Phloroglucinol Attenuates DNA Damage and Apoptosis Induced by Oxidative Stress in Human Retinal Pigment Epithelium ARPE-19 Cells by Blocking the Production of Mitochondrial ROS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. Cell Viability Assay
2.3. Cytotoxicity Assay
2.4. Quantitative Assessment of Apoptosis
2.5. DNA Fragmentation Assay
2.6. Protein Isolation and Western Blotting
2.7. Caspase-3 Activity Assay
2.8. Assessment of ROS Generation
2.9. Comet Assay
2.10. γH2AX Immunofluorescence Assay
2.11. Measurement of 8-Hydroxy-2′-Deoxyguanosine (8-OHdG)
2.12. Mitochondrial Membrane Potential (MMP) Measurement
2.13. Autophagy Detection
2.14. Statistical Analysis
3. Results
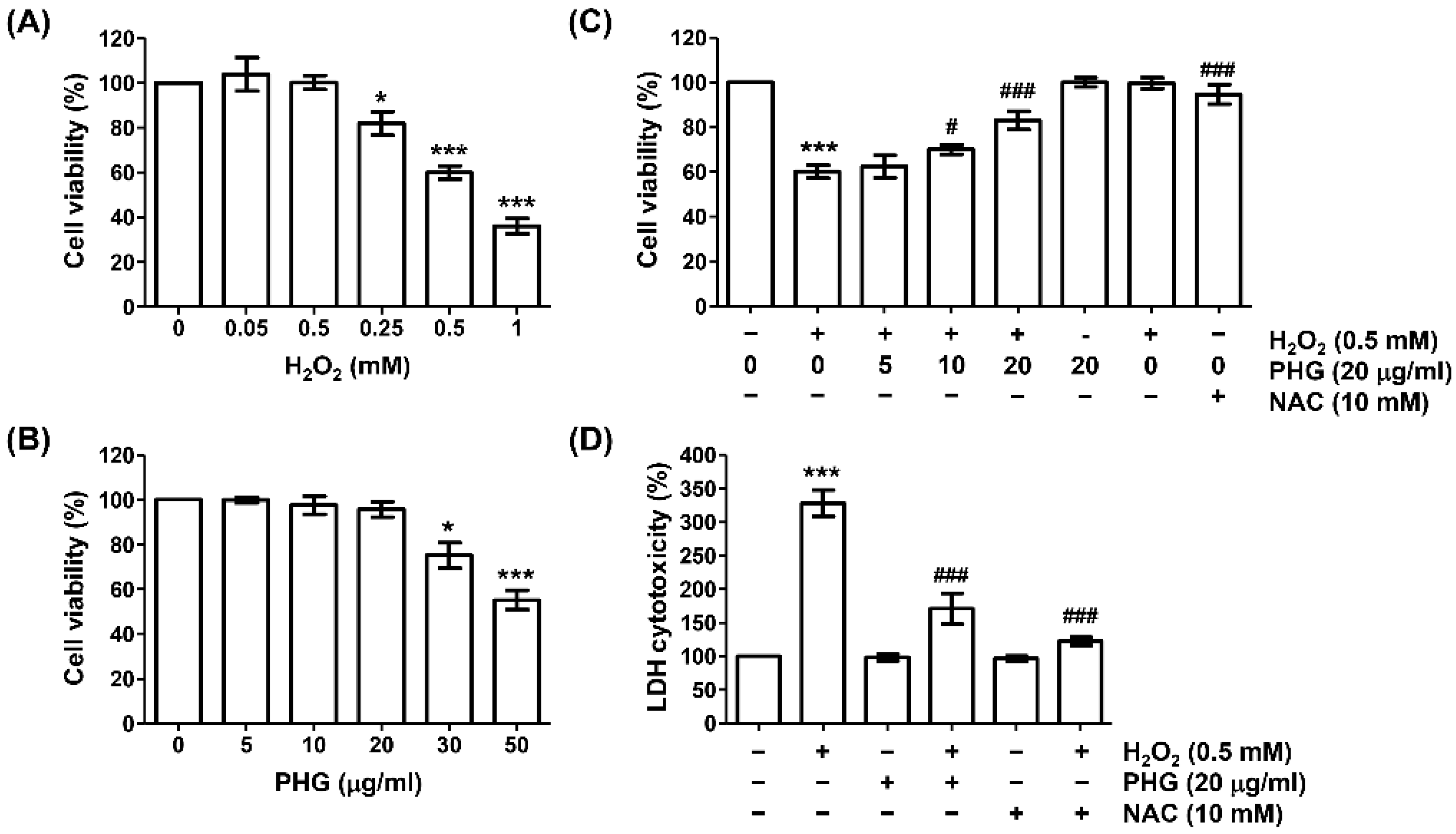
3.1. Phloroglucinol Restores Reduced Cell Viability and Increased Cytotoxicity Caused by H2O2
3.2. Phloroglucinol Reveres H2O2-Induced Apoptosis
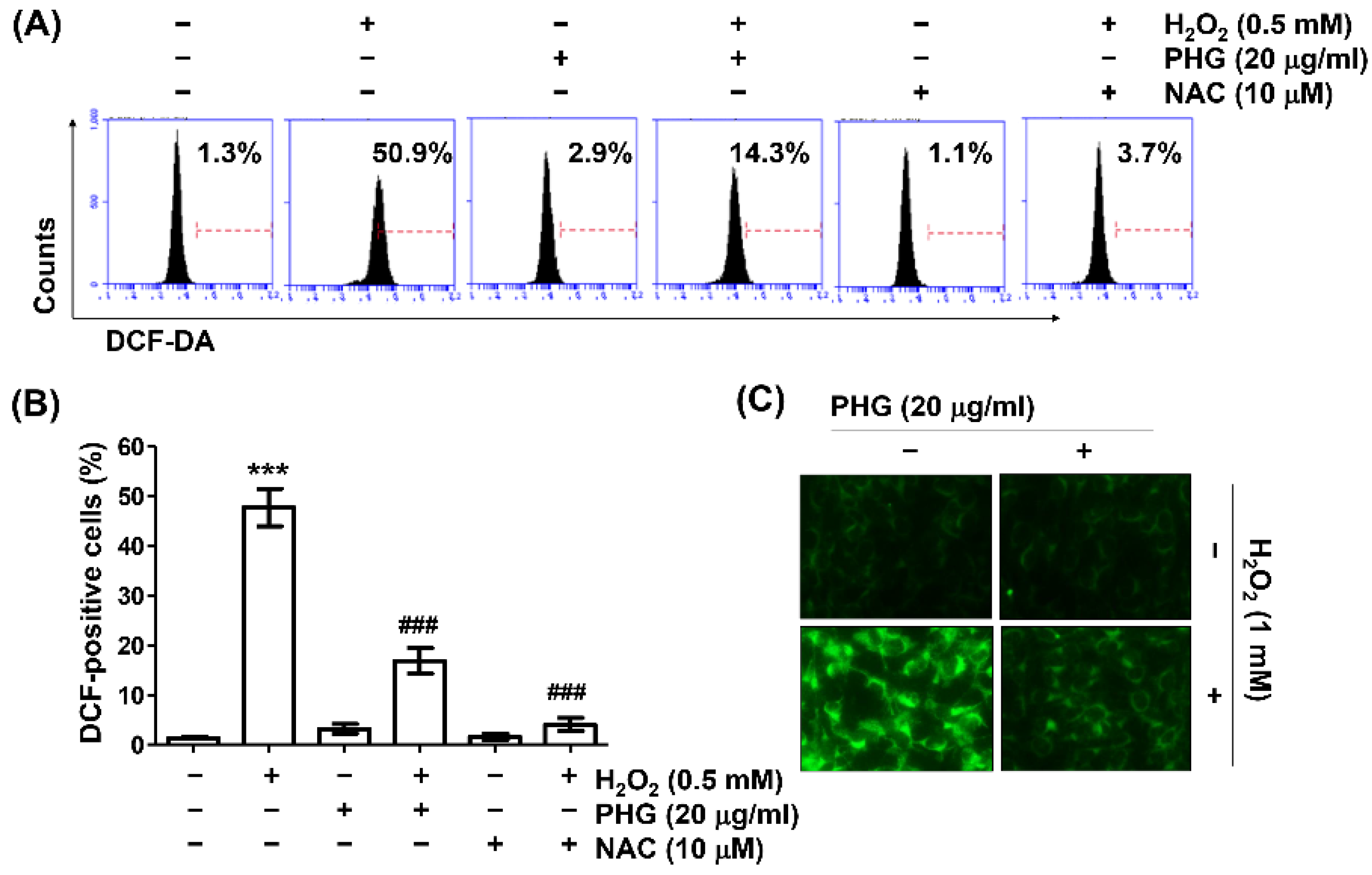
3.3. Phloroglucinol Abrogates H2O2-Induced ROS Generation
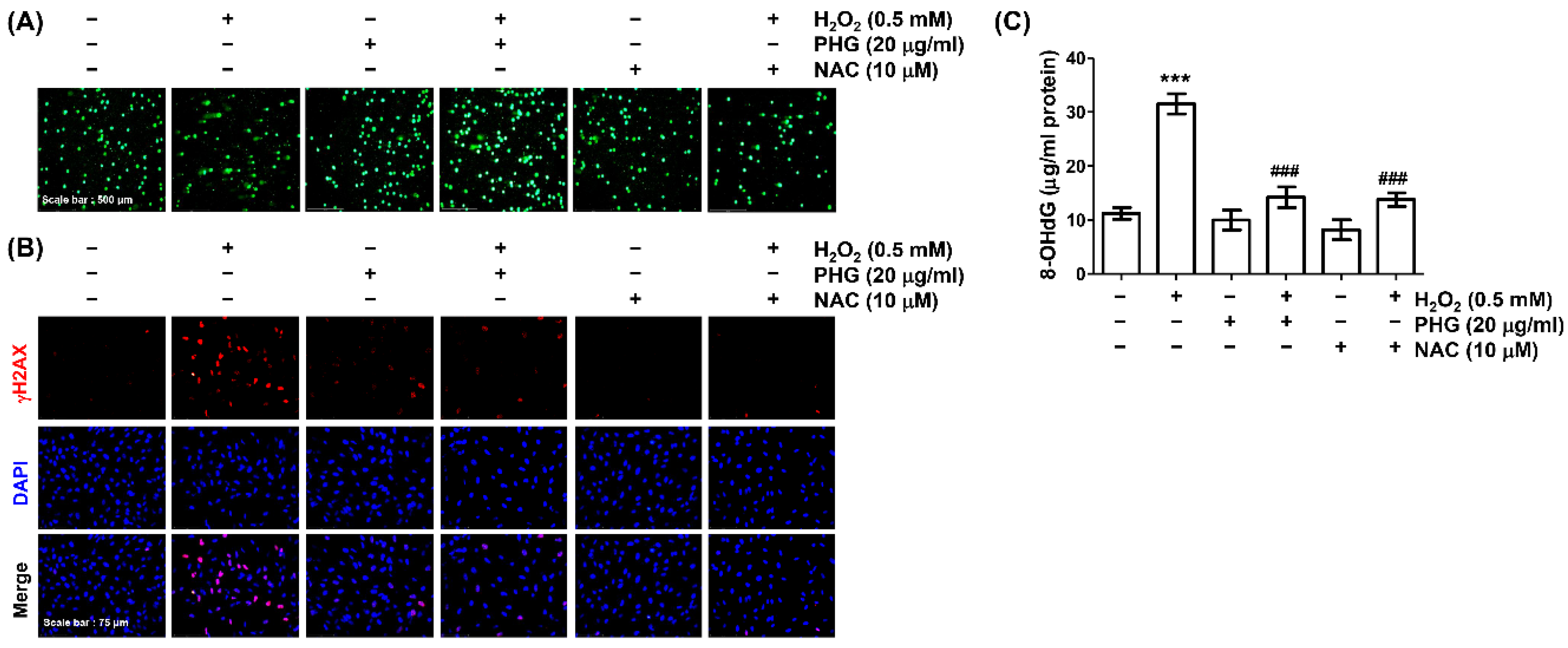
3.4. Phloroglucinol Abolishes H2O2-Induced DNA Damage
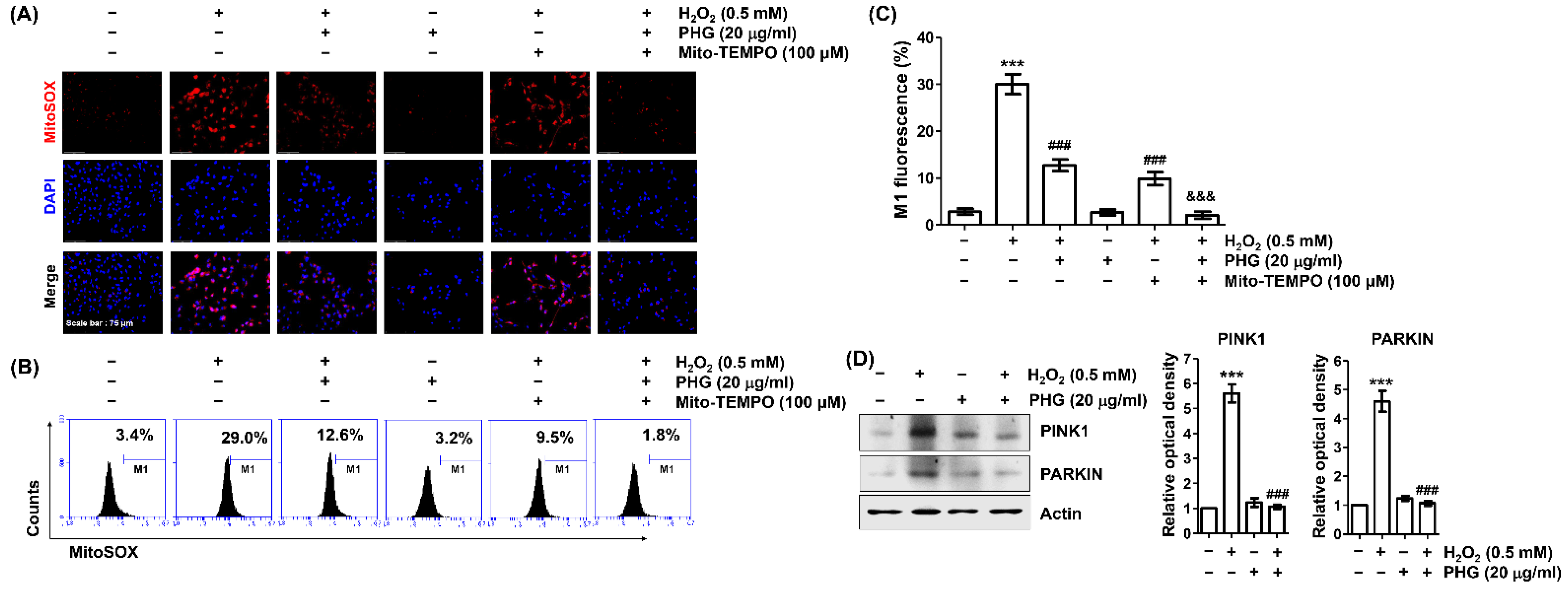
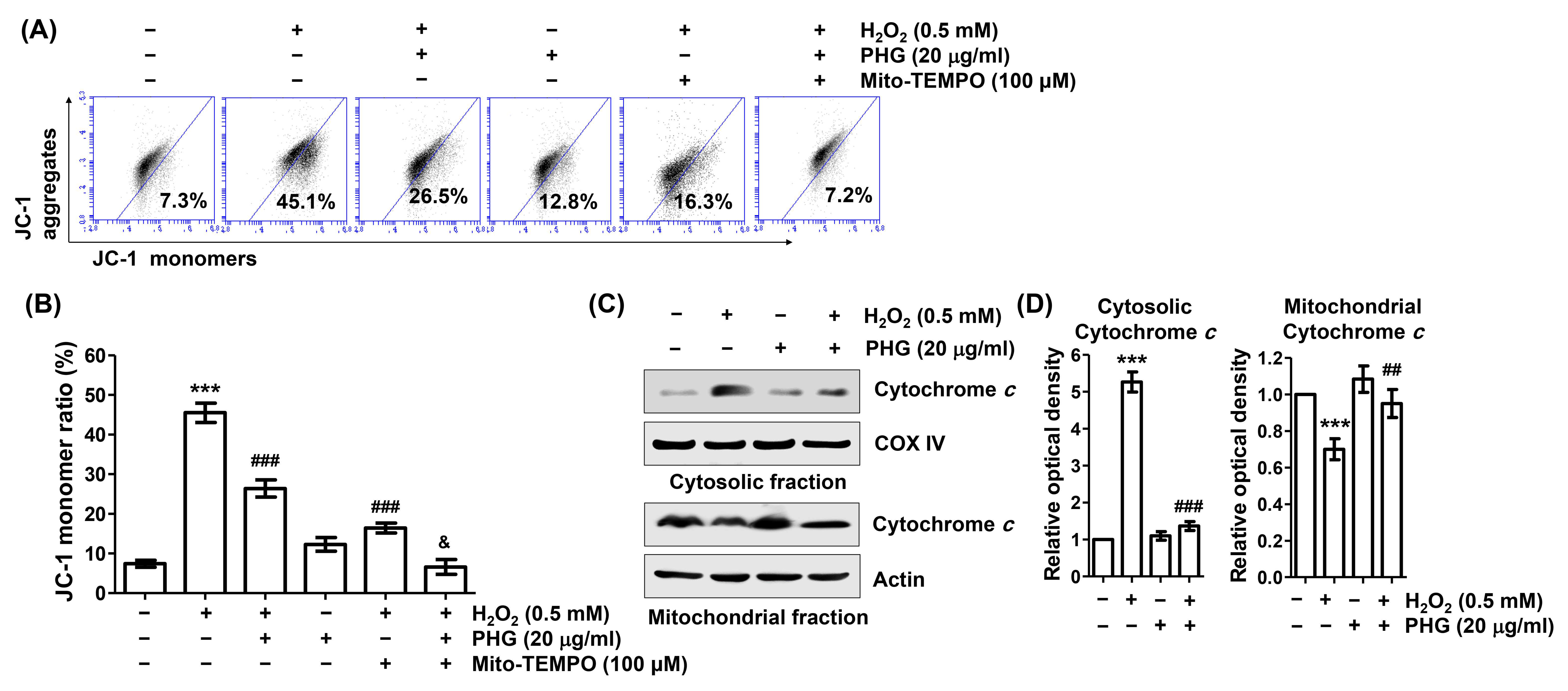
3.5. Phloroglucinol Reduces H2O2-Induced mtROS Production
3.6. Phloroglucinol Protects H2O2-Induced Mitochondrial Impairment
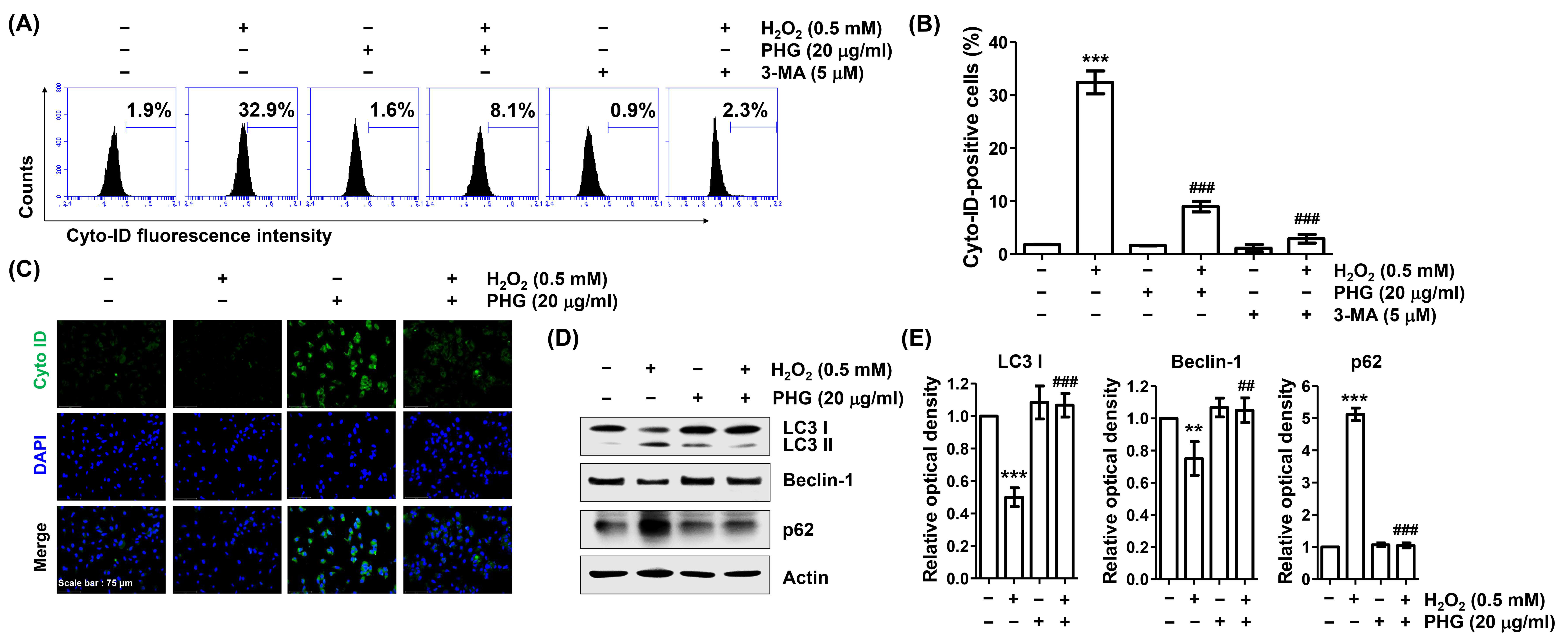
3.7. Phloroglucinol Abrogates H2O2-Induced Autophagy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nebbioso, M.; Franzone, F.; Lambiase, A.; Bonfiglio, V.; Limoli, P.G.; Artico, M.; Taurone, S.; Vingolo, E.M.; Greco, A.; Polimeni, A. Oxidative stress implication in retinal diseases—A review. Antioxidants 2022, 11, 1790. [Google Scholar] [CrossRef]
- Ozawa, Y. Oxidative stress in the light-exposed retina and its implication in age-related macular degeneration. Redox. Biol. 2020, 37, 101779. [Google Scholar] [CrossRef] [PubMed]
- Nashine, S. Potential therapeutic candidates for age-related macular degeneration (AMD). Cells 2021, 10, 2483. [Google Scholar] [CrossRef] [PubMed]
- Kaarniranta, K.; Uusitalo, H.; Blasiak, J.; Felszeghy, S.; Kannan, R.; Kauppinen, A.; Salminen, A.; Sinha, D.; Ferrington, D. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog. Retin. Eye Res. 2020, 79, 100858. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Bhardwaj, K.; Nepovimova, E.; Kuča, K.; Dhanjal, D.S.; Bhardwaj, S.; Bhatia, S.K.; Verma, R.; Kumar, D. Antioxidant functionalized nanoparticles: A combat against oxidative stress. Nanomaterials 2020, 10, 1334. [Google Scholar] [CrossRef]
