Cytokine Adsorber Use during DCD Heart Perfusion Counteracts Coronary Microvascular Dysfunction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Anesthesia
2.2. Donation after Circulatory Death Model and Harvesting of Hearts
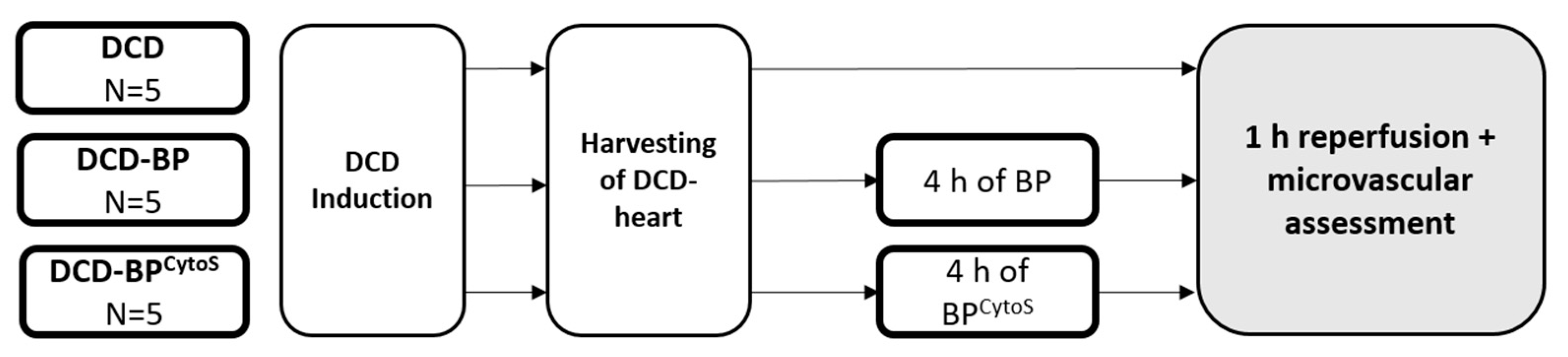
2.3. Blood Perfusion and Study Groups
2.4. Microvascular Functional Assessment
2.5. Cytokine Profiling
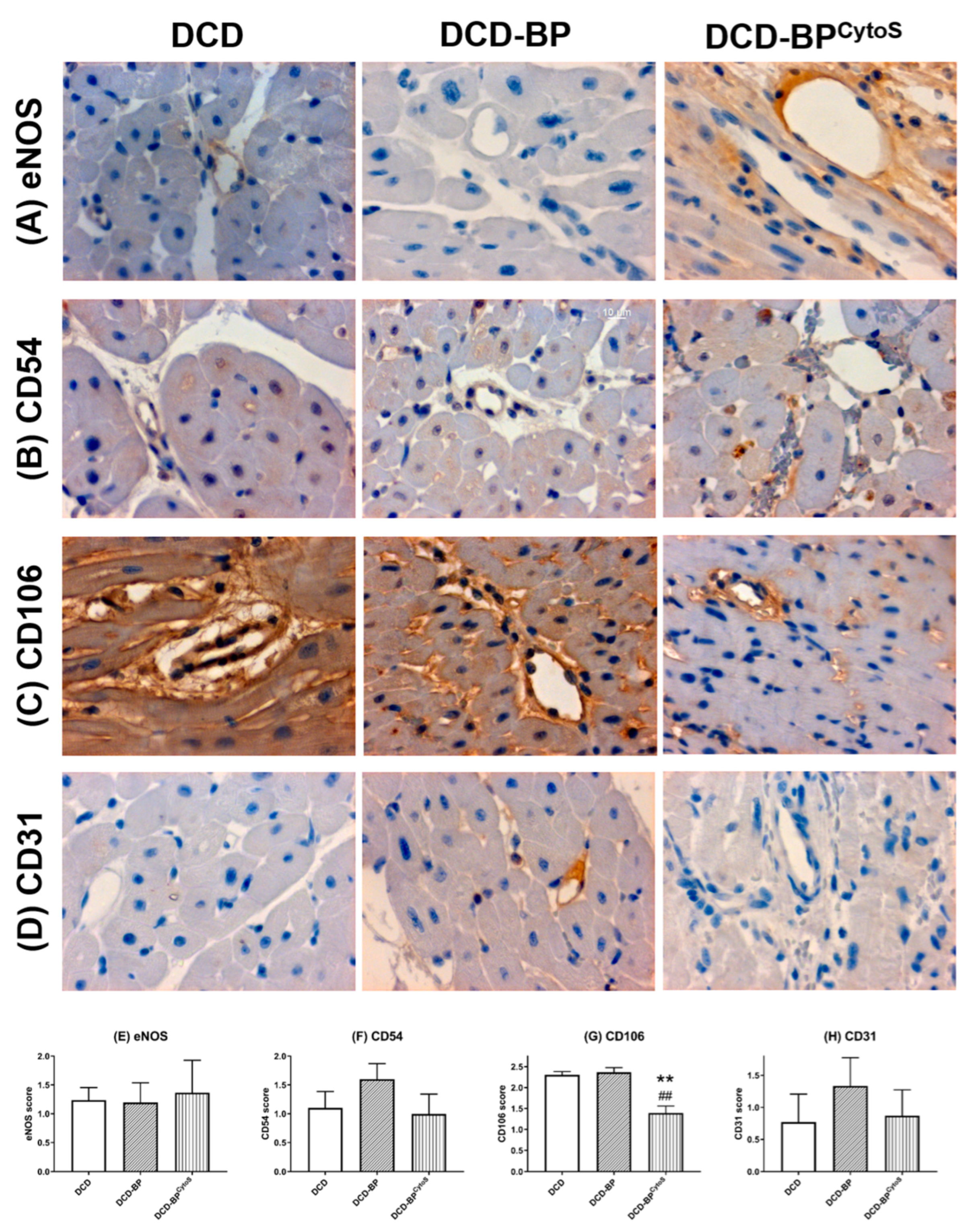
2.6. Immunohistochemical Staining
2.7. Gene Expression Analysis
2.8. Statistical Analysis and Machine Learning Algorithm
3. Results
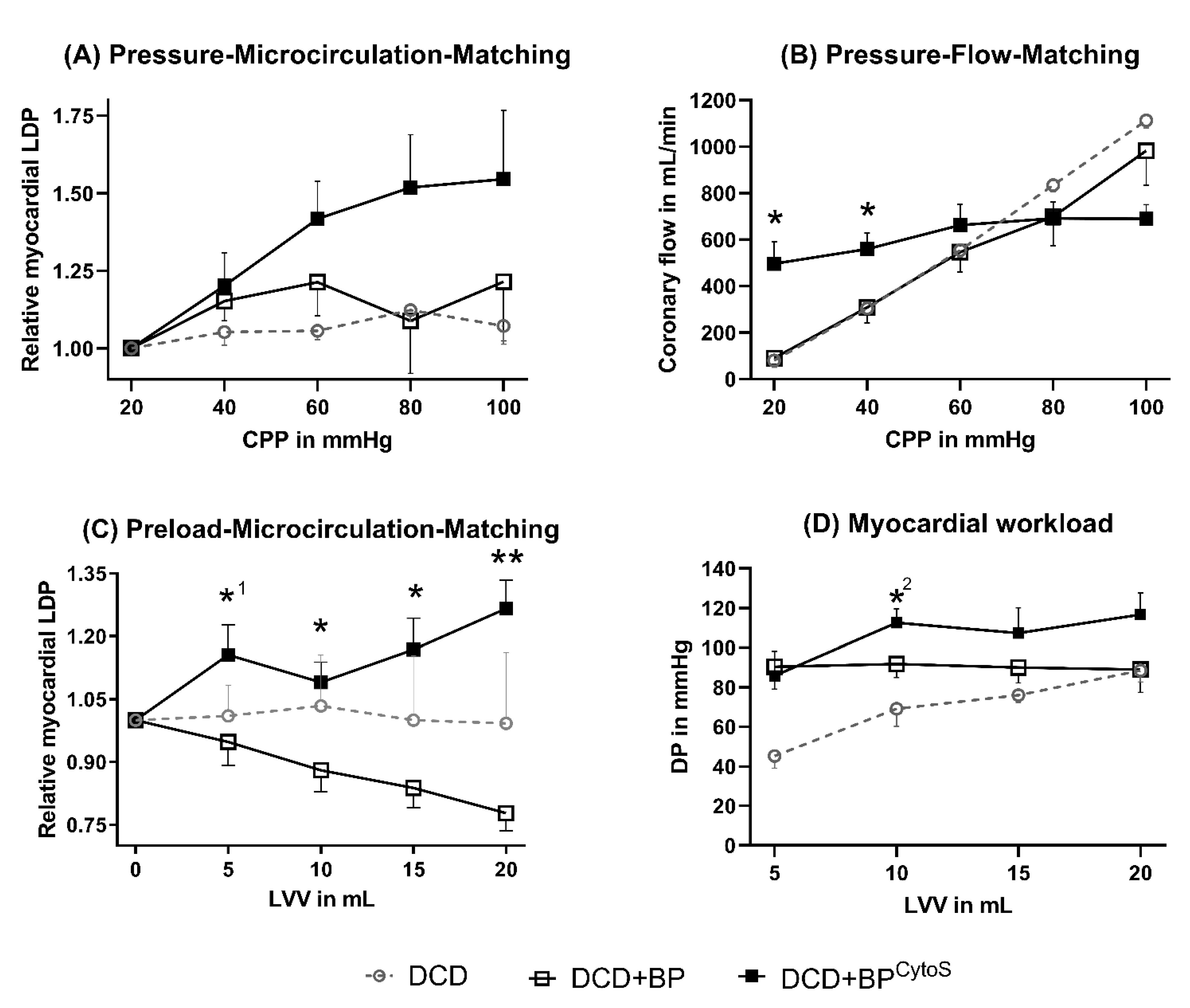
3.1. Microvascular Function
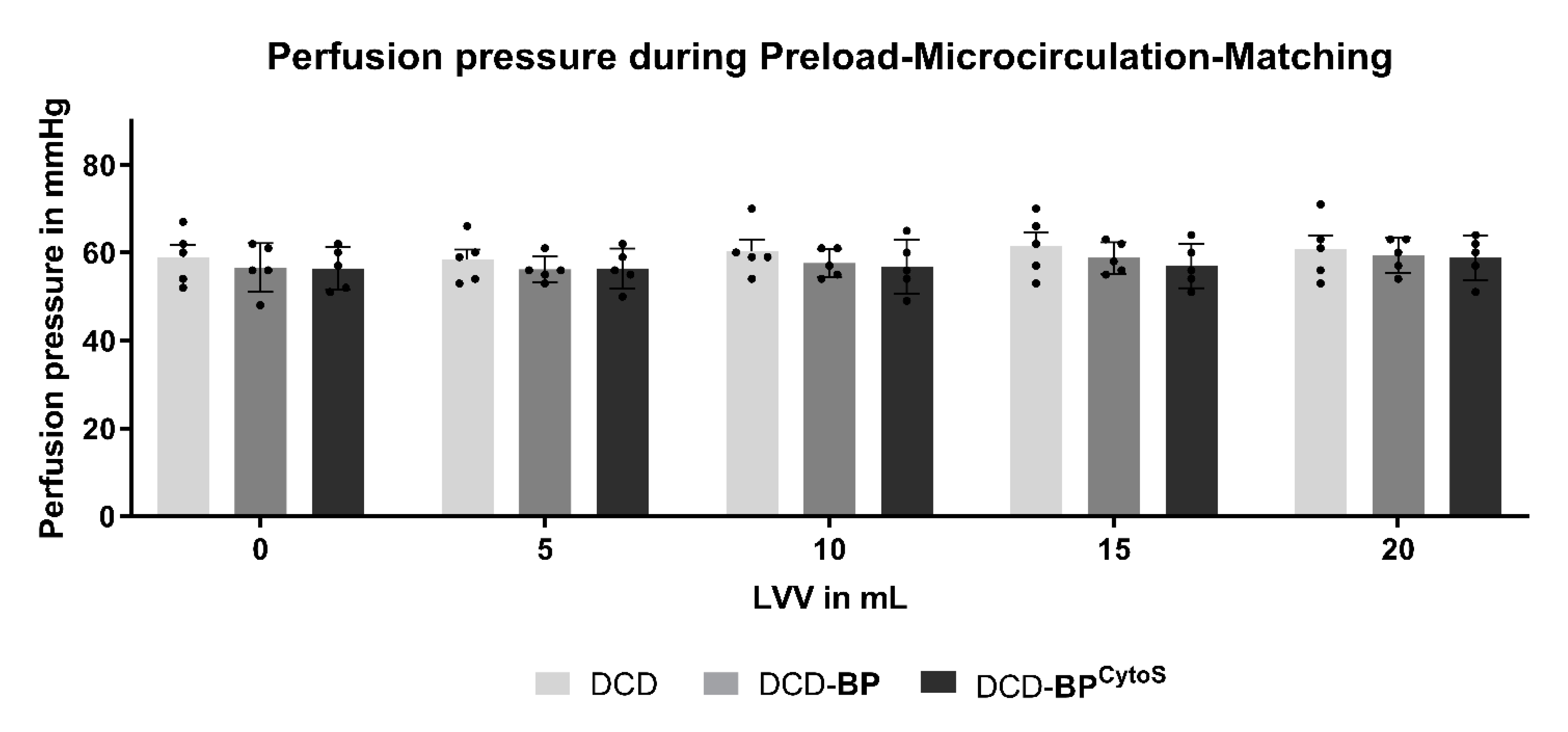
3.2. Perfusion Pressure
3.3. Cytokine Profile
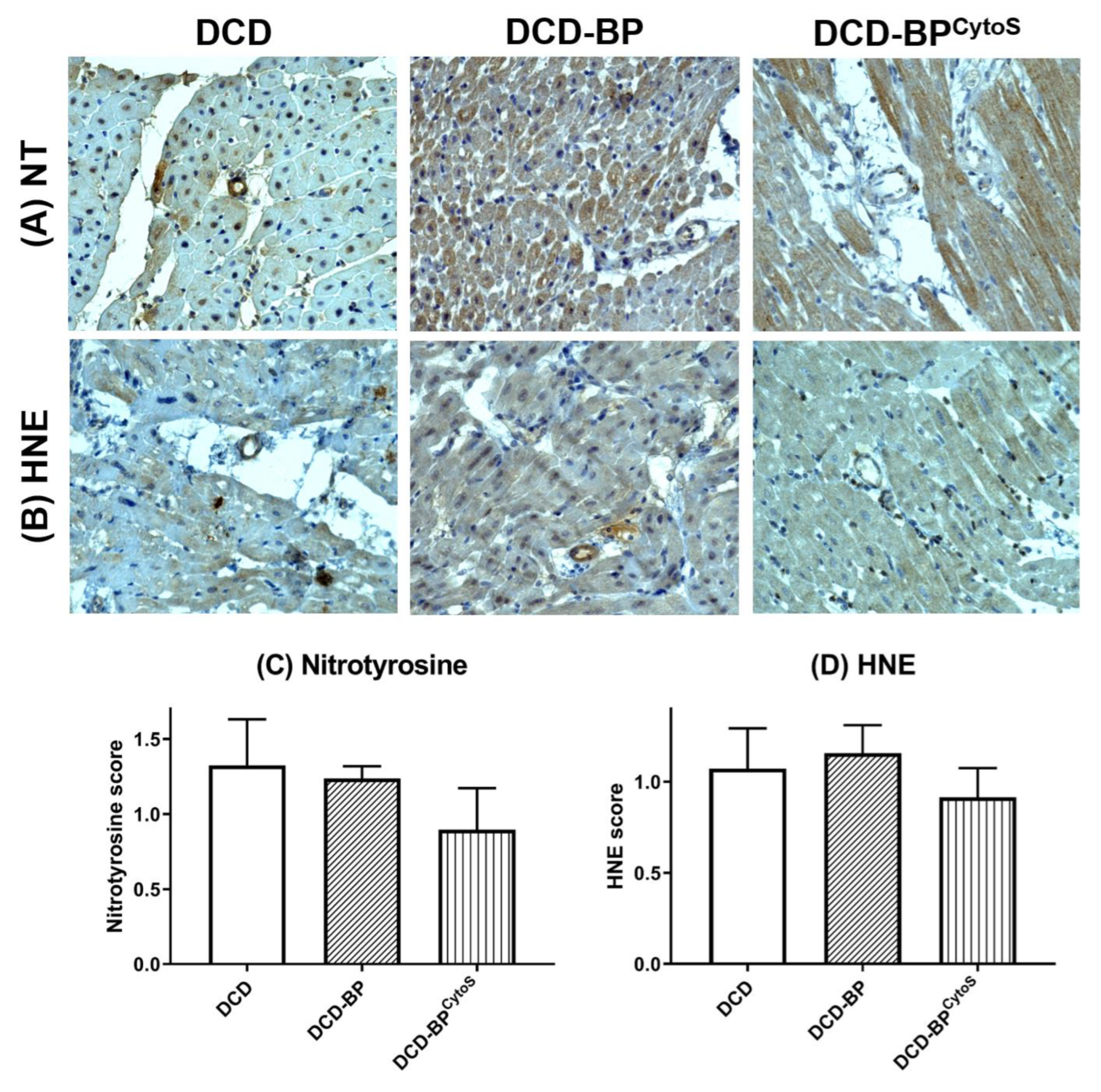
3.4. Oxidative Stress and Endothelial Injury
3.5. Gene Expression
4. Discussion
4.1. Microvascular Functional Effects
4.2. Cytokine Profile
4.3. Oxidative Stress and Endothelial Injury
4.4. Gene Expression
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BP | blood perfusion |
| CAV1 | caveolin 1 |
| CD-31 | cluster of differentiation-31 |
| CPP | coronary perfusion pressure |
| DCD | donation after circulatory death |
| eNOS | endothelial nitric oxide synthase |
| FGF2 | fibroblast growth factor 2 |
| HIF1A | hypoxia inducible factor 1a |
| ICAM | intracellular cell adhesion molecule-1 |
| IL | interleukin, interleukine |
