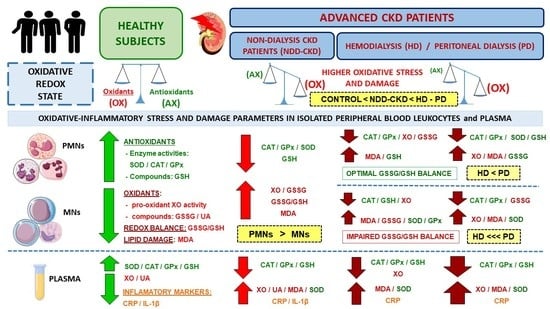
Oxidative Stress in Patients with Advanced CKD and Renal Replacement Therapy: The Key Role of Peripheral Blood Leukocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Participants
2.2. Collection of Blood Samples and Processing
2.3. Peripheral Blood Lymphocytes Population Subtyping
2.4. Superoxide Dismutase Activity
2.5. Catalase Activity
2.6. Gluthathione Peroxidase Activity
2.7. Xanthine Oxidase Activity
2.8. Glutathione Content Assay
2.9. Lipid Peroxidation Assay
2.10. Protein Content Assay
2.11. Cytokine IL-1β Measurement
2.12. Statistical Analysis
3. Results
3.1. Demographical, Clinical and Biochemical Characteristics
3.2. Changes in Oxidative Stress and Lipid Damage Parameters in Plasma
3.3. Changes in Oxidative Stress and Lipid Damage Parameters in Isolated Peripheral Blood Leukocytes
3.4. Correlations Between Biochemical, Inflammatory and Oxidative Stress Parameters in Plasma
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.R. Global Prevalence of Chronic Kidney Disease-A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef]
- Ling, X.C.; Kuo, K. Oxidative stress in chronic kidney disease. Ren. Replace. Ther. 2018, 4, 53. [Google Scholar] [CrossRef] [Green Version]
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [Green Version]
- Stenvinkel, P.; Larsson, T.E. Chronic kidney disease: A clinical model of premature aging. Am. J. Kidney Dis. 2013, 62, 339–351. [Google Scholar] [CrossRef] [Green Version]
- Carracedo, J.; Alique, M.; Vida, C.; Bodega, G.; Ceprián, N.; Morales, E.; Praga, M.; de Sequera, P.; Ramírez, R. Mechanisms of Cardiovascular Disorders in Patients with Chronic Kidney Disease: A Process Related to Accelerated Senescence. Front. Cell Dev. Biol. 2020, 8, 185. [Google Scholar] [CrossRef] [Green Version]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and Oxidative Stress in Chronic Kidney Disease-Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci. 2020, 21, 263. [Google Scholar] [CrossRef] [Green Version]
- Zijlstra, L.E.; Trompet, S.; Mooijaart, S.P.; Van Buren, M.; Sattar, N.; Stott, D.J.; Jukema, W. The association of kidney function and cognitive decline in older patients at risk of cardiovascular disease: A longitudinal data analysis. BMC Nephrol. 2020, 21, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Rysz, J.; Franczyk, B.; Lawinski, J.; Gluba-Brzózka, A. Oxidative Stress in ESRD Patients on Dialysis and the Risk of Cardiovascular Diseases. Antioxidants 2020, 9, 1079. [Google Scholar] [CrossRef]
- De la Fuente, M.; Miquel, J. An update of the Oxidation-Inflammation theory of aging: The involvement of the immune system in oxi-inflamm-aging. Curr. Pharm. Des. 2009, 15, 3003–3026. [Google Scholar] [CrossRef] [PubMed]
- Vida, C.; de Toda, I.M.; Cruces, J.; Garrido, A.; Gónzalez-Sánchez, M.; De la Fuente, M. Role of macrophages in age-related oxidative stress and lipofuscin accumulation in mice. Redox Biol. 2017, 12, 423–437. [Google Scholar] [CrossRef] [Green Version]