- Chan, T.C.; Wilkinson Berka, J.L.; Deliyanti, D.; Hunter, D.; Fung, A.; Liew, G.; White, A. The role of reactive oxygen species in the pathogenesis and treatment of retinal diseases. Exp. Eye Res. 2020, 201, 108255. [Google Scholar] [CrossRef] [PubMed]
- Kaarniranta, K.; Blasiak, J.; Liton, P.; Boulton, M.; Klionsky, D.J.; Sinha, D. Autophagy in age-related macular degeneration. Autophagy 2022, 1, 1–13. [Google Scholar] [CrossRef]
- Brook, N.; Brook, E.; Dharmarajan, A.; Chan, A.; Dass, C.R. The role of pigment epithelium-derived factor in protecting against cellular stress. Free Radic. Res. 2019, 53, 1166–1180. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, S.; Park, H.J.; Kim, S.H.; Lew, H.; Kim, G.J. PEDF-mediated mitophagy triggers the visual cycle by enhancing mitochondrial functions in a H2O2-injured rat model. Cells 2021, 10, 1117. [Google Scholar] [CrossRef]
- Sridevi Gurubaran, I.; Viiri, J.; Koskela, A.; Hyttinen, J.M.T.; Paterno, J.J.; Kis, G.; Antal, M.; Urtti, A.; Kauppinen, A.; Felszeghy, S.; et al. Mitophagy in the retinal pigment epithelium of dry age-related macular degeneration investigated in the NFE2L2/PGC-1α−/− mouse model. Int. J. Mol. Sci. 2020, 21, 1976. [Google Scholar] [CrossRef]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 13, e14264. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Luo, M.; Wei, S. The bioprotective effects of polyphenols on metabolic syndrome against oxidative stress: Evidences and perspectives. Oxid. Med. Cell. Longev. 2019, 2019, 6713194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phang, Y.L.; Liu, S.; Zheng, C.; Xu, H. Recent advances in the synthesis of natural products containing the phloroglucinol motif. Nat. Prod. Rep. 2022, 39, 1766–1802. [Google Scholar] [CrossRef]
- Monteiro, P.; Lomartire, S.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Call the eckols: Present and future potential cancer therapies. Mar. Drugs 2022, 20, 387. [Google Scholar] [CrossRef]
- Khan, F.; Tabassum, N.; Bamunuarachchi, N.I.; Kim, Y.M. Phloroglucinol and its derivatives: Antimicrobial properties toward microbial pathogens. J. Agric. Food Chem. 2022, 70, 4817–4838. [Google Scholar] [CrossRef] [PubMed]
