The Role of H2O2-Scavenging Enzymes (Ascorbate Peroxidase and Catalase) in the Tolerance of Lemna minor to Antibiotics: Implications for Phytoremediation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Bioassays
2.2. Physiological Responses
2.3. Statistical Analyses
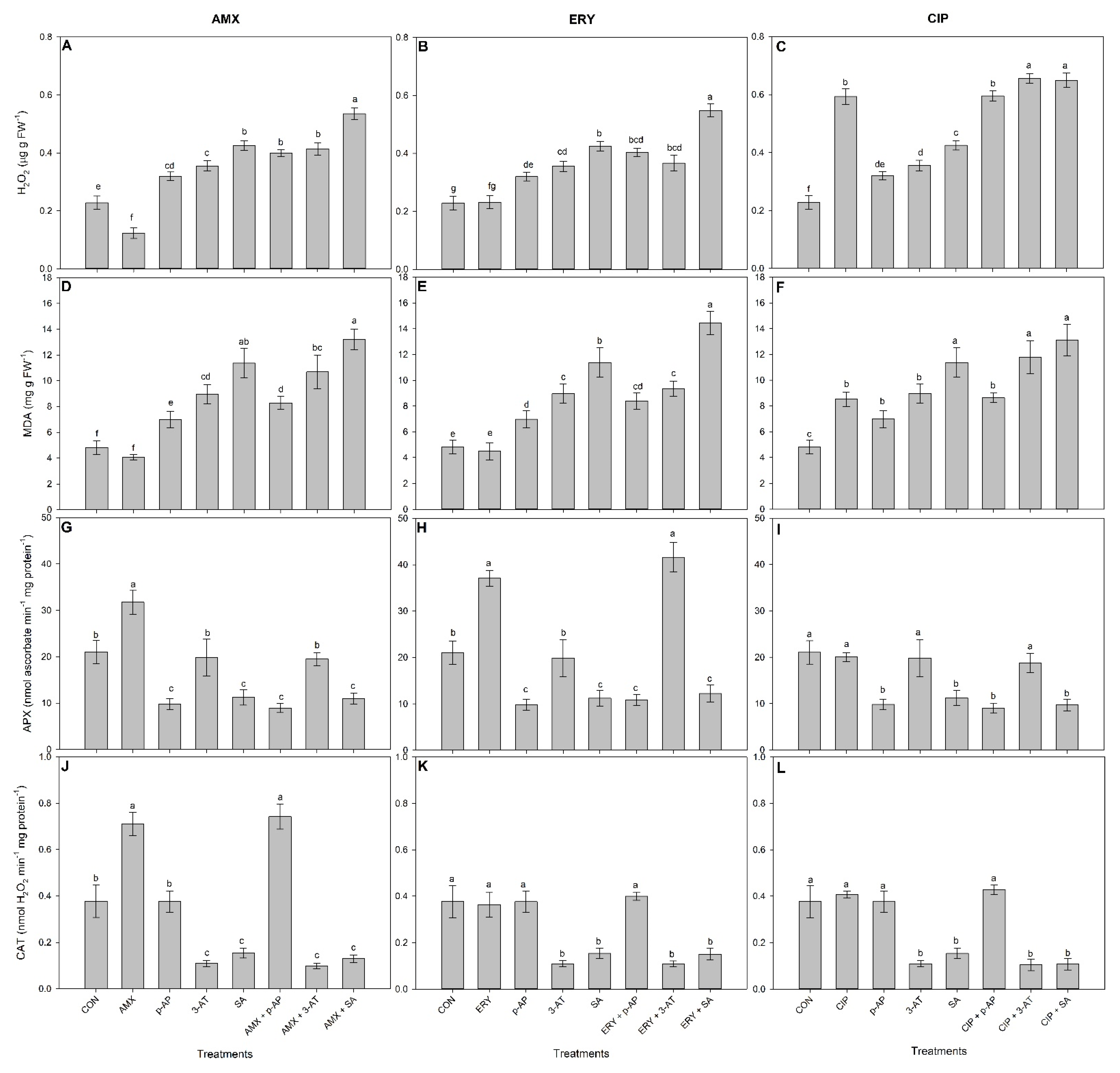
3. Results
3.1. Effects of Antioxidant Enzyme Inhibitors on Enzyme Activities
3.2. The Isolated and Combined Effects of Antibiotics and Antioxidant-Enzyme Inhibitors on Plant Physiology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Zhang, J. The effect of acute erythromycin exposure on the swimming ability of Zebrafish (Danio rerio) and medaka (Oryzias latipes). Int. J. Environ. Res. Public Health 2020, 17, 3389. [Google Scholar] [CrossRef]
- Wu, Y.; Wan, L.; Zhang, W.; Ding, H.; Yang, W. Resistance of cyanobacteria Microcystis aeruginosa to erythromycin with multiple exposure. Chemosphere 2020, 249, 126147. [Google Scholar] [CrossRef] [PubMed]
- Rocha, D.C.D.C.; da Silva Rocha, C.; Tavares, D.S.D.S.; de Morais Calado, S.L.S.L.; Gomes, M.P.M.P. Veterinary antibiotics and plant physiology: An overview. Sci. Total Environ. 2021, 767, 144902. [Google Scholar] [CrossRef]
- Karkman, A.; Do, T.T.; Walsh, F.; Virta, M.P.J. Antibiotic-Resistance Genes in Waste Water. Trends Microbiol. 2018, 26, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Gomes, M.P.; Brito, J.C.M.; Rocha, D.C.; Navarro-Silva, M.; Juneau, P. Individual and combined effects of amoxicillin, enrofloxacin, and oxytetracycline on Lemna minor physiology. Ecotoxicol. Environ. Saf. 2020, 203, 111025. [Google Scholar] [CrossRef]
- Gomes, M.P.; Brito, J.C.M.; Carneiro, M.M.L.C.; Cunha, M.R.R.; Garcia, Q.S.; Figueredo, C.C. Responses of the nitrogen-fixing aquatic fern Azolla to water contaminated with ciprofloxacin: Impacts on biofertilization. Environ. Pollut. 2017, 232, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.P.; de Brito, J.C.M.; Bicalho, E.M.; Silva, J.G.; de Fátima Gomides, M.; Garcia, Q.S.; Figueredo, C.C.; Brito, J.C.M.; Bicalho, E.M.; Silva, J.G.; et al. Ciprofloxacin vs. temperature: Antibiotic toxicity in the free-floating liverwort Ricciocarpus natans from a climate change perspective. Chemosphere 2018, 202, 410–419. [Google Scholar] [CrossRef]
- Gomes, M.P.; Gonçalves, C.A.; de Brito, J.C.M.; Souza, A.M.; da Silva Cruz, F.V.; Bicalho, E.M.; Figueredo, C.C.; Garcia, Q.S. Ciprofloxacin induces oxidative stress in duckweed (Lemna minor L.): Implications for energy metabolism and antibiotic-uptake ability. J. Hazard. Mater. 2017, 328, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.S.; Kochi, L.Y.; Ribeiro, G.B.; Rocha, D.C.; Carneiro, D.N.M.; Gomes, M.P. Evaluating aquatic macrophytes for removing erythromycin from contaminated water: Floating or submerged? Int. J. Phytoremediat. 2021. [Google Scholar] [CrossRef]