- Vida, C.; de Toda, I.M.; Garrido, A.; Carro, E.; Molina, J.A.; De la Fuente, M. Impairment of Several Immune Functions and Redox State in Blood Cells of Alzheimer’s Disease Patients. Relevant Role of Neutrophils in Oxidative Stress. Front. Immunol. 2018, 8, 1974. [Google Scholar] [CrossRef] [Green Version]
- Liakopoulos, V.; Roumeliotis, S.; Gorny, X.; Eleftheriadis, T.; Mertens, P.R. Oxidative stress in patients undergoing peritoneal dialysis: A current review of the literature. Oxid. Med. Cell. Longev. 2017, 2017, 3494867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liakopoulos, V.; Roumeliotis, S.; Bozikas, A.; Eleftheriadis, T.; Dounousi, E. Antioxidant supplementation in renal replacement therapy patients: Is there evidence? Oxid. Med. Cell. Longev. 2019, 2019, 9109473. [Google Scholar] [CrossRef] [Green Version]
- Liakopoulos, V.; Roumeliotis, S.; Zarogiannis, S.; Eleftheriadis, T.; Mertens, P.R. Oxidative stress in hemodialysis: Causative mechanisms, clinical implications, and possible therapeutic interventions. Semin. Dial. 2019, 32, 58–71. [Google Scholar] [CrossRef]
- Duni, A.; Liakopoulos, V.; Roumeliotis, S.; Peschos, D.; Dounousi, E. Oxidative Stress in the Pathogenesis and Evolution of Chronic Kidney Disease: Untangling Ariadne’s Thread. Int. J. Mol. Sci. 2019, 20, 3711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podkowinska, A.; Formanowicz, D. Chronic Kidney Disease as Oxidative Stress- and Inflammatory-Mediated Cardiovascular Disease. Antioxidants 2020, 9, 752. [Google Scholar] [CrossRef]
- Grune, T.; Sommerburg, O.; Siems, W.G. Oxidative stress in anemia. Clin. Nephrol. 2000, 53 (Suppl. 1), S18–S22. [Google Scholar]
- Nuhul, F.; Bhandari, S. Oxidative Stress and Cardiovascular Complications in Chronic Kidney Disease, the Impact of Anaemia. Pharmaceuticals 2018, 11, 103. [Google Scholar]
- Maraj, M.; Kusnierz-Cabala, B.; Dumnicka, P.; Gawlik, K.; Pawlica-Gosiewska, D.; Gala-Badzinska, A.; Zabek-Adamska, A. Redox balance correlates with nutritional status among patients with End-Stage Renal Disease treated with maintenance hemodialysis. Oxid. Med. Cell. Longev. 2019, 2019, 6309465. [Google Scholar] [CrossRef]
- Garibotto, G.; Picciotto, D.; Saio, M.; Esposito, P.; Verzola, D. Muscle protein turnover and low-protein diets in patients with chronic kidney disease. Nephrol. Dial. Transplant. 2020, 35, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Davis, K.L.; Fernelius, C.; Eliason, N.B.; Wilson, A.; Beddhu, S.; Roberts, W.L. Blood enzymes and oxidative stress in chronic kidney disease: A cross sectional study. Ann. Clin. Lab. Sci. 2011, 41, 331–339. [Google Scholar]
- Liakopoulos, V.; Jeron, A.; Shah, A.; Bruder, D.; Mertens, P.R.; Gorny, X. Hemodialysis-related changes in phenotypical features of monocytes. Sci. Rep. 2018, 8, 13964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihai, S.; Codrici, E.; Popescu, I.D.; Enciu, A.M.; Albulescu, L.; Necula, L.G.; Mambet, C.; Anton, G.; Tanase, C. Inflammation-related mechanisms in chronic kidney disease prediction, progression, and outcome. J. Immunol. Res. 2018, 2018, 2180373. [Google Scholar] [CrossRef] [PubMed]