- Clara, B.; Paul, V.; Denis, P.; Stéphanie, M.; Hélène, V.R.; Rémy, B. Efficacy of phloroglucinol for the treatment of pain of gynaecologic or obstetrical origin: A systematic review of literature of randomised controlled trials. Eur. J. Clin. Pharmacol. 2020, 76, 1–6. [Google Scholar] [CrossRef]
- Drygalski, K.; Fereniec, E.; Zalewska, A.; Krętowski, A.; Żendzian-Piotrowska, M.; Maciejczyk, M. Phloroglucinol prevents albumin glycation as well as diminishes ROS production, glycooxidative damage, nitrosative stress and inflammation in hepatocytes treated with high glucose. Biomed. Pharmacother. 2021, 142, 111958. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Zhang, R.; Chae, S.; Lee, S.J.; Kim, J.; Kim, J.; Jeong, J.; Lee, J.; Shin, T.; Lee, N.H.; et al. Phloroglucinol (1,3,5-trihydroxybenzene) protects against ionizing radiation-induced cell damage through inhibition of oxidative stress in vitro and in vivo. Chem. Biol. Interact. 2010, 185, 215–226. [Google Scholar] [CrossRef]
- Cia, D.; Cubizolle, A.; Crauste, C.; Jacquemot, N.; Guillou, L.; Vigor, C.; Angebault, C.; Hamel, C.P.; Vercauteren, J.; Brabet, P. Phloroglucinol protects retinal pigment epithelium and photoreceptor against all-trans-retinal-induced toxicity and inhibits A2E formation. J. Cell. Mol. Med. 2016, 20, 1651–1663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- So, M.J.; Cho, E.J. Phloroglucinol attenuates free radical-induced oxidative stress. Prev. Nutr. Food Sci. 2014, 19, 129–135. [Google Scholar] [CrossRef]
- Yang, E.J.; Kim, H.; Kim, H.S.; Chang, M.J. Phloroglucinol attenuates oligomeric amyloid beta peptide1-42-induced astrocytic activation by reducing oxidative stress. J. Pharmacol. Sci. 2021, 145, 308–312. [Google Scholar] [CrossRef]
- Ryu, J.; Zhang, R.; Hong, B.H.; Yang, E.J.; Kang, K.A.; Choi, M.; Kim, K.C.; Noh, S.J.; Kim, H.S.; Lee, N.H.; et al. Phloroglucinol attenuates motor functional deficits in an animal model of Parkinson’s disease by enhancing Nrf2 activity. PLoS ONE 2013, 8, e71178. [Google Scholar] [CrossRef]