| IRI | ischemia/reperfusion injury |
| ITGB1 | integrin beta 1 |
| LDP | laser-doppler-perfusion |
| CMVD | coronary microvascular dysfunction |
| NOS3 | nitric oxide synthase 3, eNOS |
| PECAM-1 | platelet endothelial cell adhesion molecule-1 |
| PLAT | plasminogen activator |
| PLAU | plasminogen activator urokinase |
| RLDP | relative LDP |
| ROS | reactive oxygen species |
| SERPRINE1 | plasminogen activator inhibitor 1 |
| VCAM | vascular cell adhesion molecule-1 |
References
- Haddad, F.; Khazanie, P.; Deuse, T.; Weisshaar, D.; Zhou, J.; Nam, C.W.; Vu, T.A.; Gomari, F.A.; Skhiri, M.; Simos, A.; et al. Clinical and Functional Correlates of Early Microvascular Dysfunction After Heart Transplantation. Circ. Hear. Fail. 2012, 5, 759–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camici, P.G.; Crea, F. Coronary Microvascular Dysfunction. N. Engl. J. Med. 2007, 356, 830–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chih, S.; Chong, A.Y.; Mielniczuk, L.M.; Bhatt, D.L.; Beanlands, R.S. Allograft Vasculopathy: The Achilles’ Heel of Heart Transplantation. J. Am. Coll. Cardiol. 2016, 68, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Syrjälä, S.O.; Nykänen, A.I.; Tuuminen, R.; Raissadati, A.; Keränen, M.A.I.; Arnaudova, R.; Krebs, R.; Koh, G.Y.; Alitalo, K.; Lemström, K.B. Donor Heart Treatment With COMP-Ang1 Limits Ischemia-Reperfusion Injury and Rejection of Cardiac Allografts. Am. J. Transplant. 2015, 15, 2075–2084. [Google Scholar] [CrossRef] [PubMed]