- Fortuño, A.; Beloqui, O.; San José, G.; Moreno, M.U.; Zalba, G.; Díez, J. Increased phagocytic nicotinamide adenine dinucleotide phosphate oxidase dependent superoxide production in patients with early chronic kidney disease. Kidney Int. Suppl. 2005, 22, S71–S75. [Google Scholar] [CrossRef] [Green Version]
- Puchades-Montesa, M.J.; González-Ricoa, M.A.; Solís-Salgueroa, M.A.; Maicasa-Torregrosa, I.; Juan-García, I.; Miquel-Carrascoa, A.; Tormos-Muñoz, M.C.; Sáez-Tormoc, G. Study of oxidative stress in advanced kidney disease. Nefrología 2009, 29, 502. [Google Scholar]
- Popolo, A.; Autore, G.; Pinto, A.; Marzocco, S. Oxidative stress in patients with cardiovascular disease and chronic renal failure. Free Radic. Res. 2013, 47, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Gondouin, B.; Jourde-Chiche, N.; Sallee, M.; Dou, L.; Cerini, C.; Loundou, A.; Morange, S.; Berland, Y.; Burtey, S.; Brunet, P.; et al. Plasma xanthine oxidase activity is predictive of cardiovascular disease in patients with chronic kidney disease, independently of uric acid levels. Nephron 2015, 131, 167–174. [Google Scholar] [CrossRef]
- Duni, A.; Liakopoulos, V.; Rapsomanikis, K.P.; Dounousi, E. Chronic Kidney Disease and Disproportionally Increased Cardiovascular Damage: Does Oxidative Stress Explain the Burden? Oxid. Med. Cell. Longev. 2017, 2017, 9036450. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wu, S.; Jiang, J.; Tian, J.; Chen, J.; Yu, X.; Chen, P.; Mei, C.; Xiong, F.; Shi, W.; et al. Accumulation of circulating advanced oxidation protein products is an independent risk factor for ischaemic heart disease in maintenance haemodialysis patients. Nephrology 2012, 17, 642–649. [Google Scholar] [CrossRef]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef] [Green Version]
- Liakopoulos, V.; Roumeliotis, S.; Gorny, X.; Dounousi, E.; Mertens, P.R. Oxidative Stress in Hemodialysis Patients: A Review of the Literature. Oxid. Med. Cell. Longev. 2017, 2017, 3081856. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Eleftheriadis, T.; Liakopoulos, V. Is oxidative stress an issue in peritoneal dialysis? Semin. Dial. 2019, 32, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha-Lakshmi, B.; Harini-Devi, N.; Suchitra, M.M.; Srinivasa-Rao, P.; Siva-Kumar, V. Changes in the inflammatory and oxidative stress markers during a single hemodialysis session in patients with chronic kidney disease. Ren. Fail. 2018, 40, 534–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-García, R.; Ramírez, R.; de Sequera, P.; Albalate, M.; Puerta, M.; Ortega, M.; Ruiz, M.C.; Alcazar-Arroyo, R. Citrate dialysate does not induce oxidative stress or inflammation in vitro as compared to acetate dialysate. Nefrología 2017, 37, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Vida, C.; Carracedo, J.; Sequera, P.; Bodega, G.; Pérez, R.; Alique, M.; Ramírez, R. Increasing the Magnesium Concentration in Various Dialysate Solutions Differentially Modulates Oxidative Stress in a Human Monocyte Cell Line. Antioxidants 2020, 9, 319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Dai, H.; Liu, L.; Xu, C.; Yin, Y.; Yi, J.; Bielec, M.D.; Han, Y.; Lia, S. Citrate reduced oxidative damage in stem cells by regulating cellular redox signaling pathways and represent a potential treatment for oxidative stress-induced diseases. Redox Biol. 2019, 21, 101057. [Google Scholar] [CrossRef]