- Park, C.; Cha, H.J.; Hong, S.H.; Kim, G.Y.; Kim, S.; Kim, H.S.; Kim, B.W.; Jeon, Y.J.; Choi, Y.H. Protective effect of phloroglucinol on oxidative stress-induced DNA damage and apoptosis through activation of the Nrf2/HO-1 signaling pathway in HaCaT human keratinocytes. Mar. Drugs 2019, 17, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piao, M.J.; Ahn, M.J.; Kang, K.A.; Kim, K.C.; Zheng, J.; Yao, C.W.; Cha, J.W.; Hyun, C.L.; Kang, H.K.; Lee, N.H.; et al. Phloroglucinol inhibits ultraviolet B radiation-induced oxidative stress in the mouse skin. Int. J. Radiat. Biol. 2014, 90, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.C.; Li, Y.; Cheng, K.C.; Hsu, C.C.; Cheng, J.T.; Lau, H.H. Potassium bromate-induced cell model of age-related macular degeneration in vitro. Mol. Med. Rep. 2021, 23, 216. [Google Scholar] [CrossRef] [PubMed]
- Moine, E.; Brabet, P.; Guillou, L.; Durand, T.; Vercauteren, J.; Crauste, C. New lipophenol antioxidants reduce oxidative damage in retina pigment epithelial cells. Antioxidants 2018, 7, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.; Noh, J.S.; Jung, Y.; Leem, S.H.; Hyun, J.W.; Chang, Y.C.; Kwon, T.K.; Kim, G.Y.; Lee, H.; Choi, Y.H. Fisetin attenuated oxidative stress-induced cellular damage in ARPE-19 human retinal pigment epithelial cells through Nrf2-mediated activation of heme oxygenase-1. Front. Pharmacol. 2022, 13, 927898. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.J.; Lim, D.S.; Kim, S.O.; Park, C.; Leem, S.H.; Lee, H.; Kim, G.Y.; Jeong, S.J.; Choi, Y.H. Protection of oxidative stress-induced DNA damage and apoptosis by rosmarinic acid in murine myoblast C2C12 cells. Biotechnol. Bioprocess Eng. 2022, 27, 171–182. [Google Scholar] [CrossRef]
- Choi, Y.H. Trans-cinnamaldehyde protects C2C12 myoblasts from DNA damage, mitochondrial dysfunction and apoptosis caused by oxidative stress through inhibiting ROS production. Genes Genom. 2021, 43, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Park, J.P.; Yun, J.W. Carboxylesterase3 (Ces3) interacts with bone morphogenetic protein 11 and promotes differentiation of osteoblasts via Smad1/5/9 pathway. Biotechnol. Bioprocess Eng. 2022, 27, 1–16. [Google Scholar] [CrossRef]
- Kim, C.; Kong, G.; Lee, H.; Tran, Q.; Vo, T.T.; Kwon, S.H.; Park, J.; Kim, S.H.; Park, J. Scavenger receptor class F member 2 (SCARF2) as a novel therapeutic target in glioblastoma. Toxicol. Res. 2022, 38, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Sim, K.H.; Shu, M.S.; Kim, S.; Kim, J.Y.; Choi, B.H.; Lee, Y.J. Cilostazol induces apoptosis and inhibits proliferation of hepatocellular carcinoma cells by activating AMPK. Biotechnol. Bioprocess Eng. 2021, 26, 776–785. [Google Scholar] [CrossRef]
