Prevention of Autoimmune Diabetes in NOD Mice by Dimethyl Fumarate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Rat Islet Isolation
2.4. Quantitative Real-Time Reverse Transcription–PCR for Rat Islets
2.5. Immunofluorescence and Confocal Microscopy
2.6. NOD Mouse Diabetes Monitoring
2.7. Histopathology
2.8. Serum Cytokine Determination
2.9. Statistical Analysis
3. Results
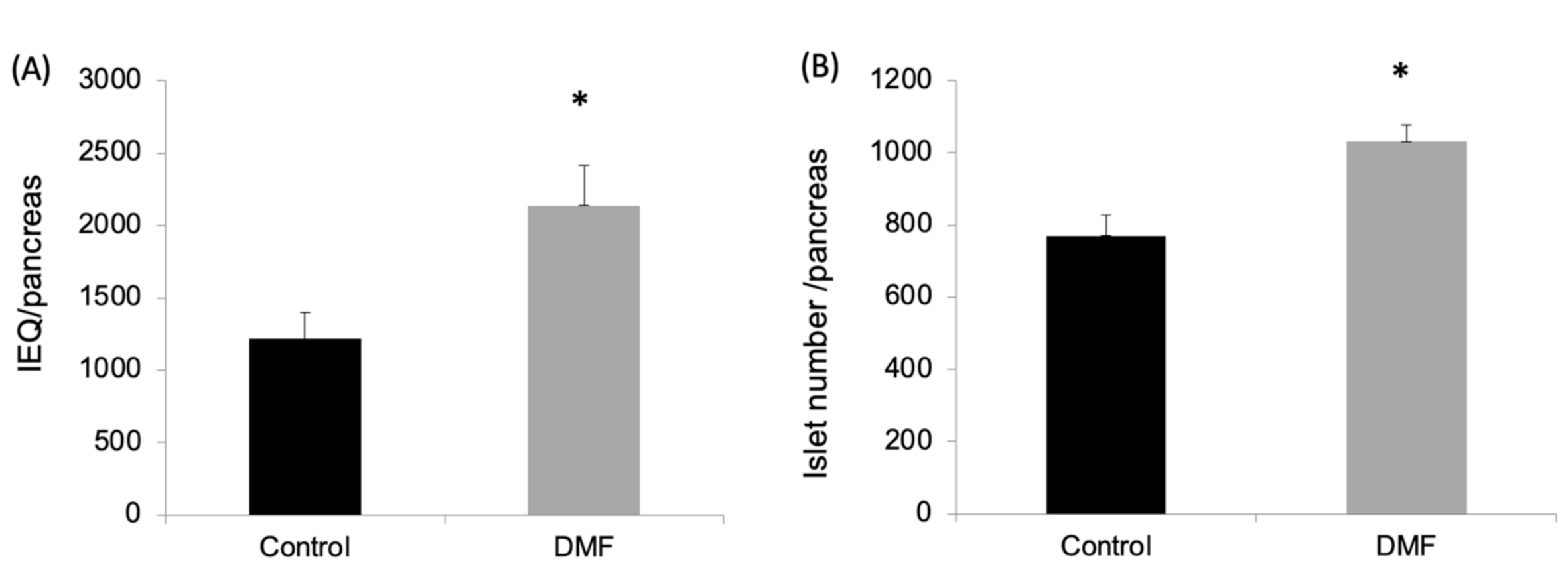
3.1. DMF Administration Improved Isolation Yield, Attenuated Oxidative Stress and Enhanced GCLC and NQO1 Expression in Pancreatic Islets
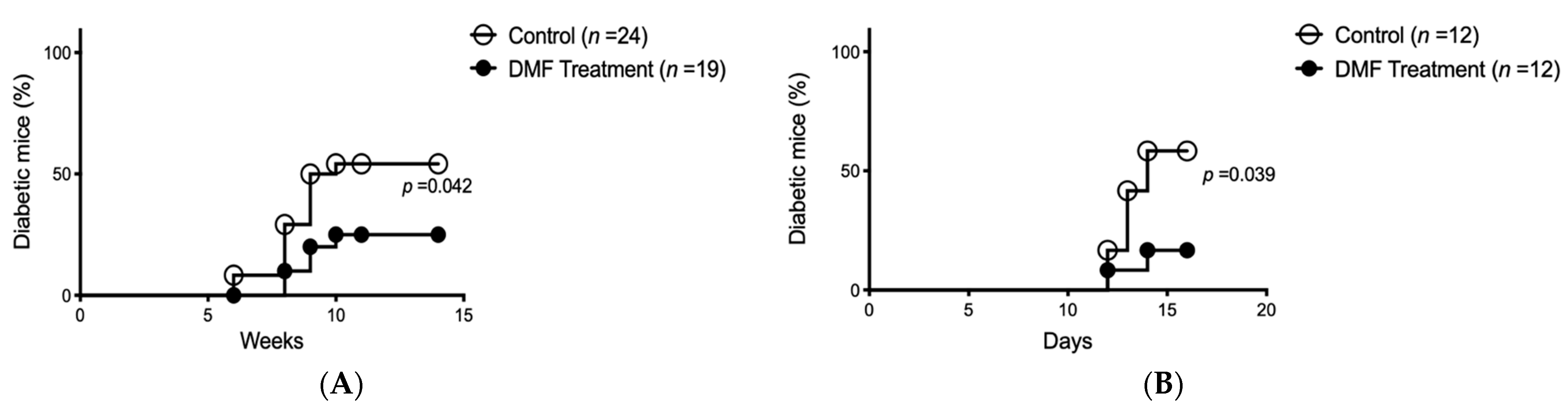
3.2. DMF Administration Retarded/Prevented the Onset of Autoimmune Diabetes in NOD Mice
3.3. DMF Administration Prevented Accelerated Autoimmune Diabetes Onset in NOD Mice
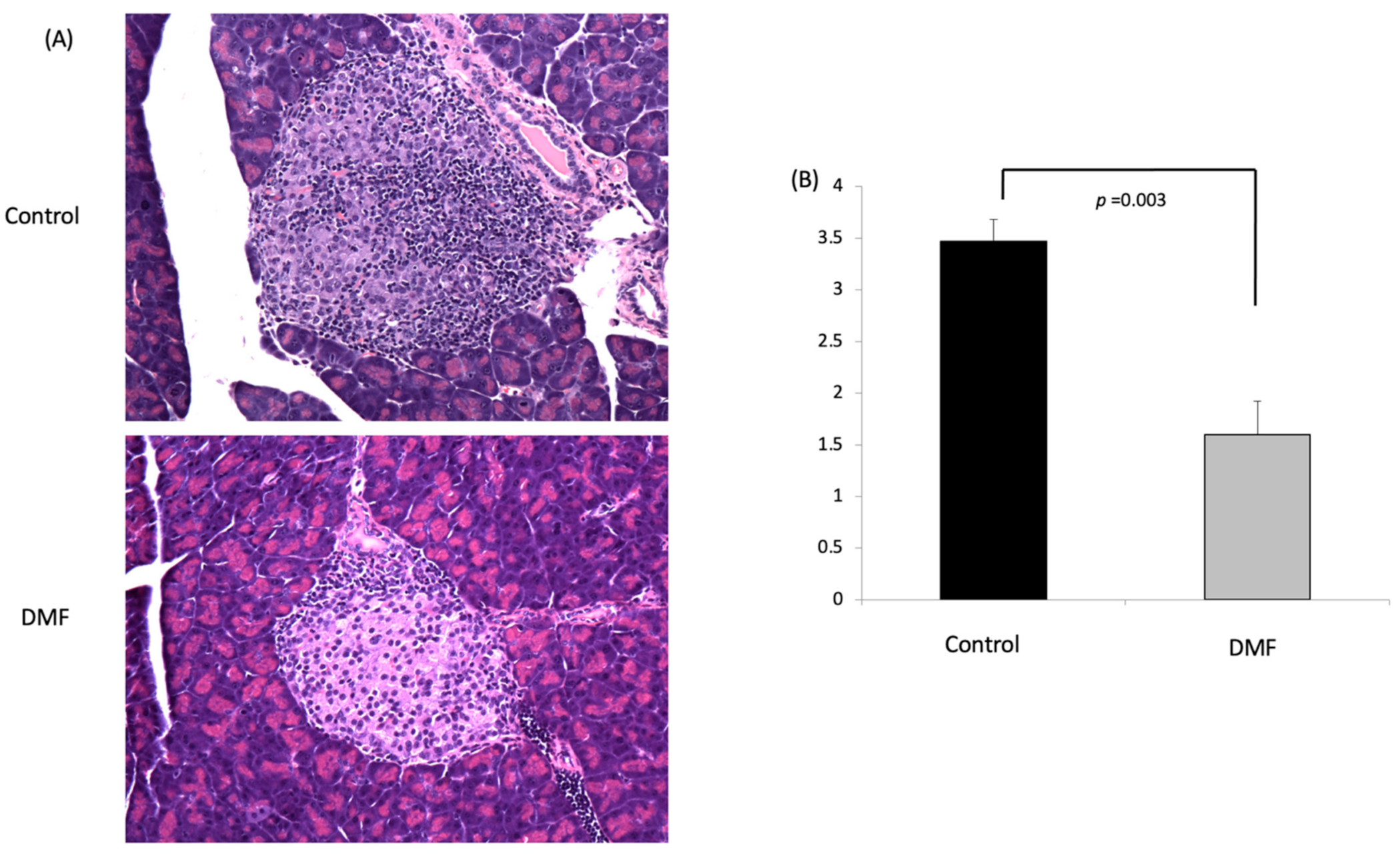
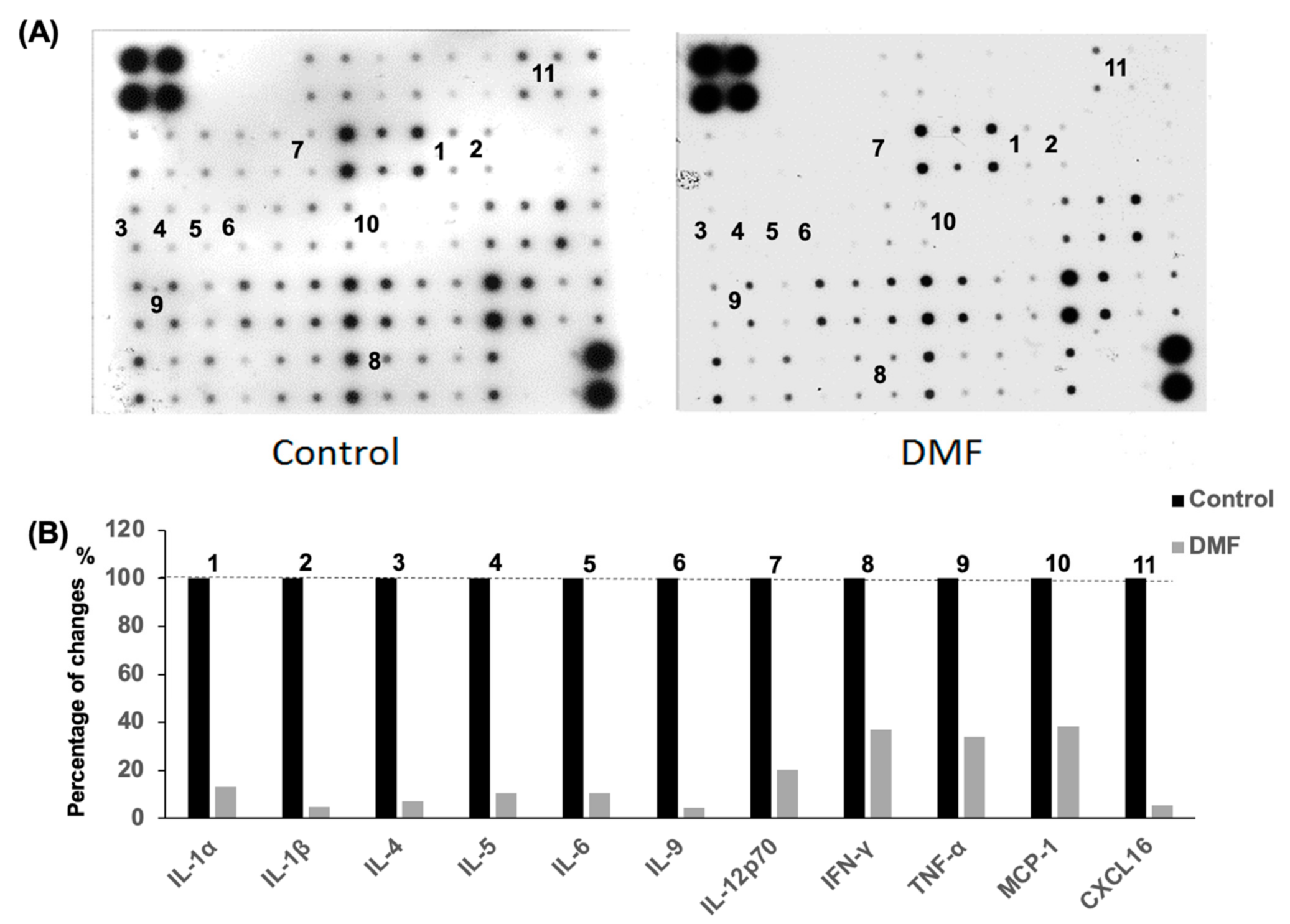
3.4. DMF Attenuated Insulitis and Decreased Serum Cytokine Levels in NOD Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Nrf2 | nuclear factor erythroid-derived 2-related factor; |
| DMF | Total phenolic content; |
| GCLC | Total flavonoid content; |
| NQO1 | NAD(P)H quinone dehydrogenase 1; |
| Nrf2 | nuclear factor erythroid-derived 2-related factor; |
| ROS | reactive oxygen species; |
| SD | standard deviation; |
| NOD | non-obese diabetic mice. |
References
- Chen, J.; Stimpson, S.E.; Fernandez-Bueno, G.A.; Mathews, C. Mitochondrial Reactive Oxygen Species and Type 1 Diabetes. Antioxidants Redox Signal. 2018, 29, 1361–1372. [Google Scholar] [CrossRef]
- Lee, K.U.; Amano, K.; Yoon, J.W. Evidence for initial involvement of macrophage in development of insulitis in NOD mice. Diabetes 1988, 37, 989–991. [Google Scholar] [CrossRef] [Green Version]
- O’Reilly, L.A.; Hutchings, P.R.; Crocker, P.R.; Simpson, E.; Lund, T.; Kioussis, D.; Takei, F.; Baird, J.; Cooke, A. Characterization of pancreatic islet cell infiltrates in NOD mice: effect of cell transfer and transgene expression. Eur. J. Immunol. 