- Ogunro, P.S.; Olujombo, F.A.; Ajala, M.O.; Oshodi, T.T. The effect of a membrane dialyzer during hemodialysis on the antioxidant status and lipid peroxidation of patients with end-stage renal disease. Saudi J. Kidney Dis. Transpl. 2014, 25, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Poulianiti, K.P.; Kaltsatou, A.; Mitrou, G.I.; Jamurtas, A.Z.; Koutedakis, Y.; Maridaki, M.; Stefanidis, I.; Sakkas, G.K.; Karatzaferi, C. Systemic redox imbalance in chronic kidney disease. Oxid. Med. Cell. Longev. 2016, 2016, 8598253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sepe, V.; Gregorini, M.; Rampino, T.; Esposito, P.; Coppo, R.; Galli, F.; Libetta, C. Vitamin e-loaded membrane dialyzers reduce hemodialysis inflammaging. BMC Nephrol. 2019, 20, 412. [Google Scholar] [CrossRef] [PubMed]
- Yeter, H.H.; Korucu, B.; Akcay, O.F.; Derici, K.; Derici, U.; Arinsoy, T. Effects of medium cut-off dialysis membranes on inflammation and oxidative stress in patients on maintenance hemodialysis. Int. Urol. Nephrol. 2020, 52, 1779–1789. [Google Scholar] [CrossRef] [PubMed]
- Terawaki, H.; Matsuyama, Y.; Era, S.; Matsuo, N.; Ikeda, M.; Ogura, M.; Yokoyama, K.; Yamamoto, H.; Hosoya, T.; Nakayama, M. Elevated oxidative stress measured as albumin redox state in continuous ambulatory peritoneal dialysis patients correlates with small uraemic solutes. Nephrol. Dial. Transplant. 2007, 22, 968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ignace, S.; Fouque, D.; Arkouche, W.; Steghens, J.P.; Guebre-Egziabher, F. Preserved residual renal function is associated with lower oxidative stress in peritoneal dialysis patients. Nephrol. Dial. Transplant. 2009, 24, 1685–1689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuya, R.; Kumagai, H.; Odamaki, M.; Takahashi, M.; Miyaki, A.; Hishida, A. Impact of residual renal function on plasma levels of advanced oxidation protein products and pentosidine in peritoneal dialysis patients. Nephron Clin. Pract. 2009, 112, c255–c261. [Google Scholar] [CrossRef]
- Krata, N.; Zagozdzon, R.; Foroncewicz, B.; Mucha, K. Oxidative Stress in Kidney Diseases: The Cause or the Consequence? Arch. Immunol. Ther. Exp. (Warsz.) 2018, 66, 211–220. [Google Scholar] [CrossRef] [Green Version]
- Dixon, J.R. The international conference on harmonization good clinical practice guideline. Qual. Assur. 1998, 6, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.; Allende, L.M.; Ramos, L.E.; Gutiérrez, E.; Pleguezuelo, D.E.; Hernández, E.R.; Ríos, F.; Fernández, C.; Praga, M.; Morales, E. CD19+ B-Cells, a new biomarker of mortality in hemodialysis patients. Front. Immunol. 2018, 9, 1221. [Google Scholar] [CrossRef] [PubMed]
- Hissin, P.J.; Hilf, R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
- Russa, D.; Pellegrino, D.; Montesanto, A.; Gigliotti, P.; Perri, A.; Russa, A.; Bonofiglio, R. Oxidative Balance and Inflammation in Hemodialysis Patients: Biomarkers of Cardiovascular Risk? Oxid. Med. Cell. Longev. 2019, 2019, 8567275. [Google Scholar] [CrossRef]