- Pernow, J.; Gonon, A.; Gourine, A. The role of the endothelium for reperfusion injury. Eur. Hear. J. Suppl. 2001, 3, C22–C27. [Google Scholar] [CrossRef] [Green Version]
- Messer, S.; Page, A.; Axell, R.; Berman, M.; Hernández-Sánchez, J.; Colah, S.; Parizkova, B.; Valchanov, K.; Dunning, J.; Pavlushkov, E.; et al. Outcome after heart transplantation from donation after circulatory-determined death donors. J. Hear. Lung Transplant. 2017, 36, 1311–1318. [Google Scholar] [CrossRef]
- Saemann, L.; Wenzel, F.; Kohl, M.; Korkmaz-Icöz, S.; Hoorn, F.; Loganathan, S.; Guo, Y.; Ding, Q.; Zhou, P.; Veres, G.; et al. Monitoring of perfusion quality and prediction of donor heart function during ex-vivo machine perfusion by myocardial microcirculation versus surrogate parameters. J. Hear. Lung Transplant. 2021, 40, 387–391. [Google Scholar] [CrossRef]
- Saemann, L.; Großkopf, A.; Hoorn, F.; Veres, G.; Guo, Y.; Korkmaz-Icöz, S.; Karck, M.; Simm, A.; Wenzel, F.; Szabó, G. Relationship of Laser-Doppler-Flow and coronary perfusion and a concise update on the importance of coronary microcirculation in donor heart machine perfusion. Clin. Hemorheol. Microcirc. 2021, 79, 121–128. [Google Scholar] [CrossRef]