1991, 21, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Staal, F.J.; Roederer, M.; Herzenberg, L.A. Intracellular thiols regulate activation of nuclear factor kappa B and transcription of human immunodeficiency virus. Proc. Nat. Acad. Sci. USA 1990, 87, 9943–9947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Held, W.; Macdonald, H.R.; Weissman, I.L.; Hess, M.W.; Mueller, C. Genes encoding tumor necrosis factor alpha and granzyme A are expressed during development of autoimmune diabetes. Proc. Nat. Acad. Sci. USA 1990, 87, 2239–2243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandrup-Poulsen, T.; Spinas, G.A.; Prowse, S.J.; Hansen, B.S.; Jorgensen, D.W.; Bendtzen, K.; Nielsen, J.H.; Nerup, J. Islet cytotoxicity of interleukin 1. Influence of culture conditions and islet donor characteristics. Diabetes 1987, 36, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Woda, B.A. Cytokine gene expression in the islets of the diabetic Biobreeding/Worcester rat. J. Immunol. 1991, 146, 2990–2994. [Google Scholar] [PubMed]
- Delmastro, M.M.; Piganelli, J.D. Oxidative Stress and Redox Modulation Potential in Type 1 Diabetes. Clin. Dev. Immunol. 2011, 2011, 1–15. [Google Scholar] [CrossRef]
- Miki, A.; Ricordi, C.; Sakuma, Y.; Yamamoto, T.; Misawa, R.; Mita, A.; Molano, R.D.; Vaziri, N.D.; Pileggi, A.; Ichii, H. Divergent antioxidant capacity of human islet cell subsets: A potential cause of beta-cell vulnerability in diabetes and islet transplantation. PLoS ONE 2018, 13, e0196570. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Vaziri, N.D.; Masuda, Y.; Hajighasemi-Ossareh, M.; Robles, L.; Le, A.; Vo, K.; Chan, J.Y.; Foster, C.E.; Stamos, M.J.; et al. Pharmacological Activation of Nrf2 Pathway Improves Pancreatic Islet Isolation and Transplantation. Cell Transplant. 2015, 24, 2273–2283. [Google Scholar] [CrossRef] [Green Version]
- Yagishita, Y.; Uruno, A.; Chartoumpekis, D.V.; Kensler, T.W.; Yamamoto, M. Nrf2 represses the onset of type 1 diabetes in non-obese diabetic mice. J. Endocrinol. 2019, 240, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Rajesh, M.; Zhang, J.; Zhou, S.; Wang, S.; Sun, J.; Tan, Y.; Zheng, Y.; Cai, L. Protection by dimethyl fumarate against diabetic cardiomyopathy in type 1 diabetic mice likely via activation of nuclear factor erythroid-2 related factor 2. Toxicol. Lett. 2018, 287, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Della Giustina, A.; Bonfante, S.; Zarbato, G.F.; Danielski, L.G.; Mathias, K.; De