraction vessel. J. Supercrit. Fluids 2019, 144, 108–121. [Google Scholar] [CrossRef]
- Salehi, H.; Karimi, M.; Raofie, F. Micronization and coating of bioflavonoids extracted from Citrus sinensis L. peels to preparation of sustained release pellets using supercritical technique. J. Iran. Chem. Soc. 2021, 18, 3235–3248. [Google Scholar] [CrossRef]
- Tsitsagi, M.; Ebralidze, K.; ChkhaidzeImeda, M.; Rubashvili, I.; Tsitsishvili, V. Sequential extraction of bioactive compounds from tangerine (Citrus Unshiu) peel. Ann. Agrar. Sci. 2018, 16, 236–241. [Google Scholar] [CrossRef]
- Šafranko, S.; Ćorković, I.; Jerković, I.; Jakovljević, M.; Aladić, K.; Šubarić, D.; Jokić, S. Green Extraction Techniques for Obtaining Bioactive Compounds from Mandarin Peel (Citrus unshiu var. Kuno): Phytochemical Analysis and Process Optimization. Foods 2021, 10, 1043. [Google Scholar] [CrossRef]
- Jokić, S.; Molnar, M.; Cikoš, A.-M.; Jakovljević, M.; Šafranko, S.; Jerković, I. Separation of selected bioactive compounds from orange peel using the sequence of supercritical CO2 extraction and ultrasound solvent extraction: Optimization of limonene and hesperidin content. Sep. Sci. Technol. 2020, 55, 2799–2811. [Google Scholar] [CrossRef]
- Mahato, N.; Sharma, K.; Koteswararao, R.; Sinha, M.; Baral, E.; Cho, M.H. Citrus essential oils: Extraction, authentication and application in food preservation. Crit. Rev. Food Sci. Nutr. 2017, 59, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, M.; Floros, J.D. Active food packaging technologies. Crit. Rev. Food Sci. Nutr. 2004, 44, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zuo, G.; Chen, F. Effect of essential oil and surfactant on the physical and antimicrobial properties of corn and wheat starch films. Int. J. Biol. Macromol. 2018, 107, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Santos, R.; Motta, J.F.G.; Teodoro, C.E.S.; Melo, N.R. Antimicrobial effectiveness and color stability of protein-based films incorporated with essential oils. Int. Food Res. J. 2017, 24, 2201–2206. [Google Scholar]
- Oluwasina, O.O.; Awonyemi, I.O. Citrus peel extract starch-based bioplastic: Effect of extract concentration on packed fish and bioplastic properties. J. Polym. Environ. 2021, 29, 1706–1716. [Google Scholar] [CrossRef]
- Khalid, S.; Yu, L.; Feng, M.; Meng, L.; Bai, Y.; Ali, A.; Liu, H.; Chen, L. Development and characterization of biodegradable antimicrobial packaging films based on polycaprolactone, starch and pomegranate rind hybrids. Food Packag. Shelf Life 2018, 18, 71–79. [Google Scholar] [CrossRef]
- Rodríguez, F.J.; Torres, A.; Peñaloza, A.; Sepulveda, H.; Galotto, M.J.; Guarda, A.; Bruna, J. Development of an antimicrobial material based on a nanocomposite cellulose acetate film for active food packaging. Food Addit. Contam. A 2014, 31, 342–353. [Google Scholar] [CrossRef]
- Hauser, C.; Wunderlich, J. Antimicrobial packaging films with a sorbic acid based coating. Procedia Food Sci. 2011, 1, 197–202. [Google Scholar] [CrossRef]
- Zhang, W.; Ronca, S.; Mele, E. Electrospun nanofibres containing antimicrobial plant extracts. Nanomaterials 2017, 7, 42. [Google Scholar] [CrossRef] [Green Version]
- Torres, A.; Ilabaca, E.; Rojas, A.; Rodríguez, F.; Galotto, M.J.; Guarda, A.; Villegas, C.; Romero, J. Effect of processing conditions on the physical, chemical and transport properties of polylactic acid films containing thymol incorporated by supercritical impregnation. Eur. Polym. J. 2017, 89, 195–210. [Google Scholar] [CrossRef]
- Bastante, C.C.; Silva, N.H.; Cardoso, L.C.; Serrano, C.M.; de la Ossa, E.J.M.; Freire, C.S.; Vilela, C. Biobased films of nanocellulose and mango leaf extract for active food packaging: Supercritical impregnation versus solvent casting. Food Hydrocoll. 2021, 117, 106709. [Google Scholar] [CrossRef]