- Saemann, L.; Korkmaz, S.; Hoorn, F.; Zhou, P.; Ding, Q.; Guo, Y.; Veres, G.; Loganathan, S.; Brune, M.; Wenzel, F.; et al. Reconditioning of Donation after Cardiac Death Hearts by Ex-Vivo Machine Perfusion with a Novel HTK-N Preservation Solution. J. Hear. Lung Transplant. 2021, 40, S29. [Google Scholar] [CrossRef]
- Kursa, M.B.; Rudnicki, W.R. Feature Selection with theBorutaPackage. J. Stat. Softw. 2010, 36, 1–13. [Google Scholar] [CrossRef]
- Bayliss, W.M. On the local reactions of the arterial wall to changes of internal pressure. J. Physiol. 1902, 28, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Ardehali, A.; Esmailian, F.; Deng, M.; Soltesz, E.; Hsich, E.; Naka, Y.; Mancini, D.; Camacho, M.; Zucker, M.; Leprince, P.; et al. Ex-vivo perfusion of donor hearts for human heart transplantation (PROCEED II): A prospective, open-label, multicentre, randomised non-inferiority trial. Lancet 2015, 385, 2577–2584. [Google Scholar] [CrossRef]
- Kjekshus, J.K. Mechanism for Flow Distribution in Normal and Ischemic Myocardium during Increased Ventricular Preload in the Dog. Circ. Res. 1973, 33, 489–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Méndez-Carmona, N.; Wyss, R.K.; Arnold, M.; Joachimbauer, A.; Segiser, A.; Fiedler, G.M.; Carrel, T.P.; Stahel, H.T.T.; Longnus, S.L. Differential effects of ischemia/reperfusion on endothelial function and contractility in donation after circulatory death. J. Hear. Lung Transplant. 2019, 38, 767–777. [Google Scholar] [CrossRef]