- Gomes, M.P.; Tavares, D.S.; Richardi, V.S.; Marques, R.Z.; Wistuba, N.; Moreira de Brito, J.C.; Soffiatti, P.; Sant’Anna-Santos, B.F.; Navarro da Silva, M.A.; Juneau, P. Enrofloxacin and Roundup® interactive effects on the aquatic macrophyte Elodea canadensis physiology. Environ. Pollut. 2019, 249, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Nunes, B.; Veiga, V.; Frankenbach, S.; Serôdio, J.; Pinto, G. Evaluation of physiological changes induced by the fluoroquinolone antibiotic ciprofloxacin in the freshwater macrophyte species Lemna minor and Lemna gibba. Environ. Toxicol. Pharmacol. 2019, 72, 103242. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Pandey, B.; Suthar, S. Phytotoxicity of amoxicillin to the duckweed Spirodela polyrhiza: Growth, oxidative stress, biochemical traits and antibiotic degradation. Chemosphere 2018, 201, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, D.; Chang, F.; Dang, C.; Fu, J. Combined Effects of Sulfamethoxazole and Erythromycin on a Freshwater Microalga, Raphidocelis subcapitata: Toxicity and Oxidative Stress. Antibiotics 2021, 10, 576. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.P.; Carvalho, M.; Carvalho, G.S.; Marques, T.C.L.L.S.M.; Garcia, Q.S.; Guilherme, L.R.G.; Soares, A.M. Phosphorus improves arsenic phytoremediation by Anadenanthera peregrina by alleviating induced oxidative stress. Int. J. Phytoremediat. 2013, 15, 633–646. [Google Scholar] [CrossRef]
- Gomes, M.P.; Juneau, P. Oxidative stress in duckweed (Lemna minor L.) induced by glyphosate: Is the mitochondrial electron transport chain a target of this herbicide? Environ. Pollut. 2016, 218, 402–409. [Google Scholar] [CrossRef]
- Bischoff, H.; Bold, H. Phycological Studies. In University of Texas Publication 6924; University of Texas: Austin, TX, USA, 1963; p. 96. [Google Scholar]
- Durner, J.; Klessig, D.F. Inhibition of ascorbate peroxidase by salicylic acid and 2,6-dichloroisonicotinic acid, two inducers of plant defense responses. Proc. Natl. Acad. Sci. USA 1995, 92, 11312–11316. [Google Scholar] [CrossRef] [Green Version]
- Margoliash, E.; Novogrodsky, A. A study of the inhibition of catalase by 3-amino-1,2,4-triazole. Biochem. J. 1957, 68, 468–475. [Google Scholar] [CrossRef] [Green Version]
- Zámocký, M.; Koller, F. Understanding the structure and function of catalases: Clues from molecular evolution and in vitro mutagenesis. Prog. Biophys. Mol. Biol. 1999, 72, 19–66. [Google Scholar] [CrossRef]