Oliveira, A.N.; Garbossa, L.; Cardoso, T.; Fileti, M.E.; De Carli, R.J.; et al. Dimethyl Fumarate Modulates Oxidative Stress and Inflammation in Organs After Sepsis in Rats. Inflammation 2018, 41, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Robles, L.; Vaziri, N.D.; Li, S.; Masuda, Y.; Takasu, C.; Takasu, M.; Vo, K.; Farzaneh, S.H.; Stamos, M.J.; Ichii, H. Dimethyl Fumarate Protects Pancreatic Islet Cells and Non-Endocrine Tissue in L-Arginine-Induced Chronic Pancreatitis. PLoS ONE 2014, 9, e107111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takasu, C.; Vaziri, N.D.; Li, S.; Robles, L.; Vo, K.; Takasu, M.; Pham, C.; Farzaneh, S.H.; Shimada, M.; Stamos, M.J.; et al. Treatment with dimethyl fumarate ameliorates liver ischemia/reperfusion injury. World J. Gastroenterol. 2017, 23, 4508–4516. [Google Scholar] [CrossRef] [PubMed]
- Zi, X.; Guo, Y.; Simoneau, A.R.; Hope, C.; Xie, J.; Holcombe, R.F.; Hoang, B.H. Expression of Frzb/Secreted Frizzled-Related Protein 3, a Secreted Wnt Antagonist, in Human Androgen-Independent Prostate Cancer PC-3 Cells Suppresses Tumor Growth and Cellular Invasiveness. Cancer Res. 2005, 65, 9762–9770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Xie, J.; Rubin, E.; Tang, Y.-X.; Xiaolin, Z.; Zi, X.; Hoang, B.H. Frzb, a Secreted Wnt Antagonist, Decreases Growth and Invasiveness of Fibrosarcoma Cells Associated with Inhibition of Met Signaling. Cancer Res. 2008, 68, 3350–3360. [Google Scholar] [CrossRef] [Green Version]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar]
- Fiorina, P.; Vergani, A.; Dada, S.; Jurewicz, M.M.; Wong, M.; Law, K.; Wu, E.; Tian, Z.; Abdi, R.; Guleria, I.; et al. Targeting CD22 Reprograms B-Cells and Reverses Autoimmune Diabetes. Diabetes 2008, 57, 3013–3024. [Google Scholar] [CrossRef] [Green Version]
- Robles, L.; Vaziri, N.D.; Li, S.; Takasu, C.; Masuda, Y.; Vo, K.; Farzaneh, S.H.; Stamos, M.J.; Ichii, H. Dimethyl Fumarate Ameliorates Acute Pancreatitis in Rodent. Pancreas 2014, 44, 441–447. [Google Scholar] [CrossRef] [Green Version]