- Rezkalla, S.H.; Dharmashankar, K.C.; Abdalrahman, I.B.; Kloner, R.A. No-Reflow Phenomenon Following Percutaneous Coronary Intervention for Acute Myocardial Infarction: Incidence, Outcome, and Effect of Pharmacologic Therapy. J. Interv. Cardiol. 2010, 23, 429–436. [Google Scholar] [CrossRef]
- Chen, X.; Andresen, B.; Hill, M.; Zhang, J.; Booth, F.; Zhang, C. Role of Reactive Oxygen Species in Tumor Necrosis Factor-alpha Induced Endothelial Dysfunction. Curr. Hypertens. Rev. 2008, 4, 245–255. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Hein, T.W.; Wang, W.; Ren, Y.; Shipley, R.D.; Kuo, L. Activation of JNK and xanthine oxidase by TNF-α impairs nitric oxide-mediated dilation of coronary arterioles. J. Mol. Cell. Cardiol. 2006, 40, 247–257. [Google Scholar] [CrossRef]
- Roebuck, K.A. Oxidant stress regulation of IL-8 and ICAM-1 gene expression: Differential activation and binding of the transcription factors AP-1 and NF-kappaB (Review). Int. J. Mol. Med. 1999, 4, 223–253. [Google Scholar] [CrossRef]
- Yeh, M.; Leitinger, N.; de Martin, R.; Onai, N.; Matsushima, K.; Vora, D.K.; Berliner, J.A.; Reddy, S.T. Increased Transcription of IL-8 in Endothelial Cells Is Differentially Regulated by TNF-α and Oxidized Phospholipids. Arter. Thromb. Vasc. Biol. 2001, 21, 1585–1591. [Google Scholar] [CrossRef] [Green Version]