- Tjahjono, E.; Kirienko, D.R.; Kirienko, N.V. The emergent role of mitochondrial surveillance in cellular health. Aging Cell 2022, 11, e13710. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, P.; Sulejczak, D.; Kleczkowska, P.; Bukowska-Ośko, I.; Kucia, M.; Popiel, M.; Wietrak, E.; Kramkowski, K.; Wrzosek, K.; Kaczyńska, K. Mitochondrial oxidative stress—A causative factor and therapeutic target in many diseases. Int. J. Mol. Sci. 2021, 22, 13384. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Zhang, Z.; Wang, S. Role of mitochondria in retinal pigment epithelial aging and degeneration. Front. Aging 2022, 3, 926627. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, C.K.; Tan, L.T.H.; Pusparajah, P.; Htar, T.T.; Chuah, L.H.; Lee, V.S.; Low, L.E.; Tang, S.Y.; Chan, K.G.; Goh, B.H. Detrimental effects of UVB on retinal pigment epithelial cells and its role in age-related macular degeneration. Oxid. Med. Cell. Longev. 2020, 2020, 1904178. [Google Scholar] [CrossRef] [PubMed]
- Møller, P.; Stopper, H.; Collins, A.R. Measurement of DNA damage with the comet assay in high-prevalence diseases: Current status and future directions. Mutagenesis 2020, 35, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Rahmanian, N.; Shokrzadeh, M.; Eskandani, M. Recent advances in γH2AX biomarker-based genotoxicity assays: A marker of DNA damage and repair. DNA Repair 2021, 108, 103243. [Google Scholar] [CrossRef] [PubMed]
- Goh, X.X.; Tang, P.Y.; Tee, S.F. 8-Hydroxy-2′-deoxyguanosine and reactive oxygen species as biomarkers of oxidative stress in mental illnesses: A meta-analysis. Psychiatry Investig. 2021, 18, 603–618. [Google Scholar] [CrossRef]
- Tiwari, S.; Dewry, R.K.; Srivastava, R.; Nath, S.; Mohanty, T.K. Targeted antioxidant delivery modulates mitochondrial functions, ameliorates oxidative stress and preserve sperm quality during cryopreservation. Theriogenology 2022, 179, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Urbani, A.; Prosdocimi, E.; Carrer, A.; Checchetto, V.; Szabò, I. Mitochondrial ion channels of the inner membrane and their regulation in cell death signaling. Front. Cell. Dev. Biol. 2021, 8, 620081. [Google Scholar] [CrossRef]