- Maiese, K. New Insights for Oxidative Stress and Diabetes Mellitus. Oxidative Med. Cell. Longev. 2015, 2015, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B. 3rd. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Calkins, M.J.; Johnson, D.A.; Townsend, J.A.; Vargas, M.R.; Dowell, J.A.; Williamson, T.P.; Kraft, A.D.; Lee, J.-M.; Li, J.; Johnson, J.A. The Nrf2/ARE Pathway as a Potential Therapeutic Target in Neurodegenerative Disease. Antioxidants Redox Signal. 2009, 11, 497–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Scannevin, R.H.; Chollate, S.; Jung, M.-Y.; Shackett, M.; Patel, H.; Bista, P.; Zeng, W.; Ryan, S.; Yamamoto, M.; Lukashev, M.; et al. Fumarates Promote Cytoprotection of Central Nervous System Cells against Oxidative Stress via the Nuclear Factor (Erythroid-Derived 2)-Like 2 Pathway. J. Pharmacol. Exp. Ther. 2012, 341, 274–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, R.J.; Miller, D.H.; Phillips, J.T.; Hutchinson, M.; Havrdova, E.; Kita, M.; Yang, M.; Raghupathi, K.; Novas, M.; Sweetser, M.T.; et al. Placebo-Controlled Phase 3 Study of Oral BG-12 or Glatiramer in Multiple Sclerosis. New Engl. J. Med. 2012, 367, 1087–1097. [Google Scholar] [CrossRef] [Green Version]
- Gold, R.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Giovannoni, G.; Selmaj, K.; Tornatore, C.; Sweetser, M.T.; Yang, M.S.M.; Sheikh, S.I.; et al. Placebo-Controlled Phase 3 Study of Oral BG-12 for Relapsing Multiple Sclerosis. New Engl. J. Med. 2012, 367, 1098–1107. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Takasu, C.; Lau, H.; Robles, L.; Vo, K.; Farzaneh, T.; Vaziri, N.; Stamos, M.J.; Ichii, H. Dimethyl Fumarate Alleviates Dextran Sulfate Sodium-Induced Colitis, through the Activation of Nrf2-Mediated Antioxidant and Anti-inflammatory Pathways. Antioxidants 2020, 9, 354. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, W.; Zhang, X.; Lu, P.; Du, Q.; Tao, L.; Ding, Y.; Wang, Y.; Hu, R. Dimethyl fumarate ameliorates dextran sulfate sodium-induced murine experimental colitis by activating Nrf2 and suppressing NLRP3 inflammasome activation. Biochem. Pharmacol. 2016, 112, 37–49. [Google Scholar] [CrossRef]
- Hasanvand, D.; Amiri, I.; Asl, S.S.; Saidijam, M.; Shabab, N.; Artimani, T. Effects of CeO2 nanoparticles on the HO-1, NQO1, and GCLC expression in the testes of diabetic rats. Can. J. Physiol. Pharmacol. 2018, 96, 963–969. [Google Scholar] [CrossRef]