- Elices, M.J.; Osborn, L.; Takada, Y.; Crouse, C.; Luhowskyj, S.; Hemler, M.E.; Lobb, R.R. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/Fibronectin binding site. Cell 1990, 60, 577–584. [Google Scholar] [CrossRef]
- Wang, X.; Feuerstein, G.Z.; Gu, J.-L.; Lysko, P.G.; Yue, T.-L. Interleukin-1β induces expression of adhesion molecules in human vascular smooth muscle cells and enhances adhesion of leukocytes to smooth muscle cells. Atherosclerosis 1995, 115, 89–98. [Google Scholar] [CrossRef]
- Woodfin, A.; Voisin, M.-B.; Nourshargh, S. PECAM-1: A Multi-Functional Molecule in Inflammation and Vascular Biology. Arter. Thromb. Vasc. Biol. 2007, 27, 2514–2523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleming, I.; Fisslthaler, B.; Dixit, M.; Busse, R. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J. Cell Sci. 2005, 118, 4103–4111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinsky, D.J.; Patton, S.; Mesaros, S.; Brovkovych, V.; Kubaszewski, E.; Grunfeld, S.; Malinski, T. Mechanical transduction of nitric oxide synthesis in the beating heart. Circ. Res. 1997, 81, 372–379. [Google Scholar] [CrossRef]
- Dusserre, N.; L’Heureux, N.; Bell, K.; Stevens, H.; Yeh, J.; Otte, L.; Loufrani, L.; Frangos, J. PECAM-1 Interacts With Nitric Oxide Synthase in Human Endothelial Cells: Implication for flow-induced nitric oxide synthase activation. Arter. Thromb. Vasc. Biol. 2004, 24, 1796–1802. [Google Scholar] [CrossRef] [Green Version]