- de Toda, I.M.; Miguélez, L.; Vida, C.; Carro, E.; De la Fuente, M. Altered Redox State in Whole Blood Cells from Patients with Mild Cognitive Impairment and Alzheimer’s Disease. J. Alzheimers Dis. 2019, 71, 153–163. [Google Scholar] [CrossRef]
- Tbahriti, H.F.; Kaddous, A.; Bouchenak, M.; Mekki, K. Effect of Different Stages of Chronic Kidney Disease and Renal Replacement Therapies on Oxidant-Antioxidant Balance in Uremic Patients. Biochem. Res. Int. 2013, 2013, 358985. [Google Scholar] [CrossRef]
- Harrison, R. Structure and function of xanthine oxidoreductase: Where are we know? Free Radic. Biol. Med. 2002, 6, 774–796. [Google Scholar] [CrossRef]
- Vida, C.; Rodríguez-Terés, S.; Heras, V.; Corpas, I.; De la Fuente, M.; González, E. The aged-related increase in xanthine oxidase expression and activity in several tissues from mice is not shown in long-lived animals. Biogerontology 2011, 12, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Miric, D.; Kisic, B.; Stolic, R.; Miric, B.; Mitic, R.; Janicijevic-Hudomal, S. The role of xanthine oxidase in hemodialysis-induced oxidative injury: Relationship with nutritional status. Oxid. Med. Cell. Longev. 2013, 2013, 245253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boaz, M.; Matas, Z.; Biro, A.; Katzir, Z.E.; Green, M.; Fainaru, M.; Smetana, S. Serum malondialdehyde and prevalent cardiovascular disease in hemodialysis. Kidney Int. 1999, 56, 1078–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terawaki, H.; Hayashi, T.; Murase, T.; Iijima, R.; Waki, K.; Tani, Y.; Nakamura, T.; Yoshimura, K.; Uchida, S.; James, J. Relationship between Xanthine Oxidoreductase Redox and Oxidative Stress among Chronic Kidney Disease Patients. Oxid. Med. Cell. Longev. 2018, 2018, 9714710. [Google Scholar] [CrossRef] [PubMed]
- Sproston, N.R.; Ashworth, J.J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Yoon, Y.J.; Choi, H.J.; Park, S.H.; Kim, C.D.; Kim, I.S.; Kwon, T.H.; Do, J.Y.; Kim, S.H.; Ryu, D.H.; et al. Dialysis modality-dependent changes in serum metabolites: Accumulation of inosine and hypoxanthine in patients on haemodialysis. Nephrol. Dial. Transplant. 2011, 26, 1304–1313. [Google Scholar] [CrossRef] [Green Version]
- McNally, J.S.; Davis, M.E.; Giddens, D.P.; Saha, A.; Hwang, J.; Dikalov, S.; Jo, H.; Harrison, D.G. Vascular signaling by free radicals role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stresss. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H2290–H2297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiyama, S.; Inagaki, M.; Tsuji, M.; Gotoh, H.; Gotoh, T.; Gotoh, Y.; Oguchi, K. mRNA study on Cu/Zn superoxide dismutase induction by hemodialysis treatment. Nephron Clin. Pract. 2005, 99, 107–114. [Google Scholar] [CrossRef]
- Drögue, W.; Breitkreutz, R. Glutathione and immune function. Proc. Nutr. Soc. 2000, 59, 595–600. [Google Scholar] [CrossRef]
- González-Rico, M.; Puchades-Montesa, J.; García-Ramón, R.; Sáez, G.; Tormos, M.C.; Miguel, A. Effect of hemodialysis therapy on oxidative stress in patients with chronic renal failure. Nefrología 2006, 26, 218–225. [Google Scholar] [PubMed]