- Liang, E.; Ma, M.; Wang, L.; Liu, X.; Xu, J.; Zhang, M.; Yang, R.; Zhao, Y. The BET/BRD inhibitor JQ1 attenuates diabetes-induced cognitive impairment in rats by targeting Nox4-Nrf2 redox imbalance. Biochem. Biophys. Res. Commun. 2018, 495, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.P.; Jin, M.; Kim, K.H.; Ahn, Y.J.; Yoon, S.P.; You, H.J.; Chang, I.Y. Immunolocalization of 8-OHdG and OGG1 in pancreatic islets of streptozotocin-induced diabetic rats. Acta Histochem. 2009, 111, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.A.; Wong, F.S.; Wen, L. The importance of the Non Obese Diabetic (NOD) mouse model in autoimmune diabetes. J. Autoimmun. 2016, 66, 76–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eizirik, D.L.; Mandrup-Poulsen, T. A choice of death—The signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia 2001, 44, 2115–2133. [Google Scholar] [CrossRef]
- Delaney, C.A.; Pavlovic, D.; Hoorens, A.; Pipeleers, D.; Eizirik, D.L. Cytokines Induce Deoxyribonucleic Acid Strand Breaks and Apoptosis in Human Pancreatic Islet Cells*. Endocrinology 1997, 138, 2610–2614. [Google Scholar] [CrossRef]
- Hanley, S.; Liu, S.; Lipsett, M.; Castellarin, M.; Rosenberg, L.; Tchervenkov, J.; Paraskevas, S. Tumor Necrosis Factor-?? Production by Human Islets Leads to Postisolation Cell Death. Transplantation 2006, 82, 813–818. [Google Scholar] [CrossRef]
- Arnush, M.; Heitmeier, M.R.; Scarim, A.L.; Marino, M.H.; Manning, P.T.; A Corbett, J. IL-1 produced and released endogenously within human islets inhibits beta cell function. J. Clin. Investig. 1998, 102, 516–526. [Google Scholar] [CrossRef]
- Berney, T.; Molano, R.D.; Cattan, P.; Pileggi, A.; Vizzardelli, C.; Oliver, R.; Ricordi, C.; Inverardi, L. Endotoxin-mediated delayed islet graft function is associated with increased intra-islet cytokine production and islet cell apoptosis1. Transplantation 2001, 71, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Taylor-Fishwick, D.A.; Weaver, J.R.; Grzesik, W.; Chakrabarti, S.; Green-Mitchell, S.; Imai, Y.; Kuhn, N.; Nadler, J.L. Production and function of IL-12 in islets and beta cells. Diabetologia 2013, 56, 126–135. [Google Scholar] [CrossRef]