- Sarwar, M.S.; Xia, Y.X.; Liang, Z.M.; Tsang, S.W.; Zhang, H.J. Mechanistic pathways and molecular targets of plant-derived anticancer ent-Kaurane diterpenes. Biomolecules 2020, 10, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiraz, Y.; Adan, A.; Kartal Yandim, M.; Baran, Y. Major apoptotic mechanisms and genes involved in apoptosis. Tumor Biol. 2016, 37, 8471–8486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clementi, M.E.; Pizzoferrato, M.; Bianchetti, G.; Brancato, A.; Sampaolese, B.; Maulucci, G.; Tringali, G. Cytoprotective effect of idebenone through modulation of the intrinsic mitochondrial pathway of apoptosis in human retinal pigment epithelial cells exposed to oxidative stress induced by hydrogen peroxide. Biomedicines 2022, 10, 503. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Huang, T.Y.; Chen, H.Y.; Huang, T.C.; Lin, L.C.; Chang, Y.J.; Hsia, S.M. Protective effect of melatonin against oxidative stress-induced apoptosis and enhanced autophagy in human retinal pigment epithelium cells. Oxid. Med. Cell. Longev. 2018, 2018, 9015765. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wong, H.S. Are mitochondria the main contributor of reactive oxygen species in cells? J. Exp. Biol. 2021, 224, jeb221606. [Google Scholar] [CrossRef] [PubMed]
- Brillo, V.; Chieregato, L.; Leanza, L.; Muccioli, S.; Costa, R. Mitochondrial dynamics, ROS, and cell signaling: A blended overview. Life 2021, 11, 332. [Google Scholar] [CrossRef]
- He, Y.; Chen, Z.; Zhang, R.; Quan, Z.; Xu, Y.; He, B.; Ren, Y. Mitochondrial-targeted antioxidant peptide SS31 prevents RPE cell death under oxidative stress. Biomed. Res. Int. 2022, 2022, 6180349. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Mitter, S.K.; Qi, X.; Beli, E.; Rao, H.V.; Ding, J.; Ip, C.S.; Gu, H.; Akin, D.; Dunn, W.A., Jr.; et al. Oxidative stress-mediated NFκB phosphorylation upregulates p62/SQSTM1 and promotes retinal pigmented epithelial cell survival through increased autophagy. PLoS ONE 2017, 12, e0171940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jîtcă, G.; Ősz, B.E.; Tero-Vescan, A.; Miklos, A.P.; Rusz, C.M.; Bătrînu, M.G.; Vari, C.E. Positive aspects of oxidative stress at different levels of the human body: A review. Antioxidants 2022, 11, 572. [Google Scholar] [CrossRef]