- Cejudo, C.; Arjona-Mudarra, P.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; de la Ossa, E.J.M.; Pereyra, C. Application of a natural antioxidant from grape pomace extract in the development of bioactive jute fibers for food packaging. Antioxidants 2021, 10, 216. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, G.A.; Bezerra, F.W.F.; de Oliveira, M.S.; da Costa, W.A.; de Carvalho Junior, R.N.; Joele, M.R.S.P. Supercritical CO2 Impregnation of Piper divaricatum Essential Oil in Fish (Cynoscion acoupa) Skin Gelatin Films. Food Bioproc. Technol. 2020, 13, 1765–1777. [Google Scholar] [CrossRef]
- Liu, X.; Jia, J.; Duan, S.; Zhou, X.; Xiang, A.; Lian, Z.; Ge, F. Zein/MCM-41 Nanocomposite Film Incorporated with Cinnamon Essential Oil Loaded by Modified Supercritical CO2 Impregnation for Long-Term Antibacterial Packaging. Pharmaceutics 2020, 12, 169. [Google Scholar] [CrossRef] [Green Version]
- Medeiros, G.R.; Ferreira, S.R.S.; Carciofi, B.A.M. High pressure carbon dioxide for impregnation of clove essential oil in LLDPE films. Innov. Food Sci. Emerg. Technol. 2018, 41, 206–215. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Gabrielson, J.; Hart, M.; Jarelöv, A.; Kühn, I.; McKenzie, D.; Möllby, R. Evaluation of redox indicators and the use of digital scanners and spectrophotometer for quantification of microbial growth in microplates. J. Microbiol. Methods 2002, 50, 63–73. [Google Scholar] [CrossRef]
- Moussa, S.H.; Tayel, A.A.; Al-Hassan, A.A.; Farouk, A. Tetrazolium/Formazan Test as an Efficient Method to Determine Fungal Chitosan Antimicrobial Activity. J. Mycol. 2013, 2013, 753692. [Google Scholar] [CrossRef]
- Shehata, M.; Awad, T.; Asker, D.; El Sohaimy, S.; Abd El-Aziz, N.M.; Youssef, M.M. Antioxidant and antimicrobial activities and UPLC-ESI-MS/MS polyphenolic profile of sweet orange peel extracts. Curr. Res. Food Sci. 2021, 4, 326–335. [Google Scholar] [CrossRef]
- Oliveira, D.A.; Salvador, A.A.; Smânia, A., Jr.; Smânia, E.F.; Maraschin, M.; Ferreira, S.R. Antimicrobial activity and composition profile of grape (Vitis vinifera) pomace extracts obtained by supercritical fluids. J. Biotechnol. 2013, 164, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Seabra, I.J.; Braga, M.E.; Batista, M.T.; de Sousa, H.C. Effect of solvent (CO2/ethanol/H2O) on the fractionated enhanced solvent extraction of anthocyanins from elderberry pomace. J. Supercrit. Fluids 2010, 54, 145–152. [Google Scholar] [CrossRef]
- Fuentes-Gandara, F.; Torres, A.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; Varela, R.; de la Ossa-Fernández, E.J.M.; Macías, F.A. Selective fractionation and isolation of allelopathic compounds from Helianthus annuus L. leaves by means of high-pressure techniques. J. Supercrit. Fluids 2019, 143, 32–41. [Google Scholar] [CrossRef]
- Grosso, C.; Figueiredo, A.C.; Burillo, J.; Mainar, A.M.; Urieta, J.S.; Barroso, J.G.; Coelho, J.A.; Palabra, A.M.F. Composition and antioxidant activity of Thymus vulgaris volatiles: Comparison between supercritical fluid extraction and hydrodestillation. J. Sep. Sci. 2010, 33, 2211–2218. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pan, H.; Hao, S.; Pan, D.; Wang, G.; Yu, W. Evaluation of phenolic composition and antioxidant properties of different varieties of Chinese citrus. Food Chem. 2021, 364, 130413. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Breksa, A.P., III; Pan, Z.; Ma, H. Quantitative determination of major polyphenol constituents in pomegranate products. Food Chem. 2012, 132, 1585–1591. [Google Scholar] [CrossRef]
- Selahvarzi, A.; Ramezan, Y.; Sanjabi, M.R.; Mirsaeedghazi, H.; Azarikia, F.; Abedinia, A. Investigation of antimicrobial activity of orange and pomegranate peels extracts and their use as a natural preservative in a functional beverage. Food Meas. 2021, 15, 5683–5694. [Google Scholar] [CrossRef]
