Utilizing Proteomic Approach to Analyze Potential Antioxidant Proteins in Plant against Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions and Gamma-Ray Irradiation
2.2. Protein Extraction
2.3. Protein Concentration Measurement
2.4. Determination of Malondialdehyde Concentration
2.5. Determination of Flavonoids Content
2.6. Free Radical Scavenging Activity Using DPPH
2.7. Protein Identification by Mass Spectrometry
2.8. Quality Control of Mass Spectrum
2.9. Proteomic Identification by Database Searching
2.10. Statistical Analysis
3. Results and Discussions
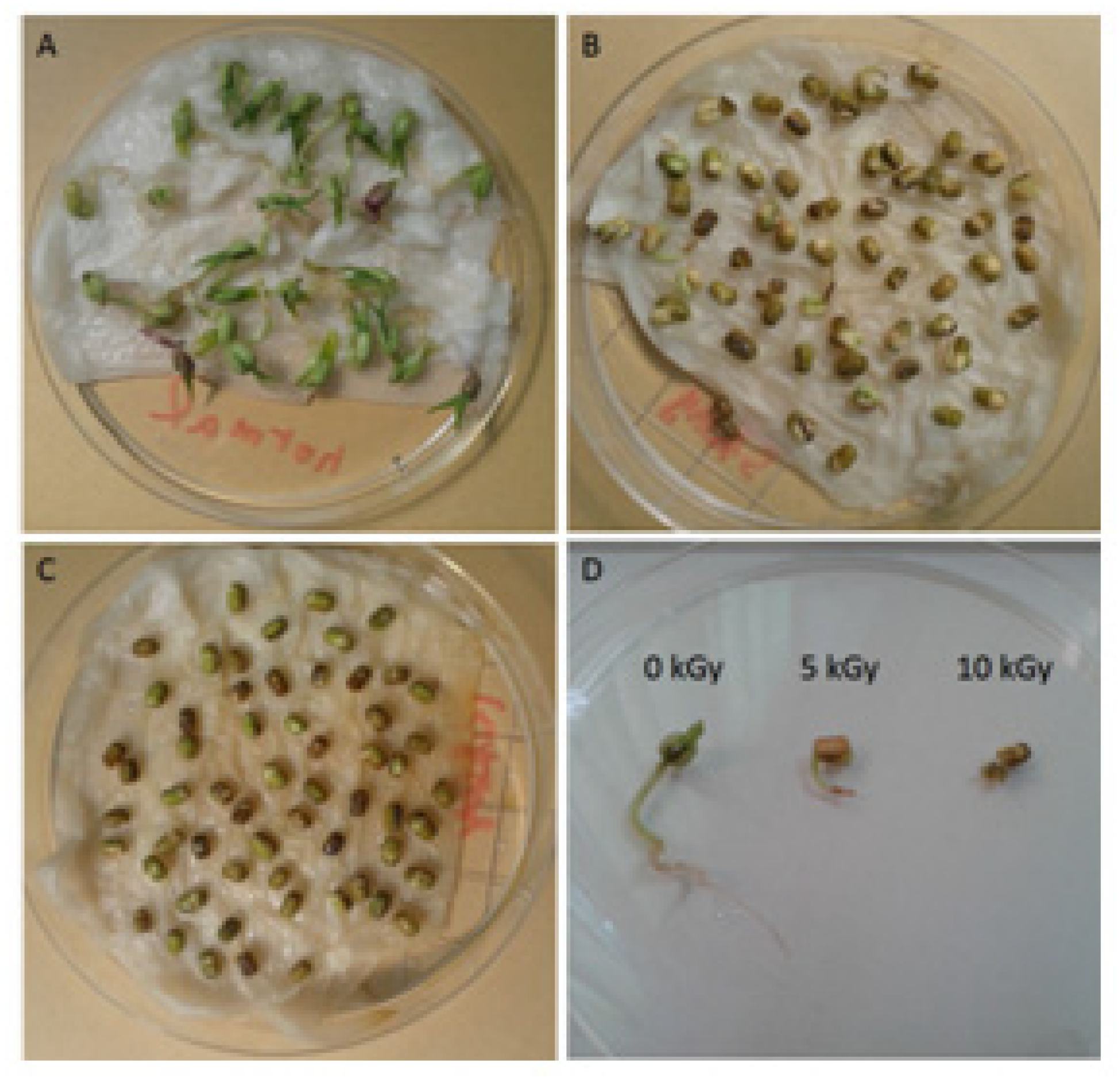
3.1. Plant Appearance and Growth Condition
3.2. Lipid Peroxidation, Flavonoid Content and Free Radical Scavenging Activity
3.3. Functional Classification and Subcellular Localization Prediction of Gamma Ray Responsive Proteins
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baalamurugan, J.; Ganesh Kumar, V.; Chandrasekaran, S.; Balasundar, S.; Venkatraman, B.; Padmapriya, R.; Bupesh Raja, V.K. Utiliza-tion of induction furnace steel slag in concrete as coarse aggregate for gamma radiation shielding. J. Hazard Mater. 2019, 369, 561–568. [Google Scholar] [CrossRef]
- Dixit, A.K.; Bhatnagar, D.; Kumar, V.; Rani, A.; Manjaya, J.G.; Bhatnagar, D. Gamma Irradiation Induced Enhancement in Isoflavones, Total Phenol, Anthocyanin and Antioxidant Properties of Varying Seed Coat Colored Soybean. J. Agric. Food Chem. 2010, 58, 4298–4302. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Rizhsky, L.; Hegie, A.; Koussevitzky, S.; Mittler, R. Double mutants deficient in cytosolic and thylakoid ascor-bate peroxidase reveal a complex mode of interaction between reactive oxygen species, plant development, and response to abiotic stresses. Plant Physiol. 2007, 144, 1777–1785. [Google Scholar] [CrossRef] [Green Version]
- Scandalios, J.G. Oxidative stress and defence mechanisms in plants: Introduction. Free Radic. Biol. Med. 1997, 23, 471–472. [Google Scholar] [CrossRef]
- Brosché, M.; Strid, Å. Molecular events following perception of ultraviolet-B radiation by plants. Physiol. Plant. 2003, 117, 1–10. [Google Scholar] [CrossRef]
- Jansen, M.A.K.; Gaba, V.; Greenberg, B.M. Higher plant and UV-B radiation: Balancing damage, repair and acclimation. Trends Plant Sci. 1998, 3, 131–135. [Google Scholar] [CrossRef]
- Santos, I.; Fidalgo, F.; Almeida, J.M.; Salema, R. Biochemical and ultrastructural changes in leaves of potato plants grown under supplementary UV-B radiation. Plant Sci. 2004, 167, 925–935. [Google Scholar] [CrossRef]
- Danon, A.; Gallois, P. UV-C radiation induces apoptotic-like changes in Arabidopsis thaliana. FEBS Lett. 1998, 437, 131–136. [Google Scholar] [CrossRef]
- Hanaealt, P. Essentials of Molecular Biology; Jones & Barlett Learning: Burlington, MA, USA, 1993; pp. 145–157. [Google Scholar]