- Tarng, D.C.; Chen, T.W.; Huang, T.P.; Chen, C.L.; Liu, T.Y.; Wei, Y.H. Increased oxidative damage to peripheral blood leukocyte DNA in chronic peritoneal dialysis patients. J. Am. Soc. Nephrol. 2002, 13, 1321–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiaoyan, J.; Rongyi, C.; Xuesen, C.; Jianzhou, Z.; Jun, J.; Xiaoquiang, D.; Xiaofang, Y. The difference of T cell phenotypes in end stage renal disease patients under different dialysis modality. BMC Nephrol. 2019, 20, 301. [Google Scholar] [CrossRef] [Green Version]
- Usta, M.; Ersoy, A.; Ayar, Y.; Budak, F. The relationship between lymphocyte subsets, nutritional status and tuberculin reactivity in continuous ambulatory peritoneal dialysis and hemodialysis patients. Int. Urol. Nephrol. 2020, 52, 1167–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ducloux, D.; Legendre, M.; Bamoulid, J.; Rebibou, J.M.; Saas, P.; Courivaud, C.; Crepin, T. ESRD-associated immune phenotype depends on dialysis modality and iron status: Clinical implications. Immun. Ageing 2018, 15, 16. [Google Scholar] [CrossRef]



| Characteristics | Control | NDD-CKD | HD | PD |
|---|---|---|---|---|
| Demographic data | n = 17 | n = 39 | n = 39 | n = 22 |
| Male, n (%) | 8 (47.1) | 25 (64.1) | 27 (69.2) | 11 (50) |
| Female, n (%) | 9 (52.9) | 14 (35.9) | 12 (30.8) | 11 (50) |
| Age (years; mean ± SD) | 52.17 ± 15.33 | 60.41 ± 17.26 | 57.46 ± 14.75 | 54.63 ± 15.82 |
| BMI (kg/m2; mean ± SD) | 24.54 ± 3.55 | 25.19 ± 9.17 | 24.29 ± 4.05 | 24.54 ± 4.17 |
| eGFR (mL/min per 1.73 m2) | - | 15.84 ± 2.74 | - | - |
| Comorbidity | ||||
| Hypertension, n (%) | 1 (5.9) | 35 (89.7) | 33 (84.6) | 20 (90.9) |
| Diabetes, n (%) | 2 (11.8) | 17 (43.6) | 7 (17.9) bb | 5 (22.7) b |
| Dyslipidemia, n (%) | 0 (0) | 31 (79.5) | 23 (59.0) b | 14 (63.6) b |
| Hyperuricemia, n (%) | 0 (0) | 27 (69.2) | 10 (25.6) bb | 13 (59.1) c |
| CVD, n (%) | 0 (0) | 19 (48.7) | 19 (48.7) | 7 (31.8) |
| Peripheral vascular disease, n (%) | 0 (0) | 1 (2.6) | 9 (23.1) b | 2 (9.1) c |
| ACVA, n (%) | 0 (0) | 5 (12.8) | 2 (5.1) | 3 (13.6) |
| Tumors, n (%) | 0 (0) | 1 (2.6) | 1 (2.6) | 1 (4.5) |
| Etiology of the nephropathy | ||||
| Glomerulonephritis, n (%) | 0 (0) | 6 (15) | 11 (28) | 8 (36) |
| Hypertension/vascular, n (%) | 0 (0) | 7 (18) | 6 (15) | 4 (18) |
| Diabetes, n (%) | 0 (0) | 12 (31) | 4 (10) | 4 (18) |
| Polycystic kidneys | 0 (0) | 4 (10) | 1 (3) | 1 (5) |
| Tubulointersticial nephritis, n (%) | 0 (0) | 6 (15) | 8 (21) | 2 (5) |
| Others, n (%) | 0 (0) | 4 (10) | 9 (23) | 3 (14) |
| Dialysis data | ||||
| AVF (%) | - | - | 56.41 | - |
| OL-HDF/HFD (%) | - | - | 75/25 | - |
| APD/CAPD (%) | - | - | - | 86/14 |
| Kt/v (mean ± SD) | - | - | 1.67 ± 0.25 | 2.34 ± 0.51 |
| RRF (mL/day; median) | - | - | 400 (100–1300) | 1275 (500–3000) cc |
| Treatment | ||||
| Statins | 0 (0) | 30 (76.9) | 16 (41) b | 14 (63.6) c |
| Allopurinol | 0 (0) | 23 (59) | 8 (20.5) b | 14 (63.6) c |
| Antiplatelet | 0 (0) | 8 (20.5) | 16 (41) b | 7 (31.8) |
| Erythropoietin | 0 (0) | 18 (46.2) | 34 (87.2) bb | 16 (72.7) bb |
| Variables | CONTROL | NDD-CKD | HD | PD |
|---|---|---|---|---|
| Biochemical parameters | n = 17 | n = 39 | n = 39 | n = 22 |