- McCormick, M.E.; Goel, R.; Fulton, D.; Oess, S.; Newman, D.; Tzima, E. Platelet-Endothelial Cell Adhesion Molecule-1 Regulates Endothelial NO Synthase Activity and Localization Through Signal Transducers and Activators of Transcription 3–Dependent NOSTRIN Expression. Arter. Thromb. Vasc. Biol. 2011, 31, 643–649. [Google Scholar] [CrossRef] [Green Version]
- Valantine, H.A. Cardiac allograft vasculopathy: Central role of endothelial injury leading to transplant “atheroma”. Transplantation 2003, 76, 891–899. [Google Scholar] [CrossRef]
- Labarrere, C.A.; Lee, J.B.; Nelson, D.R.; Al-Hassani, M.; Miller, S.J.; Pitts, D.E. C-reactive protein, arterial endothelial activation, and development of transplant coronary artery disease: A prospective study. Lancet 2002, 360, 1462–1467. [Google Scholar] [CrossRef]
- Aszodi, A.; Hunziker, E.B.; Brakebusch, C.; Fässler, R. β1 integrins regulate chondrocyte rotation, G1 progression, and cytokinesis. Genes Dev. 2003, 17, 2465–2479. [Google Scholar] [CrossRef] [Green Version]
- Eckle, T.; Kohler, D.; Lehmann, R.; El Kasmi, K.C.; Eltzschig, H.K. Hypoxia-Inducible Factor-1 Is Central to Cardioprotection: A new paradigm for ischemic preconditioning. Circulation 2008, 118, 166–175. [Google Scholar] [CrossRef]