- Yun, J.; Li, X.; Fan, X.; Tang, Y.; Xiao, Y.; Wan, S. Effect of gamma irradiation on microbial load, physicochemical and sensory characteristics of soybeans (Glycine max L. Merrill). Radiat. Phys. Chem. 2012, 81, 1198–1202. [Google Scholar] [CrossRef]
- Khattak, K.F.; Simpson, T.J.; Ihasnulla. Effect of gamma irradiation on the extraction yield, total phenolic content and free radi-cal-scavenging activity of Nigella staiva seed. Food Chem. 2008, 110, 967–972. [Google Scholar] [CrossRef]
- Pagano, E.A.; Chueca, A.; López-Gorgé, J. Expression of thioredoxins f and m, and of their targets fructose-1,6-bisphosphatase and NADP-malate dehydrogenase, in pea plants grown under normal and light/temperature stress conditions. J. Exp. Bot. 2000, 51, 1299–1307. [Google Scholar] [PubMed]
- Zhang, Y.; Yang, J.; Lu, S.; Cai, J.; Guo, Z. Overexpressing SgNCED1 in Tobacco Increases ABA Level, Antioxidant Enzyme Activities, and Stress Tolerance. J. Plant Growth Regul. 2008, 27, 151–158. [Google Scholar] [CrossRef]
- Barka, E.A. Protective enzymes against reactive oxygen species during ripening of tomato (Lycopersicon esculentum) fruits in response to low amounts of UV-C. Funct. Plant Biol. 2001, 28, 785–791. [Google Scholar] [CrossRef]
- Navarro, S.; León, M.; Roca-Pérez, L.; Boluda, R.; García-Ferriz, L.; Pérez-Bermúdez, P.; Gavidia, I. Characterisation of Bobal and Crujidera grape cultivars, in comparison with Tempranillo and Cabernet Sauvignon: Evolution of leaf macronutrients and berry composition during grape ripening. Food Chem. 2008, 108, 182–190. [Google Scholar] [CrossRef]
- Graves, P.R.; Haystead, T.A. Molecular biologist’s guide to proteomics. Microbiol. Mol. Biol. Rev. 2002, 66, 39–63. [Google Scholar] [CrossRef] [Green Version]
- Aebersold, R.; Goodlett, D.R. Mass spectrometry in proteomics. Chem. Rev. 2001, 101, 269–295. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, T.; Tang, K.; Liu, R.H. Effect of Germination on Phytochemical Profiles and Antioxidant Activity of Mung Bean Sprouts (Vigna radiata). J. Agric. Food Chem. 2012, 60, 11050–11055. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.J.; Kim, D.Y.; Jo, Y.D.; Choi, H.-I.; Ahn, J.-W.; Kwon, S.-J.; Kim, S.H.; Seo, Y.W.; Kim, J.-B. Biological Effect of Gamma Rays According to Exposure Time on Germination and Plant Growth in Wheat. Appl. Sci. 2022, 12, 3208. [Google Scholar] [CrossRef]
- Nasr, G.M.; Taha, E.-K.A.; Hamza, A.M.; Negm, E.A.; Eryan, N.L.; Noureldeen, A.; Darwish, H.; Zayed, M.S.; Elnabawy, E.-S.M. Gamma Radiation: An Eco-Friendly Control Method for the Rice Weevil, Sitophilus oryzae (L.) (Coleoptera: Curculionidae). Biology 2022, 11, 1295. [Google Scholar] [CrossRef]
- Schneider, M.; Tognolli, M.; Bairoch, A. The Swiss-Prot protein knowledgebase and ExPASy: Providing the plant community with high quality proteomic data and tools. Plant Physiol. Biochem. 2004, 42, 1013–1021. [Google Scholar] [CrossRef]