| Serum creatinine (mg/dL) | 0.82 ± 0.16 | 4.17 ± 1.04 aaa | 7.79 ± 1.91 aaa,bbb | 7.46 ± 2.72 aaa,bbb |
| Albumin (g/dL) | 4.65 ± 0.28 | 4.25 ± 0.36 aa | 4.11 ± 0.35 aa | 3.82 ± 0.43 aaa,bb |
| TG (mmol/L) | 96.94 ± 43.44 | 171.02 ± 98.36 aa | 117.23 ± 44.70 bb | 132.68 ± 61.64 b |
| TC (mmol/L) | 178.41 ± 27.64 | 168.41 ± 52.30 | 147.15 ± 30.96 b | 159.45 ± 37.39 |
| HDL-C (mmol/L) | 55.17 ± 8.88 | 46.02 ± 13.43 | 48.72 ± 11.21 | 47.41 ± 13.80 |
| LDL-C (mmol/L) | 103.82 ± 26.33 | 89.85 ± 44.32 | 77.82 ± 31.30 | 90.45 ± 28.03 |
| UA (mg/dL) | 5.10 ± 1.10 | 6.32 ± 1.70 a | 5.78 ± 1.22 | 5.76 ± 1.29 |
| Hb (g/dL) | 13.61 ± 2.30 | 9.98 ± 2.82 a | 8.79 ± 2.85 aa | 10.21 ± 2.52 a |
| HbA1c (mg/dL) | 5.43 ± 0.43 | 5.95 ± 1.20 | 5.14 ± 0.70 b | 42 ± 0.49 |
| Inflammatory markers | ||||
| CRP (mg/dL) | 0.29 ± 0.09 | 0.45 ± 0.07 a | 0.86 ± 0.13 aa,b | 0.83 ± 0.16 aa,b |
| IL-1β (pg/mL) | 4247 ± 1218 | 5200 ± 2450 a | 5156 ± 2697 | 5280 ± 3031 |
| Lymphocyte populations (cells/µL) | ||||
| Total lymphocytes | 1798 ± 449 | 1585 ± 548 | 1083 ± 509 aaa,bbb | 1271 ± 293 aa,b |
| CD3+ T-cells | 1272 ± 350 | 1187 ± 440 | 761 ± 425 aaa,bbb | 916 ± 537 a |
| CD4+ T-cells | 831 ± 308 | 731 ± 295 | 430 ± 280 aaa,bbb | 603 ± 224 a |
| CD8+ T-cells | 410 ± 143 | 429 ± 250 | 301 ± 190 | 387 ± 136 |
| CD4+/CD8+ ratio | 2.23 ± 0.22 | 2.20 ± 0.17 | 1.49 ± 0.09 a,bbb | 1.71 ± 0.21 b |
| CD19+ B-cells | 194 ± 22 | 130 ± 18 | 118 ± 21 a | 81 ± 11 aa,b |
| CD3−CD56+CD16+ NK | 243 ± 23 | 204 ± 22 | 173 ± 24 a,b | 228 ± 13 |
| Group | Parameters | r | p-Value |
|---|---|---|---|
| NDD-CKD | XO; IL-1β | 0.333 | 0.048 * |
| XO; CRP | 0.776 | 0.039 * | |
| IL-1β; MDA | 0.511 | 0.036 * | |
| SOD; GPx | −0.579 | 0.002 ** | |
| SOD; LDL-C | −0.689 | 0.040 * | |
| GPx; TG | 0.810 | 0.020 * | |
| GPx; LDL-C | 0.775 | 0.041 * | |
| GSH; HDL-C | 0.651 | 0.022 * | |
| MDA; HbA1c | 0.676 | 0.010 ** | |
| HD | BMI; HbA1c | 0.357 | 0.028 * |
| IL-1β; HbA1c | 0.553 | 0.006 ** | |
| XO; GPx | 0.598 | 0.003 ** | |
| CAT; HbA1c | −0.434 | 0.043 * | |
| UA; SOD | 0.478 | 0.022 * | |
| PD | UA; HDL-C | −0.451 | 0.035 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vida, C.; Oliva, C.; Yuste, C.; Ceprián, N.; Caro, P.J.; Valera, G.; González de Pablos, I.; Morales, E.; Carracedo, J. Oxidative Stress in Patients with Advanced CKD and Renal Replacement Therapy: The Key Role of Peripheral Blood Leukocytes. Antioxidants 2021, 10, 1155. https://doi.org/10.3390/antiox10071155
Vida C, Oliva C, Yuste C, Ceprián N, Caro PJ, Valera G, González de Pablos I, Morales E, Carracedo J. Oxidative Stress in Patients with Advanced CKD and Renal Replacement Therapy: The Key Role of Peripheral Blood Leukocytes. Antioxidants. 2021; 10(7):1155. https://doi.org/10.3390/antiox10071155
Chicago/Turabian StyleVida, Carmen, Carlos Oliva, Claudia Yuste, Noemí Ceprián, Paula Jara Caro, Gemma Valera, Ignacio González de Pablos, Enrique Morales, and Julia Carracedo. 2021. "Oxidative Stress in Patients with Advanced CKD and Renal Replacement Therapy: The Key Role of Peripheral Blood Leukocytes" Antioxidants 10, no. 7: 1155. https://doi.org/10.3390/antiox10071155