- Chen, M.C.; Proost, P.; Gysemans, C.; Mathieu, C.; Eizirik, D.L. Monocyte chemoattractant protein-1 is expressed in pancreatic islets from prediabetic NOD mice and in interleukin-1 beta-exposed human and rat islet cells. Diabetologia 2001, 44, 325–332. [Google Scholar]
- Piemonti, L.; Leone, B.E.; Nano, R.; Saccani, A.; Monti, P.; Maffi, P.; Bianchi, G.; Sica, A.; Peri, G.; Melzi, R.; et al. Human Pancreatic Islets Produce and Secrete MCP-1/CCL2: Relevance in Human Islet Transplantation. Diabetes 2002, 51, 55–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandor, A.M.; Lindsay, R.S.; Dyjack, N.; Whitesell, J.C.; Rios, C.; Bradley, B.J.; Haskins, K.; Serreze, D.V.; Geurts, A.M.; Chen, Y.-G.; et al. CD11c+ Cells Are Gatekeepers for Lymphocyte Trafficking to Infiltrated Islets During Type 1 Diabetes. Front. Immunol. 2019, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, Q.; Mao, G.; Dowling, C.A.; Lundy, S.K.; Mao-Draayer, Y. Dimethyl Fumarate Selectively Reduces Memory T Cells and Shifts the Balance between Th1/Th17 and Th2 in Multiple Sclerosis Patients. J. Immunol. 2017, 198, 3069–3080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schultheis, J.; Beckmann, D.; Mulac, D.; Müller, L.; Esselen, M.; Düfer, M. Nrf2 Activation Protects Mouse Beta Cells from Glucolipotoxicity by Restoring Mitochondrial Function and Physiological Redox Balance. Oxidative Med. Cell. Longev. 2019, 2019, 1–17. [Google Scholar] [CrossRef]
- Kappos, L.; Gold, R.; Miller, D.; MacManus, D.G.; Havrdova, E.; Limmroth, V.; Polman, C.H.; Schmierer, K.; Yousry, T.A.; Yang, M.; et al. Efficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet 2008, 372, 1463–1472. [Google Scholar] [CrossRef]
- Reszke, R.; Szepietowski, J.C. A safety evaluation of dimethyl fumarate in moderate-to-severe psoriasis. Expert Opin. Drug Saf. 2020, 19, 373–380. [Google Scholar] [CrossRef]
- Mrowietz, U.; Altmeyer, P.; Bieber, T.; Röcken, M.; Schopf, R.E.; Sterry, W. Treatment of psoriasis with fumaric acid esters (Fumaderm®). J. der Dtsch. Dermatol. Ges. 2007, 5, 716–717. [Google Scholar] [CrossRef]
- Vucic, S.; Ryder, J.; Mekhael, L.; Rd, H.; Mathers, S.; Needham, M.; Dw, S.; Mc, K. Phase 2 randomized placebo controlled double blind study to assess the efficacy and safety of tecfidera in patients with amyotrophic lateral sclerosis (TEALS Study). Medicine 2020, 99, e18904. [Google Scholar] [CrossRef]