- Sengupta, M.; Chakraborty, A.; Raychaudhuri, S.S. Ionizing radiation induced changes in phenotype, photosynthetic pigments and free polyamine levels in Vigna radiata (L.) Wilczek. Appl. Radiat. Isot. 2013, 75, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Kovács, E.; Keresztes, Á. Effect of gamma and UV-B/C radiation on plant cells. Micron 2002, 33, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Robak, J.; Gryglewski, R.J. Flavonoids are scavengers of superoxide anions. Biochem. Pharmacol. 1988, 37, 837–841. [Google Scholar] [CrossRef]
- Okawa, M.; Kinjo, J.; Nohara, T.; Ono, M. DPPH (1,1-Diphenyl-2-Picrylhydrazyl) Radical Scavenging Activity of Flavonoids Obtained from Some Medicinal Plants. Biol. Pharm. Bull. 2001, 24, 1202–1205. [Google Scholar] [CrossRef] [Green Version]
- St. Angelo, A.J. Lipid oxidation on foods. Crit. Rev. Food Sci. Nutr. 1996, 36, 175–224. [Google Scholar] [CrossRef]
- Gicquel, M.; Taconnat, L.; Renou, J.P.; Esnault, M.A.; Cabello-Hurtado, F. Kinetic transcriptomic approach revealed metabolic pathways and genotoxic-related changes implied in the Arabidopsis response to ionising radiations. Plant Sci. 2012, 195, 106–119. [Google Scholar] [CrossRef]
- Suorsa, M.; Grieco, M.; Järvi, S.; Gollan, P.J.; Kangasjärvi, S.; Tikkanen, M.; Aro, E.M. PGR5 ensures photosynthetic control to safeguard photosystem I under fluctuating light conditions. Plant. Signal Behav. 2013, 8, e22741. [Google Scholar] [CrossRef] [Green Version]
- Müller-Moulé, P.; Golan, T.; Niyogi, K.K. Ascorbate-Deficient Mutants of Arabidopsis Grow in High Light Despite Chronic Photooxidative Stress. Plant Physiol. 2004, 134, 1163–1172. [Google Scholar] [CrossRef] [Green Version]
- Scheer, J.M.; Ryan, C.A., Jr. The systemin receptor SR160 from Lycopersicon peruvianum is a member of the LRR receptor kinase family. Proc. Natl. Acad. Sci. USA 2002, 99, 9585–9590. [Google Scholar] [CrossRef]
- Malinowski, R.; Higgins, R.; Luo, Y.; Piper, L.; Nazir, A.; Bajwa, V.S.; Clouse, S.D.; Thompson, P.R.; Stratmann, J.W. The tomato brassinosteroid receptor BRI1 increases binding of systemin to tobacco plasma membranes, but is not involved in systemin signaling. Plant Mol. Biol. 2009, 70, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Einig, E.; Almeida-Trapp, M.; Albert, M.; Fliegmann, J.; Mithöfer, A.; Kalbacher, H.; Felix, G. The systemin receptor SYR1 enhances resistance of tomato against herbivorous insects. Nat. Plants 2018, 4, 152–156. [Google Scholar] [CrossRef] [PubMed]
