The Protective Effects of Carrageenan Oligosaccharides on Intestinal Oxidative Stress Damage of Female Drosophila melanogaster
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fly Stocks
2.3. Generating Axenic Drosophila
2.4. Oxidative Stress Treatment and Lifespan Assay
2.5. The Oxidation Level in Drosophila
2.6. The Intestinal Oxidative Damage in Drosophila
2.6.1. The Intestinal Morphological Changes
2.6.2. Staining
2.7. Analyses of the Gut Microbiota
DNA Extraction and 16S rDNA Sequencing
2.8. Analysis of Gene Expression Relevant to Intestinal Oxidative Stress Damage
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effect of COS on the Lifespan in of Female Flies with Oxidative Stress
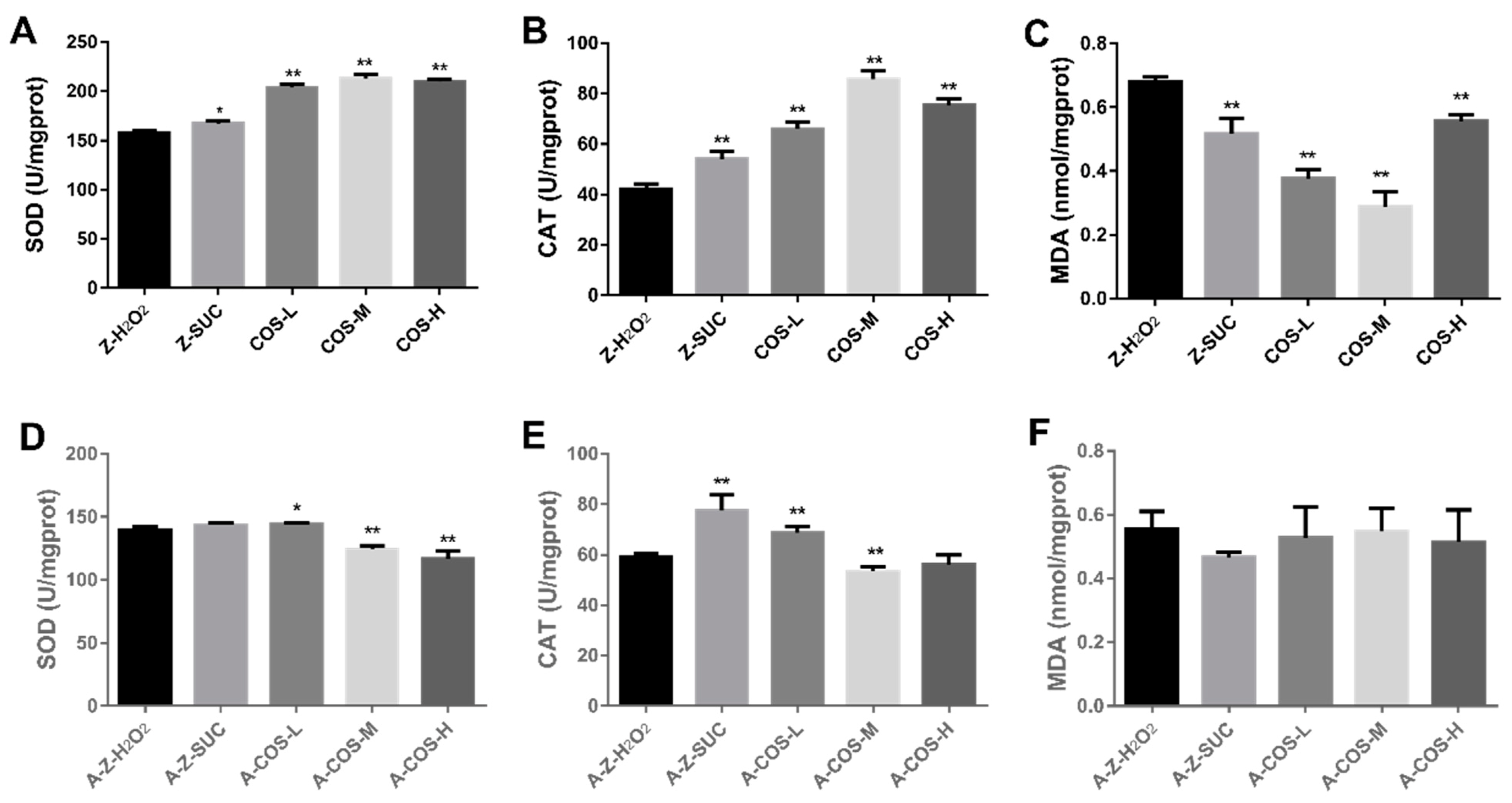
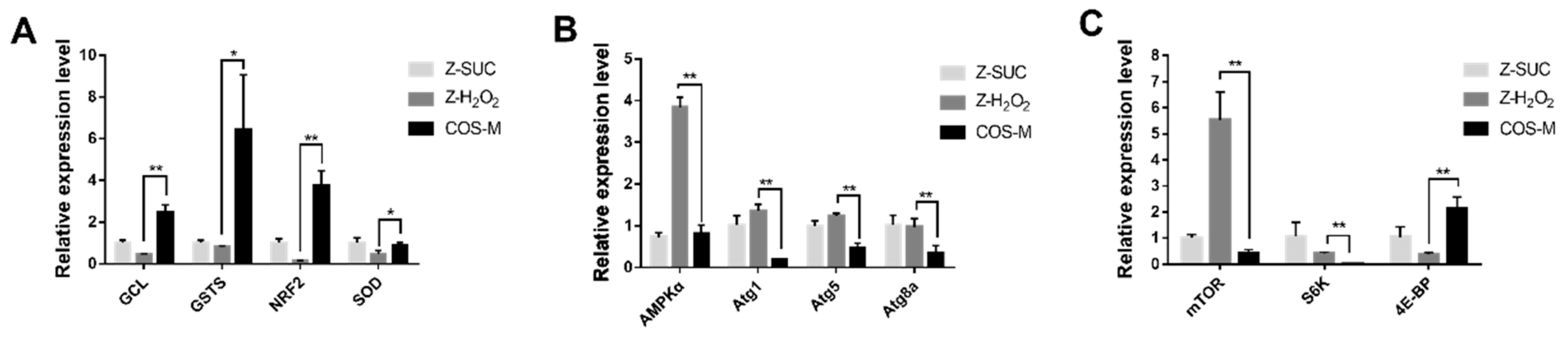