- Shohet, R.V.; Garcia, J.A. Keeping the engine primed: HIF factors as key regulators of cardiac metabolism and angiogenesis during ischemia. Klin. Wochenschr. 2007, 85, 1309–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Oliveira, S.D.S.; Zimnicka, A.M.; Jiang, Y.; Sharma, T.; Chen, S.; Lazarov, O.; Bonini, M.G.; Haus, J.M.; Minshall, R.D. Reciprocal regulation of eNOS and caveolin-1 functions in endothelial cells. Mol. Biol. Cell 2018, 29, 1190–1202. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saemann, L.; Hoorn, F.; Georgevici, A.-I.; Pohl, S.; Korkmaz-Icöz, S.; Veres, G.; Guo, Y.; Karck, M.; Simm, A.; Wenzel, F.; et al. Cytokine Adsorber Use during DCD Heart Perfusion Counteracts Coronary Microvascular Dysfunction. Antioxidants 2022, 11, 2280. https://doi.org/10.3390/antiox11112280
Saemann L, Hoorn F, Georgevici A-I, Pohl S, Korkmaz-Icöz S, Veres G, Guo Y, Karck M, Simm A, Wenzel F, et al. Cytokine Adsorber Use during DCD Heart Perfusion Counteracts Coronary Microvascular Dysfunction. Antioxidants. 2022; 11(11):2280. https://doi.org/10.3390/antiox11112280
Chicago/Turabian StyleSaemann, Lars, Fabio Hoorn, Adrian-Iustin Georgevici, Sabine Pohl, Sevil Korkmaz-Icöz, Gábor Veres, Yuxing Guo, Matthias Karck, Andreas Simm, Folker Wenzel, and et al. 2022. "Cytokine Adsorber Use during DCD Heart Perfusion Counteracts Coronary Microvascular Dysfunction" Antioxidants 11, no. 11: 2280. https://doi.org/10.3390/antiox11112280