- She, J.; Han, Z.; Kim, T.W.; Wang, J.; Cheng, W.; Chang, J.; Shi, S.; Wang, J.; Yang, M.; Wang, Z.Y.; et al. Structural insight into brassinosteroid perception by BRI1. Nature 2011, 474, 472–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandoth, P.K.; Ranf, S.; Pancholi, S.S.; Jayanty, S.; Walla, M.D.; Miller, W.; Howe, G.A.; Lincoln, D.E.; Stratmann, J.W. Tomato MAPKs LeMPK1, LeMPK2, and LeMPK3 function in the systemin-mediated defense response against herbivorous insects. Proc. Natl. Acad. Sci. USA 2007, 104, 12205–12210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweighofer, A.; Meskiene, I. Regulation of stress hormones jasmonates and ethylene by MAPK pathways in plants. Mol. BioSyst. 2008, 4, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.T.; Mattheis, J.P.; Fellman, J.K.; Patterson, M.E. Changes in jasmonic acid concentration during early development of apple fruit. Physiol. Plant. 1997, 101, 328–332. [Google Scholar] [CrossRef]
- Willige, B.C.; Ghosh, S.; Nill, C.; Zourelidou, M.; Dohmann, E.M.; Maier, A.; Schwechheimer, C. The DELLA domain of GA INSENSITIVE mediates the interaction with the GA INSENSITIVE DWARF1A gibberellin receptor of Arabidopsis. Plant Cell 2007, 19, 1209–1220. [Google Scholar] [CrossRef] [Green Version]
- Achard, P.; Vriezen, W.H.; Van Der Straeten, D.; Harberd, N.P. Ethylene Regulates Arabidopsis Development via the Modulation of DELLA Protein Growth Repressor Function. Plant Cell 2003, 15, 2816–2825. [Google Scholar] [CrossRef] [Green Version]
- Hirano, K.; Ueguchi-Tanaka, M.; Matsuoka, M. GID1-mediated gibberellin signaling in plants. Trends Plant Sci. 2008, 13, 192–199. [Google Scholar] [CrossRef]
- Thornsberry, J.M.; Goodman, M.M.; Doebley, J.; Kresovich, S.; Nielsen, D.; Buckler, E.S., IV. Dwarf8 polymorphisms associate with variation in flowering time. Nat. Genet. 2001, 28, 286–289. [Google Scholar] [CrossRef]
- Camus-Kulandaivelu, L.; Veyrieras, J.-B.; Madur, D.; Combes, V.; Fourmann, M.; Barraud, S.; Dubreuil, P.; Gouesnard, B.; Manicacci, D.; Charcosset, A. Maize Adaptation to Temperate Climate: Relationship Between Population Structure and Polymorphism in the Dwarf8 Gene. Genetics 2006, 172, 2449–2463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, J.; Richards, D.E.; Hartley, N.M.; Murphy, G.P.; Devos, K.M.; Flintham, J.E.; Beales, J.; Fish, L.J.; Worland, A.J.; Pelica, F.; et al. ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature 1999, 400, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Domonkos, I.; Kis, M.; Gombos, Z.; Ughy, B. Carotenoids, versatile components of oxygenic photosynthesis. Prog. Lipid Res. 2013, 52, 539–561. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, L.; Stange, C. Light-dependent regulation of carotenoid biosynthesis in plants. Cienc. Investig. Agrar. 2009, 36, 143–162. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. The 1-Deoxy-D-Xylulose-5-Phosphate Pathway of Isoprenoid Biosynthesis in Plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 47–65. [Google Scholar] [CrossRef]
- Götz, T.; Sandmann, G.; Römer, S. Expression of a bacterial carotene hydroxylase gene (crtZ) enhances UV tolerance in tobacco. Plant Mol. Biol. 2002, 50, 127–140. [Google Scholar] [CrossRef]