- Rakholiya, K.; Kaneria, M.; Chanda, S. Inhibition of microbial pathogens using fruit and vegetable peel extracts. Int. J. Food Sci. Nutr. 2014, 65, 733–739. [Google Scholar] [CrossRef]
- Jakovljević, M.; Jokić, S.; Molnar, M.; Jašić, M.; Babić, J.; Jukić, H.; Banjari, I. Bioactive Profile of Various Salvia officinalis L. Preparations. Plants 2019, 8, 55. [Google Scholar] [CrossRef] [Green Version]
- Ndayishimiye, J.; Lim, D.; Chun, B. Antioxidant and antimicrobial activity of oils obtained from a mixture of citrus by-products using a modified supercritical carbon dioxide. J. Ind. Eng. Chem. 2018, 57, 339–348. [Google Scholar] [CrossRef]
- García-Casas, I.; Crampon, C.; Montes, A.; Pereyra, C.; de la Ossa, E.J.M.; Badens, E. Supercritical CO2 impregnation of silica microparticles with quercetin. J. Supercrit. Fluids 2019, 143, 157–161. [Google Scholar] [CrossRef]
- Cejudo Bastante, C.; Casas Cardoso, L.; Fernández-Ponce, M.T.; Mantell Serrano, C.; de la Ossa, E.J.M. Supercritical impregnation of olive leaf extract to obtain bioactive films effective in cherry tomato preservation. Food Packag. Shelf Life 2019, 21, 100338. [Google Scholar] [CrossRef]
- Belizón, M.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; de la Ossa-Fernández, E.J.M. Supercritical impregnation of antioxidant mango polyphenols into a multilayer PET/PP food-grade film. J. CO2 Util. 2018, 25, 56–67. [Google Scholar] [CrossRef]
- Cejudo Bastante, C.; Casas Cardoso, L.; Mantell Serrano, C.; de la Ossa, E.J.M. Supercritical impregnation of food packaging films to provide antioxidant properties. J. Supercrit. Fluids 2017, 128, 200–207. [Google Scholar] [CrossRef]
- Fernández Ponce, M.T.; Cejudo Bastante, C.; Casas Cardoso, L.; Mantell, C.; Martínez de la Ossa, E.J.; Pereyra, C. Potential Use of Annona Genus Plants Leaf Extracts to Produce Bioactive Transdermal Patches by Supercritical Solvent Impregnation. Antioxidants 2021, 10, 1196. [Google Scholar] [CrossRef] [PubMed]


| Molecular formula | -(C3H6)-n |
| Semi-crystalline density | 0.95 g/cm3 |
| Melting point | 173 °C |
| Degradation temperature | 287 °C |
| Pressure (bar) | Temperature (°C) | MIC (µg/mL) |
|---|---|---|
| 200 | 35 | n.d. |
| 300 | n.d. | |
| 400 | 1267 ± 4.7 | |
| 200 | 45 | n.d. |
| 300 | 806 ± 2.8 | |
| 400 | 100 ± 1.1 |
| Impregnation Conditions | Extract Loading (mg Extract/100 mg Film) | |
|---|---|---|
| Extract (400 bar, 45 °C) | Fraction (FIII) (300 bar, 45 °C) | |
| 200 bar-35 °C, 1 h-1% ethanol | 0.96 ± 0.12 | 0.96 ± 0.20 |
| 200 bar-35 °C, 1 h-2% ethanol | 1.11 ± 0.18 | 0.93 ± 0.19 |
| 200 bar-35 °C, 3 h-1% ethanol | 1.56 ± 0.15 | 0.90 ± 0.12 |
| 200 bar-35 °C, 3 h-2% ethanol | 1.71 ± 0.12 | 0.92 ± 0.14 |
| 200 bar-55 °C, 3 h-1% ethanol | 1.32 ± 0.34 | 0.93 ± 0.19 |
| 400 bar-35 °C, 3 h-1% ethanol | 0.67 ± 0.29 | 0. 51 ± 0.16 |
| 400 bar-55 °C, 3 h-1% ethanol | 0.75 ± 0.22 | 0.45 ± 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casas Cardoso, L.; Cejudo Bastante, C.; Mantell Serrano, C.; Martínez de la Ossa, E.J. Application of Citrus By-Products in the Production of Active Food Packaging. Antioxidants 2022, 11, 738. https://doi.org/10.3390/antiox11040738
Casas Cardoso L, Cejudo Bastante C, Mantell Serrano C, Martínez de la Ossa EJ. Application of Citrus By-Products in the Production of Active Food Packaging. Antioxidants. 2022; 11(4):738. https://doi.org/10.3390/antiox11040738
Chicago/Turabian StyleCasas Cardoso, Lourdes, Cristina Cejudo Bastante, Casimiro Mantell Serrano, and Enrique J. Martínez de la Ossa. 2022. "Application of Citrus By-Products in the Production of Active Food Packaging" Antioxidants 11, no. 4: 738. https://doi.org/10.3390/antiox11040738