- Nicolay, J.P.; Muller-Decker, K.; Schroeder, A.; Brechmann, M.; Mobs, M.; Geraud, C.; Assaf, C.; Goerdt, S.; Krammer, P.H.; Gulow, K. Dimethyl fumarate restores apoptosis sensitivity and inhibits tumor growth and metastasis in CTCL by targeting NF-kappaB. Blood 2016, 128, 805–815. [Google Scholar] [CrossRef] [Green Version]
- Casili, G.; Cordaro, M.; Impellizzeri, D.; Bsh, G.B.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. Dimethyl Fumarate Reduces Inflammatory Responses in Experimental Colitis. J Crohns Colitis 2016, 10, 472–483. [Google Scholar] [CrossRef]
- Campolo, M.; Casili, G.; Biundo, F.; Crupi, R.; Cordaro, M.; Cuzzocrea, S.; Esposito, E. The Neuroprotective Effect of Dimethyl Fumarate in an MPTP-Mouse Model of Parkinson’s Disease: Involvement of Reactive Oxygen Species/Nuclear Factor-kappaB/Nuclear Transcription Factor Related to NF-E2. Antioxid. Redox Signal. 2017, 27, 453–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lastres-Becker, I.; Garcia-Yague, A.J.; Scannevin, R.H.; Casarejos, M.J.; Kugler, S.; Rabano, A.; Cuadrado, A. Repurposing the NRF2 Activator Dimethyl Fumarate as Therapy Against Synucleinopathy in Parkinson’s Disease. Antioxid. Redox Signal. 2016, 25, 61–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loewe, R.; Valero, T.; Kremling, S.; Pratscher, B.; Kunstfeld, R.; Pehamberger, H.; Petzelbauer, P. Dimethylfumarate Impairs Melanoma Growth and Metastasis. Cancer Res. 2006, 66, 11888–11896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamazoe, Y.; Tsubaki, M.; Matsuoka, H.; Satou, T.; Itoh, T.; Kusunoki, T.; Kidera, Y.; Tanimori, Y.; Shoji, K.; Nakamura, H. Dimethylfumarate inhibits tumor cell invasion and metastasis by suppressing the expression and activities of matrix metalloproteinases in melanoma cells. Cell Biol. Int. 2009, 33, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Tsubaki, M.; Asano, R.; Itoh, T.; Imano, M.; Satou, T.; Nishida, S. Dimethyl fumarate suppresses metastasis and growth of melanoma cells by inhibiting the nuclear translocation of NF-kappaB. J. Dermatol. Sci. 2020, 99, 168–176. [Google Scholar] [CrossRef]