- Cazzonelli, C.; Pogson, B. Source to sink: Regulation of carotenoid biosynthesis in plants. Trends Plant Sci. 2010, 15, 266–274. [Google Scholar] [CrossRef]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid Metabolism in Plants. Mol. Plant 2015, 8, 68–82. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Yamamura, S.; Koiwa, H.; Nishihara, M.; Sandmann, G. cDNA cloning and expression of carotenogenic genes during flower development in Gentiana lutea. Plant Mol. Biol. 2002, 48, 277–285. [Google Scholar] [CrossRef]
- Cong, L.; Wang, C.; Li, Z.; Chen, L.; Yang, G.; Wang, Y.; He, G. cDNA cloning and expression analysis of wheat (Triticum aestivum L.) phytoene and ζ-carotene desaturase genes. Mol. Biol. Rep. 2010, 37, 3351–3361. [Google Scholar] [CrossRef]
- Li, R.; Kang, C.; Song, X.; Yu, L.; Liu, D.; He, S.; Zhai, H.; Liu, Q. A ζ-carotene desaturase gene, IbZDS, increases β-carotene and lutein contents and enhances salt tolerance in transgenic sweetpotato. Plant Sci. 2017, 262, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Prasad, P.; Lokesh, V.; Shang, X.; Shin, J.; Keum, Y.S.; Lee, J.H. Carotenoids: Dietary Sources, Extraction, Encapsulation, Bioavailability, and Health Benefits—A Review of Recent Advancements. Antioxidants 2022, 11, 795. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, G.; Kang, H. Chloroplast- or Mitochondria-Targeted DEAD-Box RNA Helicases Play Essential Roles in Organellar RNA Metabolism and Abiotic Stress Responses. Front. Plant Sci. 2017, 8, 871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Floris, M.; Mahgoub, H.; Lanet, E.; Robaglia, C.; Menand, B. Post-transcriptional Regulation of Gene Expression in Plants during Abiotic Stress. Int. J. Mol. Sci. 2009, 10, 3168–3185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthes, A.; Schmidt-Gattung, S.; Köhler, D.; Forner, J.; Wildum, S.; Raabe, M.; Urlaub, H.; Binder, S. Two DEAD-Box Proteins May Be Part of RNA-Dependent High-Molecular-Mass Protein Complexes in Arabidopsis Mitochondria. Plant Physiol. 2007, 145, 1637–1646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.S.; Kim, K.A.; Oh, T.R.; Park, C.M.; Kang, H. Functional Characterization of DEAD-Box RNA Helicases in Arabidopsis thaliana under Abiotic Stress Conditions. Plant Cell Physiol. 2008, 49, 1563–1571. [Google Scholar] [CrossRef] [Green Version]
- Marcotuli, I.; Mazzeo, A.; Colasuonno, P.; Terzano, R.; Nigro, D.; Porfido, C.; Tarantino, A.; Cigliano, R.A.; Sanseverino, W.; Gadaleta, A.; et al. Fruit Development in Ficus carica L.: Morphological and Genetic Approaches to Fig Buds for an Evolution from Monoecy Toward Dioecy. Front. Plant Sci. 2020, 11, 1208. [Google Scholar] [CrossRef]
- Pelayo, M.A.; Yamaguchi, N.; Ito, T. One factor, many systems: The floral homeotic protein AGAMOUS and its epigenetic regulatory mechanisms. Curr. Opin. Plant Biol. 2021, 61, 102009. [Google Scholar] [CrossRef]