- Terman, A.; Gustafsson, B.; Brunk, U.T. Autophagy, organelles and ageing. J. Pathol. 2007, 211, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Rohrer, B.; Bandyopadhyay, M.; Beeson, C. Reduced metabolic capacity in aged primary retinal pigment epithelium (RPE) is correlated with increased susceptibility to oxidative stress. Adv. Exp. Med. Biol. 2016, 854, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yao, T.; Chen, J.; Zhang, Z.; Yang, W.; Gao, X.; Dan, Y.; He, Y. Effect of Sirt3 on retinal pigment epithelial cells in high glucose through Foxo3a/ PINK1-Parkin pathway mediated mitophagy. Exp. Eye Res. 2022, 218, 109015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xi, X.; Mei, Y.; Zhao, X.; Zhou, L.; Ma, M.; Liu, S.; Zha, X.; Yang, Y. High-glucose induces retinal pigment epithelium mitochondrial pathways of apoptosis and inhibits mitophagy by regulating ROS/PINK1/Parkin signal pathway. Biomed. Pharmacother. 2019, 111, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Dammak, A.; Huete-Toral, F.; Carpena-Torres, C.; Martin-Gil, A.; Pastrana, C.; Carracedo, G. From oxidative stress to inflammation in the posterior ocular diseases: Diagnosis and treatment. Pharmaceutics 2021, 13, 1376. [Google Scholar] [CrossRef] [PubMed]
- Nita, M.; Grzybowski, A. Interplay between reactive oxygen species and autophagy in the course of age-related macular degeneration. EXCLI J. 2020, 19, 1353–1371. [Google Scholar] [CrossRef]
- Blasiak, J.; Pawlowska, E.; Sobczuk, A.; Szczepanska, J.; Kaarniranta, K. The aging stress response and its implication for AMD pathogenesis. Int. J. Mol. Sci. 2020, 21, 8840. [Google Scholar] [CrossRef] [PubMed]
- Mitter, S.K.; Rao, H.V.; Qi, X.; Cai, J.; Sugrue, A.; Dunn, W.A., Jr.; Grant, M.B.; Boulton, M.E. Autophagy in the retina: A potential role in age-related macular degeneration. Adv. Exp. Med. Biol. 2012, 723, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Li, D.; Cui, Y.; Xie, T.; Cai, J.; Yao, Y. Decorin protects retinal pigment epithelium cells from oxidative stress and apoptosis via AMPK-mTOR-regulated autophagy. Oxid. Med. Cell, Longev. 2022, 2022, 3955748. [Google Scholar] [CrossRef] [PubMed]
- Buzun, K.; Gornowicz, A.; Lesyk, R.; Bielawski, K.; Bielawska, A. Autophagy modulators in cancer therapy. Int. J. Mol. Sci. 2021, 22, 5804. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Huang, T.; Tong, Y.; Fan, Z.; Yang, Z.; Yang, D.; Mao, X.; Yang, M. p62 works as a hub modulation in the ageing process. Ageing Res. Rev. 2022, 73, 101538. [Google Scholar] [CrossRef]