- Choi, S.; Sim, W.; Jang, D.; Yoon, Y.; Ryu, J.; Oh, J.; Woo, J.S.; Kim, Y.M.; Lee, Y. Antibiotics in coastal aquaculture waters: Occurrence and elimination efficiency in oxidative water treatment processes. J. Hazard. Mater. 2020, 396, 122585. [Google Scholar] [CrossRef]
- Anawar, H.M.; Rengel, Z.; Damon, P.; Tibbett, M. Arsenic-phosphorus interactions in the soil-plant-microbe system: Dynamics of uptake, suppression and toxicity to plants. Environ. Pollut. 2018, 233, 1003–1012. [Google Scholar] [CrossRef]
- Gomes, M.P.; Brito, J.C.M.; Vieira, F.; Kitamura, R.; Juneau, P. Emerging contaminants in streams of Doce river watershed, Minas Gerais, Brazil. Front. Environ. Sci. 2022, 9, 801599. [Google Scholar] [CrossRef]
- Organization for Economic Co-operation and Development. Test No. 221: Lemna sp. Growth Inhibition Test. Guidel. Test. Chem. 2006, 1–26. [Google Scholar] [CrossRef]
- van Kooten, O.; Snel, J. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth. Res. 1990, 25, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Hodges, D.M.; Delong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Beyer, W.F., Jr.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Chovanová, K.; Böhmer, M.; Poljovka, A.; Budiš, J.; Harichová, J.; Szemeš, T.; Zámocký, M. Parallel Molecular Evolution of Catalases and Superoxide Dismutases—Focus on Thermophilic Fungal Genomes. Antioxidants 2020, 9, 1047. [Google Scholar] [CrossRef]
- Gomes, M.P.; Garcia, Q.S. Reactive oxygen species and seed germination. Biologia 2013, 68, 351–357. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci. Rep. 2018, 8, 16496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mani, F.; Bettaieb, T.; Doudech, N.; Hannachi, C. Effect of hydrogen peroxide and thiourea on dormancy breaking of microtubers and field-grown tubers of potato. Afr. Crop Sci. J. 2013, 21, 221–234. [Google Scholar]
- Chen, G.; Asada, K. Hydroxyurea and p-aminophenol are the suicide inhibitors of ascorbate peroxidase. J. Biol. Chem. 1990, 265, 2775–2781. [Google Scholar] [CrossRef]
- Margoliash, E.; Novogrodsky, A.; Schejter, A. Irreversible reaction of 3-amino-1,2,4-triazole andrelated inhibitors with the protein of catalase. Biochem. J. 1959, 74, 339–350. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Silva, H.; Klessig, D.F. Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science 1993, 262, 1883–1886. [Google Scholar] [CrossRef]
- Gomes, M.P.; Le Manac’h, S.G.; Hénault-Ethier, L.; Labrecque, M.; Lucotte, M.; Juneau, P. Glyphosate-dependent inhibition of photosynthesis in willow. Front. Plant Sci. 2017, 8, 207. [Google Scholar] [CrossRef]