- Kastrati, I.; Siklos, M.I.; Calderon-Gierszal, E.L.; El-Shennawy, L.; Georgieva, G.; Thayer, E.N.; Thatcher, G.R.; Frasor, J. Dimethyl Fumarate Inhibits the Nuclear Factor kappaB Pathway in Breast Cancer Cells by Covalent Modification of p65 Protein. J. Biol. Chem. 2016, 291, 3639–3647. [Google Scholar] [CrossRef] [Green Version]
- Grzegorzewska, A.P.; Seta, F.; Han, R.; Czajka, C.A.; Makino, K.; Stawski, L.; Isenberg, J.S.; Browning, J.L.; Trojanowska, M. Dimethyl Fumarate ameliorates pulmonary arterial hypertension and lung fibrosis by targeting multiple pathways. Sci. Rep. 2017, 7, 41605. [Google Scholar] [CrossRef] [Green Version]
- Abdelrahman, R.S.; Abdel-Rahman, N. Dimethyl fumarate ameliorates acetaminophen-induced hepatic injury in mice dependent of Nrf-2/HO-1 pathway. Life Sci. 2019, 217, 251–260. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, G.; Zhang, J.; Ting, S.-M.; Gonzales, N.; Aronowski, J. Dimethyl Fumarate Protects Brain From Damage Produced by Intracerebral Hemorrhage by Mechanism Involving Nrf2. Stroke 2015, 46, 1923–1928. [Google Scholar] [CrossRef] [Green Version]
- Krishnamoorthy, S.; Pace, B.S.; Gupta, D.; Sturtevant, S.; Li, B.; Makala, L.; Brittain, J.E.; Moore, N.; Vieira, B.F.; Thullen, T.; et al. Dimethyl fumarate increases fetal hemoglobin, provides heme detoxification, and corrects anemia in sickle cell disease. JCI Insight 2017, 2, e96409. [Google Scholar] [CrossRef] [Green Version]
- Giacco, F.; Brownlee, M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Vaziri, N.D.; Swentek, L.; Takasu, C.; Vo, K.; Stamos, M.J.; Ricordi, C.; Ichii, H. Prevention of Autoimmune Diabetes in NOD Mice by Dimethyl Fumarate. Antioxidants 2021, 10, 193. https://doi.org/10.3390/antiox10020193
Li S, Vaziri ND, Swentek L, Takasu C, Vo K, Stamos MJ, Ricordi C, Ichii H. Prevention of Autoimmune Diabetes in NOD Mice by Dimethyl Fumarate. Antioxidants. 2021; 10(2):193. https://doi.org/10.3390/antiox10020193
Chicago/Turabian StyleLi, Shiri, Nosratola D. Vaziri, Lourdes Swentek, Chie Takasu, Kelly Vo, Michael J. Stamos, Camillo Ricordi, and Hirohito Ichii. 2021. "Prevention of Autoimmune Diabetes in NOD Mice by Dimethyl Fumarate" Antioxidants 10, no. 2: 193. https://doi.org/10.3390/antiox10020193