- Yoshii, S.R.; Mizushima, N. Monitoring and measuring autophagy. Int. J. Mol. Sci. 2017, 18, 1865. [Google Scholar] [CrossRef] [PubMed]


| Antibody | Species | Dilution | Catalog No. | Vendor |
|---|---|---|---|---|
| Bax | Mouse monoclonal | 1:1000 | sc-7480 | Santa Cruz Biotechnology Inc. |
| Bcl-2 | Mouse monoclonal | 1:1000 | sc-509 | Santa Cruz Biotechnology Inc. |
| Caspase-3 | Mouse monoclonal | 1:1000 | sc-56052 | Santa Cruz Biotechnology Inc. |
| PARP | Mouse monoclonal | 1:1000 | sc-8007 | Santa Cruz Biotechnology Inc. |
| Pink1 | Mouse monoclonal | 1:1000 | sc-517353 | Santa Cruz Biotechnology Inc. |
| Parkin | Mouse monoclonal | 1:1000 | sc-32282 | Santa Cruz Biotechnology Inc. |
| Cytochrome c | Mouse monoclonal | 1:1000 | sc-13560 | Santa Cruz Biotechnology Inc. |
| LC3 | Rabbit polyclonal | 1:1000 | 3868s | Cell Signaling Technology Inc. |
| Beclin-1 | Rabbit polyclonal | 1:1000 | 3495s | Cell Signaling Technology Inc. |
| p62 | Rabbit polyclonal | 1:1000 | 5114 | Cell Signaling Technology Inc. |
| COX IV | Rabbit polyclonal | 1:1000 | 4844 | Cell Signaling Technology Inc. |
| Actin | Mouse monoclonal | 1:1000 | sc-47778 | Santa Cruz Biotechnology Inc. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.; Cha, H.-J.; Kim, M.Y.; Bang, E.; Moon, S.-K.; Yun, S.J.; Kim, W.-J.; Noh, J.S.; Kim, G.-Y.; Cho, S.; et al. Phloroglucinol Attenuates DNA Damage and Apoptosis Induced by Oxidative Stress in Human Retinal Pigment Epithelium ARPE-19 Cells by Blocking the Production of Mitochondrial ROS. Antioxidants 2022, 11, 2353. https://doi.org/10.3390/antiox11122353
Park C, Cha H-J, Kim MY, Bang E, Moon S-K, Yun SJ, Kim W-J, Noh JS, Kim G-Y, Cho S, et al. Phloroglucinol Attenuates DNA Damage and Apoptosis Induced by Oxidative Stress in Human Retinal Pigment Epithelium ARPE-19 Cells by Blocking the Production of Mitochondrial ROS. Antioxidants. 2022; 11(12):2353. https://doi.org/10.3390/antiox11122353
Chicago/Turabian StylePark, Cheol, Hee-Jae Cha, Min Yeong Kim, EunJin Bang, Sung-Kwon Moon, Seok Joong Yun, Wun-Jae Kim, Jeong Sook Noh, Gi-Young Kim, Suengmok Cho, and et al. 2022. "Phloroglucinol Attenuates DNA Damage and Apoptosis Induced by Oxidative Stress in Human Retinal Pigment Epithelium ARPE-19 Cells by Blocking the Production of Mitochondrial ROS" Antioxidants 11, no. 12: 2353. https://doi.org/10.3390/antiox11122353