- Takahashi, S.; Murata, N. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 2008, 13, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-X.; Chen, T.-B.; Huang, Z.-C.; Lei, M.; Liao, X.-Y. Effect of arsenic on chloroplast ultrastructure and calcium distribution in arsenic hyperaccumulator Pteris vittata L. Chemosphere 2006, 62, 803–809. [Google Scholar] [CrossRef]
- Gomes, M.P.; Le Manac’h, S.G.; Moingt, M.; Smedbol, E.; Paquet, S.; Labrecque, M.; Lucotte, M.; Juneau, P. Impact of phosphate on glyphosate uptake and toxicity in willow. J. Hazard. Mater. 2016, 304, 269–279. [Google Scholar] [CrossRef] [Green Version]
- Gomes, M.P.; Soares, A.M.; Garcia, Q.S. Phosphorous and sulfur nutrition modulate antioxidant defenses in Myracrodruom urundeuva plants exposed to arsenic. J. Hazard. Mater. 2014, 276, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.P.; Carneiro, M.M.L.C.; Nogueira, C.O.G.; Soares, A.M.; Garcia, Q.S. The system modulating ROS content in germinating seeds of two Brazilian savanna tree species exposed to As and Zn. Acta Physiol. Plant. 2013, 35, 1011–1022. [Google Scholar] [CrossRef]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012, 35, 1011–1019. [Google Scholar] [CrossRef] [Green Version]
- Gomes, M.; Brito, J.; Silva, J.; Crus, F.; Bicalho, E.; Herken, D.; Garcia, Q. Temperature effects on Zn-responses and Zn-reclamation capacity of two native Brazilian plant species: Implications of climate change. Environ. Exp. Bot. 2018, 155, 589–599. [Google Scholar] [CrossRef]
- Aristilde, L.; Melis, A.; Sposito, G.; Aristilde, L.; Melis, A.; Sposito, G. Inhibition of photosynthesis by a fluoroquinolone antibiotic. Environ. Sci. Technol. 2010, 44, 1444–1450. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, M.P.; Kitamura, R.S.A.; Marques, R.Z.; Barbato, M.L.; Zámocký, M. The Role of H2O2-Scavenging Enzymes (Ascorbate Peroxidase and Catalase) in the Tolerance of Lemna minor to Antibiotics: Implications for Phytoremediation. Antioxidants 2022, 11, 151. https://doi.org/10.3390/antiox11010151
Gomes MP, Kitamura RSA, Marques RZ, Barbato ML, Zámocký M. The Role of H2O2-Scavenging Enzymes (Ascorbate Peroxidase and Catalase) in the Tolerance of Lemna minor to Antibiotics: Implications for Phytoremediation. Antioxidants. 2022; 11(1):151. https://doi.org/10.3390/antiox11010151
Chicago/Turabian StyleGomes, Marcelo Pedrosa, Rafael Shinji Akiyama Kitamura, Raizza Zorman Marques, Marcello Locatelli Barbato, and Marcel Zámocký. 2022. "The Role of H2O2-Scavenging Enzymes (Ascorbate Peroxidase and Catalase) in the Tolerance of Lemna minor to Antibiotics: Implications for Phytoremediation" Antioxidants 11, no. 1: 151. https://doi.org/10.3390/antiox11010151