- Doyle, M.R.; Amasino, R.M. A single amino acid change in the enhancer of zeste ortholog CURLY LEAF results in vernaliza-tion-independent, rapid flowering in Arabidopsis. Plant Physiol. 2009, 151, 1688–1697. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.-Q.; Jiang, H.-L.; Li, C.-Y. Systemin/Jasmonate-Mediated Systemic Defense Signaling in Tomato. Mol. Plant 2011, 4, 607–615. [Google Scholar] [CrossRef]
- Jibran, R.; Tahir, J.; Cooney, J.; Hunter, D.A.; Dijkwel, P.P. Arabidopsis AGAMOUS Regulates Sepal Senescence by Driving Jasmonate Production. Front. Plant Sci. 2017, 8, 2101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.S.; Yang, M.H.; Lu, C.Y.; Chen, C.C.; Huang, Y.F.; Hsu, Y.C.; Kuo, C.W.; Liu, Y.C.; Jong, S.B.; Tyan, Y.C. The proteomic study of the gamma ray irradiation effect for Vigna radiata. Gamma 2013, 44, 1–10. [Google Scholar]
- Katiyar, P.; Pandey, N.; Keshavkant, S. Gamma radiation: A potential tool for abiotic stress mitigation and management of agroecosystem. Plant Stress 2022, 5, 100089. [Google Scholar] [CrossRef]
- Joint FAO/IAEA/WHO Expert Committee on the Technical Basis for Legislation on Irradiated Food, World Health Organization; Food and Agriculture Organization of the United Nations, International Atomic Energy Agency. 1981. Wholesomeness of Irradiated Food: Report of a Joint FAO/IAEA/WHO Expert Committee (Meeting held in Geneva from 27 October to 3 November 1980). World Health Organization. Available online: https://apps.who.int/iris/handle/10665/41508 (accessed on 1 December 2022).





| Swiss-Port/TrEMBL Accession Number | Protein Name | MW (Da) | Score | Match Queries | Sequence Coverage (%) | Match Peptide * | Subcellular Location | Function |
|---|---|---|---|---|---|---|---|---|
| Q1ACM5 | Apocytochrome f | 35,148 | 31 | 16 | 8 | R.IVCANCHLAKK.T R.GQIYLDGSKSNNTIYSSSAEGQVVK.I K.ISLKLLK.T R.IVCANCHLAKK.S K.KNIVVVGPIPGK.T R.IQGLIAFFISVIIAQTFLVLKKK.Q K.GGLNVGAVLILPEGFQIAPADRIPEEMKSK.I R.IVCANCHLAKK.S K.GGLNVGAVLILPEGFELAPSDRISPEIKQK.I -.MQTAITKK.W -.MQTAITK.K R.IVCANCHLAKK.T K.SNNTVYTASATGK. I R.IVCANCHLAKK.A K.IPYDTQIKQVLSNGKK.G K.SNNNVFSASTAGTISQITR.Q | Plastid | Component of the cytochrome b6-f complex, which mediates electron transfer between photosystem II (PSII) and photosystem I (PSI), cyclic electron flow around PSI, and state transitions |
| Q9ST48 | DELLA protein DWARF8 | 65,988 | 35 | 6 | 7 | R.SSDMADVAQK.L R.EYQDAGGSGGDMGSSK.D R.MRTGGGSTSSSSSSSSSMDGGR.T R.MRTGGGSTSSSSSSSSSMDGGR.T R.MRTGGGSTSSSSSSSSSMDGGR.T R.MRTGGGSTSSSSSSSSSMDGGRTR.S | Nucleus | Probable transcriptional regulator that acts as a repressor of the gibberellin (GA) signaling pathway. Probably acts by participating in large multiprotein complexes that repress transcription of GA-inducible genes. Upon GA application, it is degraded by the proteasome, allowing the GA signaling pathway. |
| Q9LUW6 | DEAD-box ATP-dependent RNA helicase 9 | 63,609 | 25 | 4 | 11 | R.SSFGGFGSNDGK.R K.SLPSNSSPFGVKVR.D R.SGGGGYGSYGSSSGR.S R.SGGGSYGGYGGSSGRSGGGGGSYGGSGGSSSR.Y | mitochondrion | RH9 was participated in RNA metabolism, which associated with the cellular function of response to abiotic stress. |
| Q40168 | Floral homeotic protein AGAMOUS | 28,706 | 20 | 5 | 31 | K.NLLKKIYK.L R.IEKGISKIR.S K.LRAQIGNLMNQNR.N K.ACSDSSNTGSVSEANAQYYQQEASK.L R.AQHQHQQMNLMPGSSSNYHELVPPPQQFDTR.N | Nucleus | Probable transcription factor involved in the control of organ identity during the early development of flowers. Is required for normal development of stamens and carpels in the wild-type flower. Plays a role in maintaining the determinacy of the floral meristem. Acts as C class cadastral protein by repressing the A class floral homeotic genes such as APETALA1. Forms a heterodimer via the K-box domain with either SEPALATTA1/AGL2, SEPALATTA2/AGL4, SEPALLATA3/AGL9 or AGL6 that could be involved in genes regulation during floral meristem development. |
| Q8L899 | Systemin receptor SR160 | 131,880 | 31 | 4 | 4 | K.AQLKDGSVVAIKK.L K.QSGNIAVALLTGKR.Y K.LPVDTLLKLSNIK.T K.EDASIEIELLQHLK.V | Cell membrane; Single-pass type I membrane protein. | Receptor with a serine/threonine-protein kinase activity. Involved in the perception of systemin, a peptide hormone responsible for the systemic activation of defense genes in leaves of wounded plants. May also regulate, in response to brassinosteroid binding, a signaling cascade involved in plant development |
| O49901 | ζ-carotene desaturase, chloroplastic/chromoplastic | 63,542 | 30 | 5 | 7 | R.LPMGAPLHGIR.A K.TPVKNFFLAGSYTK.Q -.MASSTCLIHSSSFGVGGKK.V K.ADVYIAACDVPGIK.R K.SANGETYVTGLAMSK.A | Plastid, chloroplast, chromoplast | Catalyzes the conversion of zeta-carotene to lycopene via the intermediary of neurosporene. It carries out two consecutive desaturations (introduction of double bonds) at positions C-7 and C-7’ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.-H.; Lu, Y.-S.; Ho, T.-C.; Shen, D.H.-Y.; Huang, Y.-F.; Chuang, K.-P.; Yuan, C.-H.; Tyan, Y.-C. Utilizing Proteomic Approach to Analyze Potential Antioxidant Proteins in Plant against Irradiation. Antioxidants 2022, 11, 2498. https://doi.org/10.3390/antiox11122498
Yang M-H, Lu Y-S, Ho T-C, Shen DH-Y, Huang Y-F, Chuang K-P, Yuan C-H, Tyan Y-C. Utilizing Proteomic Approach to Analyze Potential Antioxidant Proteins in Plant against Irradiation. Antioxidants. 2022; 11(12):2498. https://doi.org/10.3390/antiox11122498
Chicago/Turabian StyleYang, Ming-Hui, Yi-Shan Lu, Tzu-Chuan Ho, Daniel Hueng-Yuan Shen, Ying-Fong Huang, Kuo-Pin Chuang, Cheng-Hui Yuan, and Yu-Chang Tyan. 2022. "Utilizing Proteomic Approach to Analyze Potential Antioxidant Proteins in Plant against Irradiation" Antioxidants 11, no. 12: 2498. https://doi.org/10.3390/antiox11122498