3.2. Influences of COS on the Antioxidant Ability of Female Drosophila
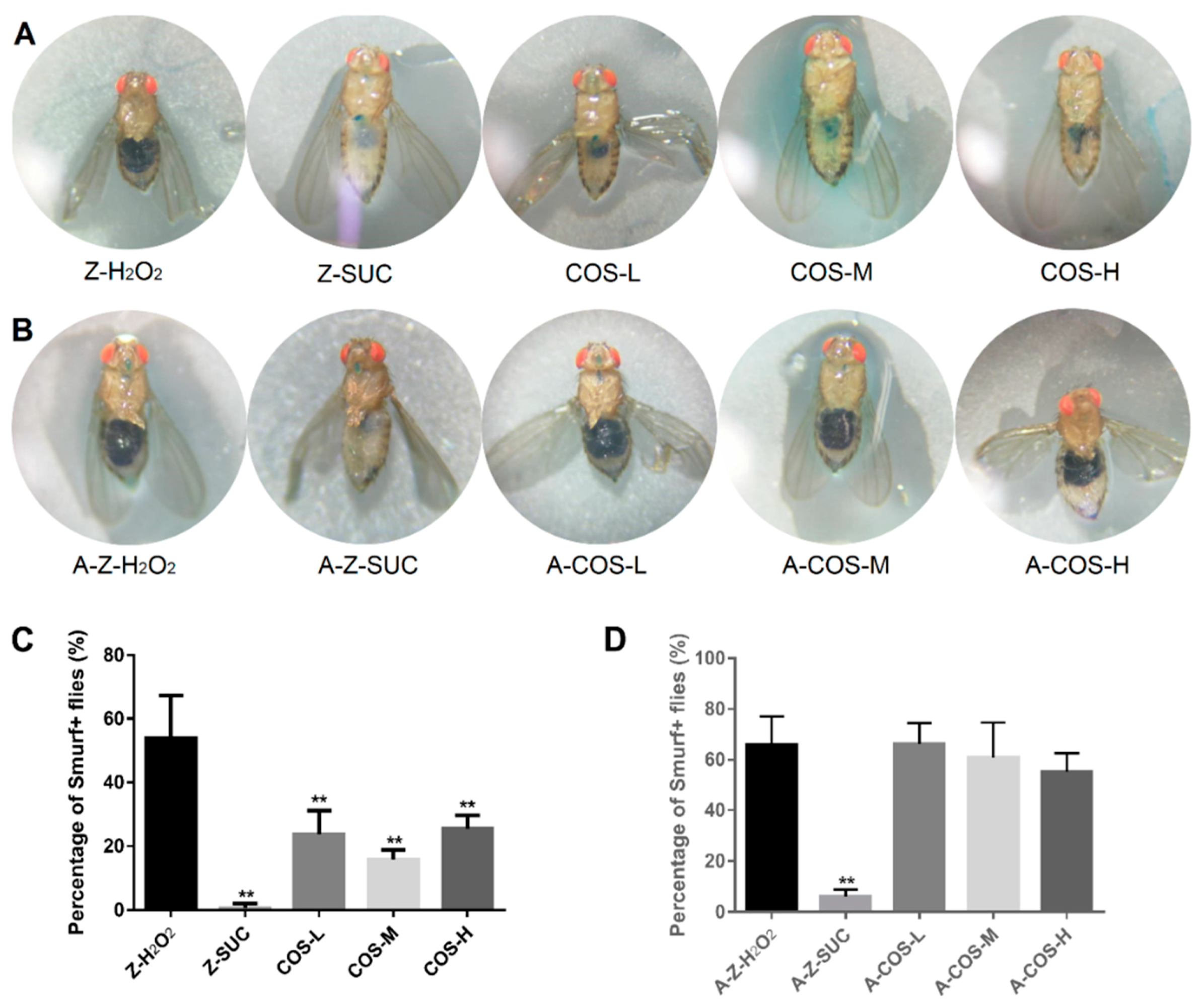
3.3. The Effect of COS on Intestinal Oxidative Damage in Drosophila
3.3.1. The Changes of Intestinal Integrity
3.3.2. The Changes of Drosophila Gut Morphology
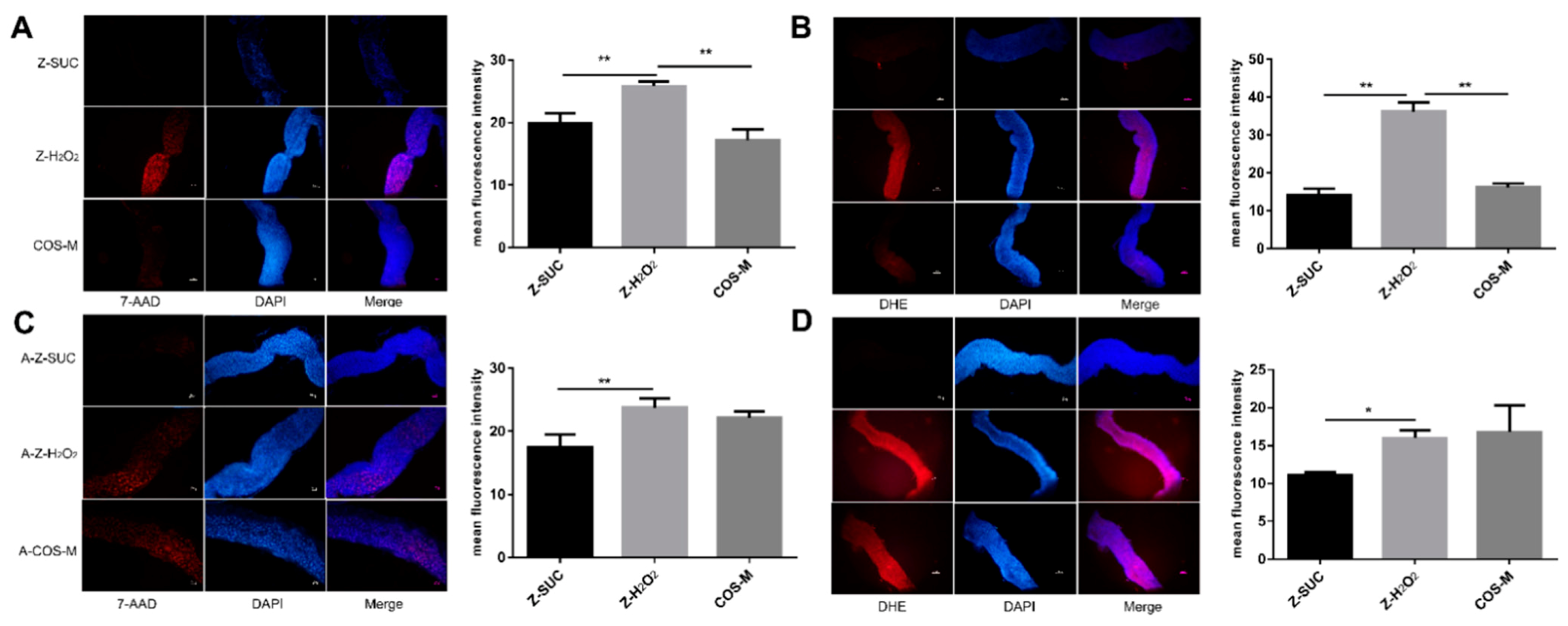
3.4. Effects of COS on the Changes of Intestinal Cell Activity and ROS Levels in Drosophila
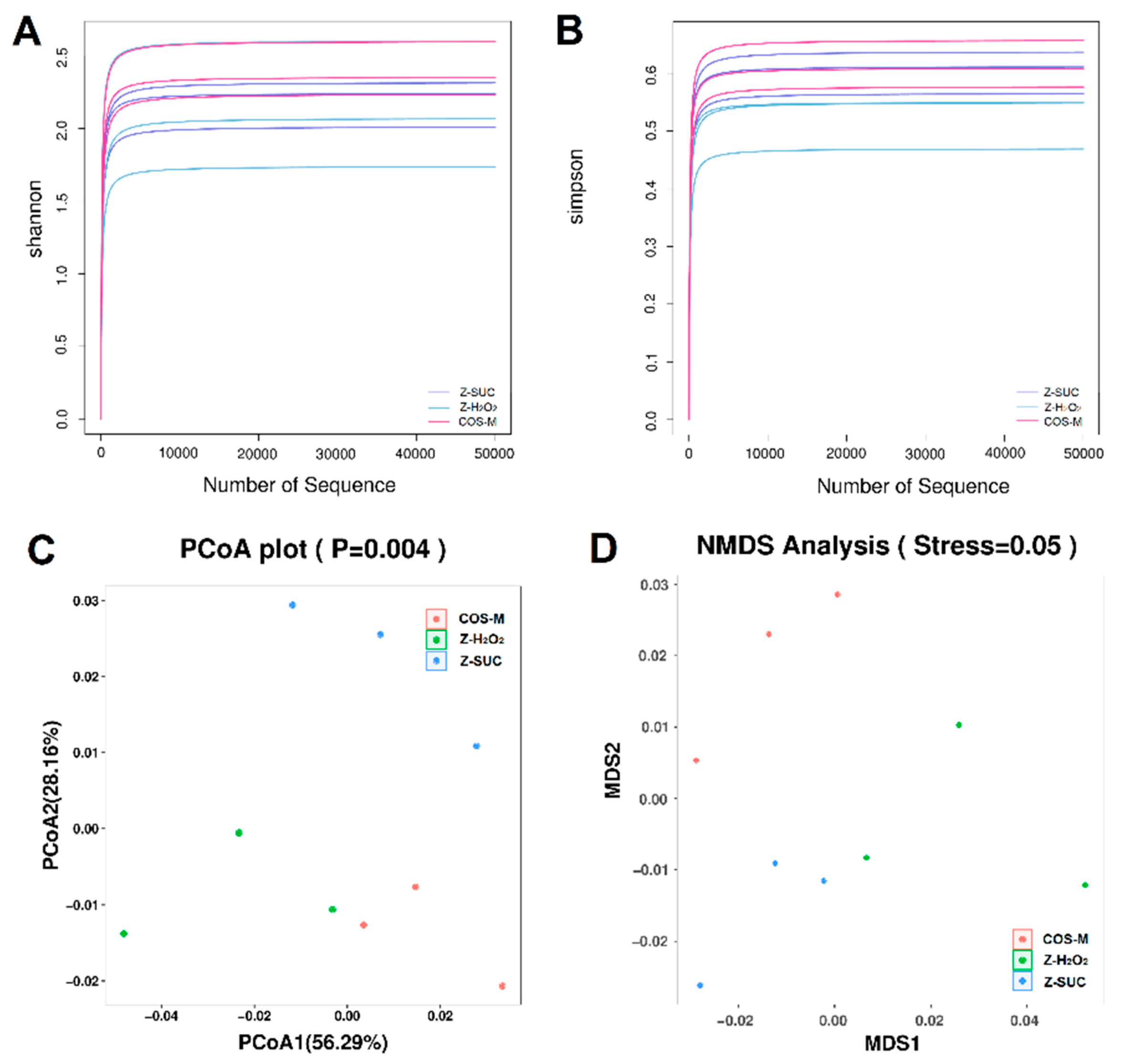
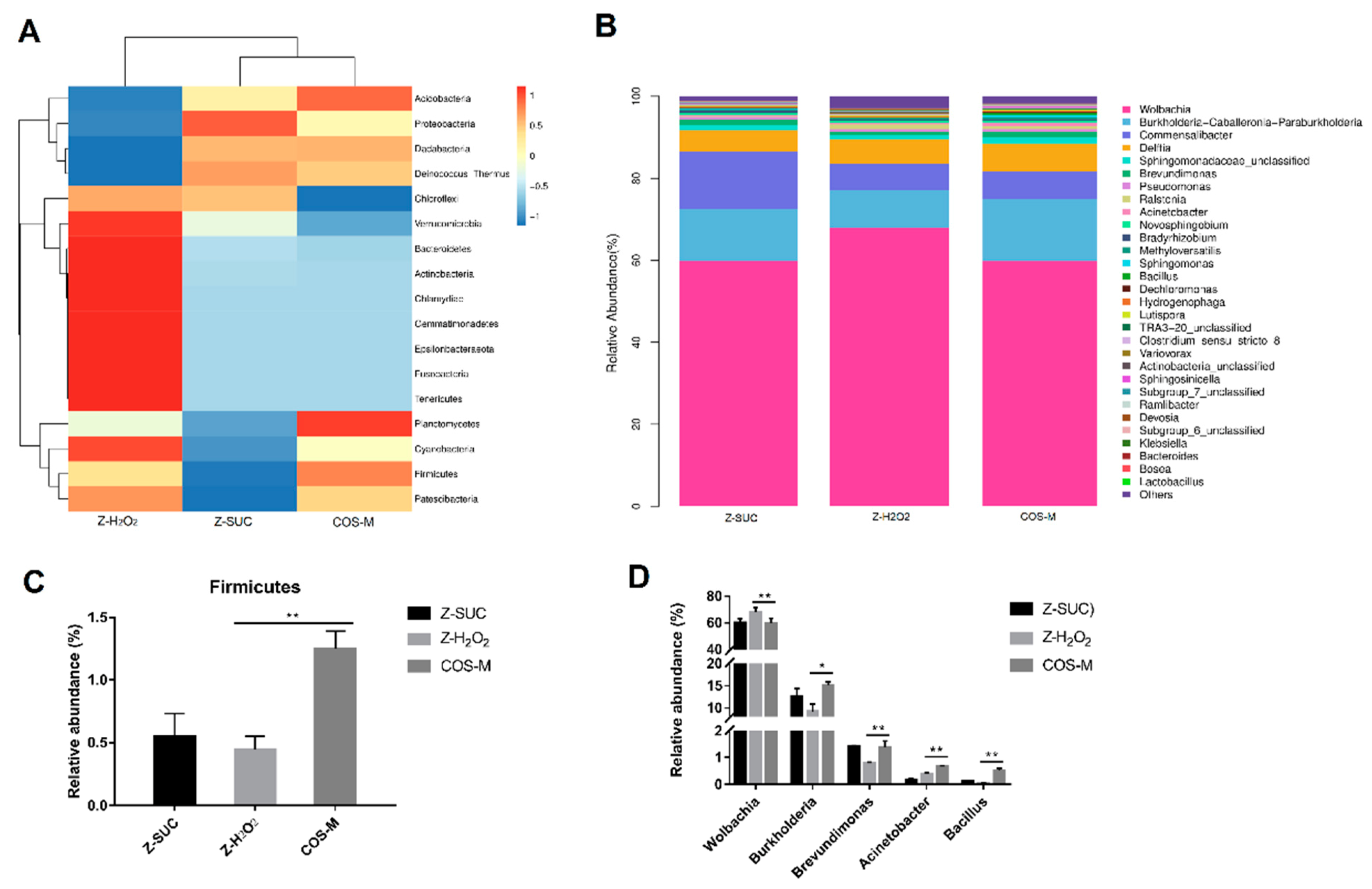
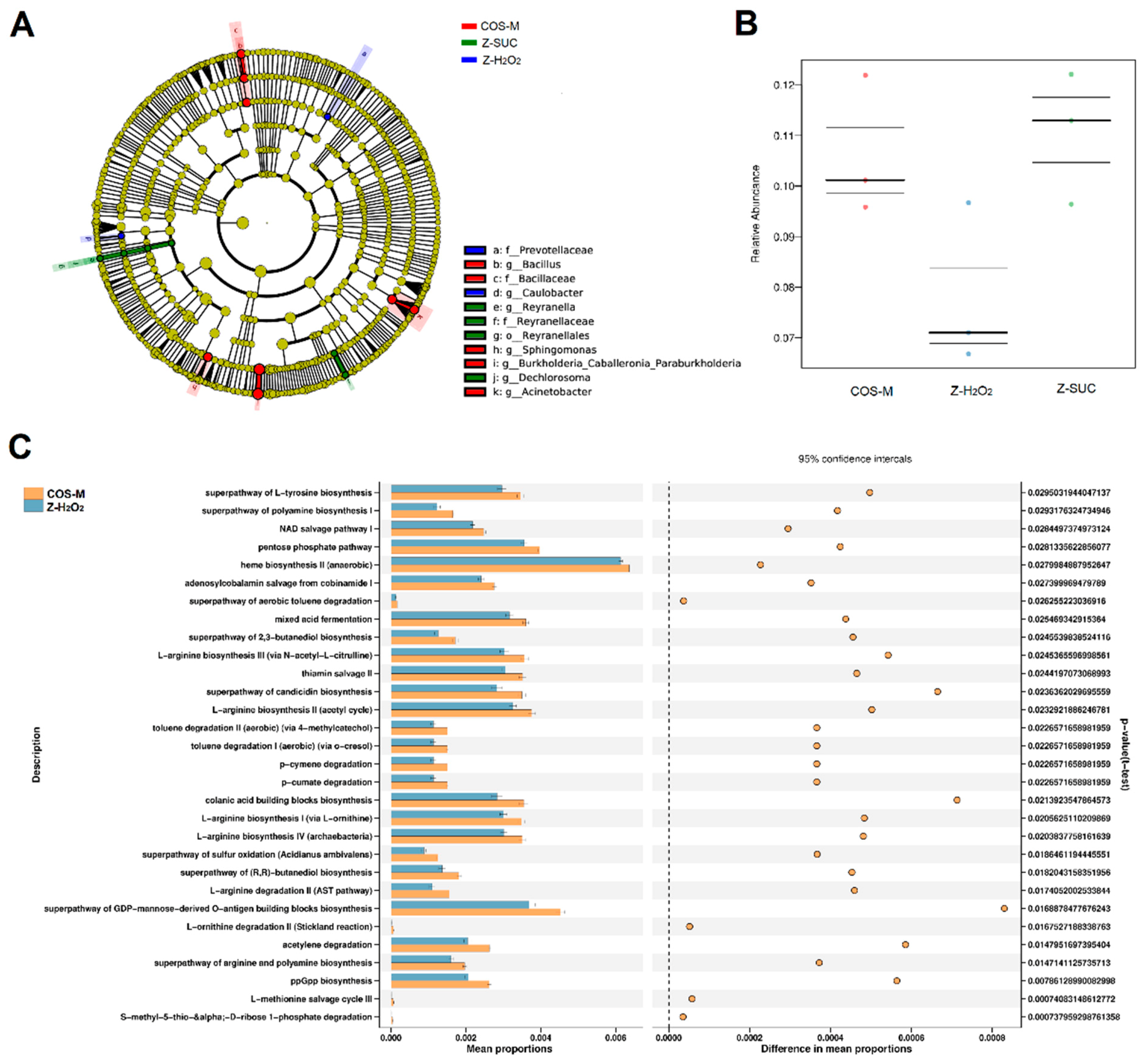
3.5. The Effects of COS on Intestinal Microbiota of Female Drosophila
3.6. Gene Expression Level of Female Drosophila Related to Immune Defense
3.7. Future Research Directions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharifi-Rad, M.; Kumar, N.; Zucca, P.; Varoni, E.M.; Sharifi-Rad, J. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alessandra, A.; Beatriz, F.; Filipe, M.G.; Aristides, T.; Elisavet, R.; Anatoly, S.; Marcelo, F.; Joao, R.; Michael, A. Oxidative stress in methylmercury-induced cell toxicity. Toxics 2018, 6, 47. [Google Scholar] [CrossRef] [Green Version]
- Mahajan, L.; Verma, P.K.; Raina, R.; Pankaj, N.K.; Sood, S.; Singh, M. Alteration in thiols homeostasis, protein and lipid peroxidation in renal tissue following subacute oral exposure of imidacloprid and arsenic in Wistar rats. Toxicol. Rep. 2018, 5, 1114–1119. [Google Scholar] [CrossRef]
- Oke, G.O.; Abiodun, A.A.; Imafidon, C.E.; Monsi, B.F. Zingiber officinale (Roscoe) mitigates CCl4-induced liver histopathology and biochemical derangements through antioxidant, membrane-stabilizing and tissue-regenerating potentials. Toxicol. Rep. 2019, 6, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Frieling, J.V.; Faisal, M.N.; Sporn, F.; Pfefferkorn, R.; Nolte, S.S.; Sommer, F.; Rosenstiel, P.; Roeder, T. A high-fat diet induces a microbiota-dependent increase in stem cell activity in the Drosophila intestine. PLoS Genet. 2020, 16, e1008789. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Ni, F.; Xiong, Q.; Yao, Z. Marine oligosaccharides originated from seaweeds: Source, preparation, structure, physiological activity and applications. Crit. Rev. Food Sci. Nutr. 2020, 61, 60–74. [Google Scholar] [CrossRef]
- Yuan, H.; Song, J.; Zhang, W.; Li, X.; Li, N.; Gao, X. Antioxidant activity and cytoprotective effect of κ-carrageenan oligosaccharides and their different derivatives. Bioorganic Med. Chem. Lett. 2006, 16, 1329–1334. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, B.; Wu, Y.; Liu, Y.; Gu, X.; Zhang, H.; Wang, C.; Cao, H.; Huang, L.; Wang, Z. Structural characterization and antioxidant activities of κ-carrageenan oligosaccharides degraded by different methods. Food Chem. 2015, 178, 311–318. [Google Scholar] [CrossRef]
- Zhu, N.N.; Yao, Z.A.; Hai-Ge, W.U.; Liu, Y.Y.; Jing-Xin, D. Research progress on carrageenan oligosaccharides. Chem. Bioeng. 2009, 26, 9–12. [Google Scholar]
- Brandt, A.; Vilcinskas, A. The fruit fly Drosophila melanogaster as a model for aging research. Adv. Biochem. Eng.-Biotechnol. 2013, 135, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yang, Q.; Chen, X.; Tian, Y.; Liu, Z.; Wang, S. Bioactive peptides derived from crimson snapper and in vivo anti-aging effects on fat diet-induced high fat Drosophila melanogaster. Food Funct. 2020, 11, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Chen, S.; Liang, J.; Tang, M.; Wang, S. Protective effects of crimson snapper scales peptides against oxidative stress on Drosophila melanogaster and the action mechanism. Food Chem. Toxicol. 2021, 148, 111965. [Google Scholar] [CrossRef]
- Ng, K.M.; Ferreyra, J.A.; Higginbottom, S.K.; Lynch, J.B.; Kashyap, P.C.; Gopinath, S.; Naidu, N.; Choudhury, B.; Weimer, B.C.; Monack, D.M. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature 2013, 502, 96–99. [Google Scholar] [CrossRef] [Green Version]
- Chassard, C.; Lacroix, C. Carbohydrates and the human gut microbiota. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 453–460. [Google Scholar] [CrossRef]
- Yadav, M.; Verma, M.K.; Chauhan, N.S. A review of metabolic potential of human gut microbiome in human nutrition. Arch. Microbiol. 2018, 200, 203–217. [Google Scholar] [CrossRef]
- Kong, Y.; Wang, L.Y.; Jiang, B.C. The role of gut microbiota in aging and aging related neurodegenerative disorders: Insights from Drosophila model. Life 2021, 11, 855. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Yang, K.; Wang, Y.F.; Dai, X.J. Anti-aging effect of agar oligosaccharide on male Drosophila melanogaster and its preliminary mechanism. Mar. Drugs 2019, 17, 632. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Li, Q.W.; Dai, X.J. Carrageenan oligosaccharides extend life span and health span in male Drosophila melanogaster by modulating antioxidant activity, immunity, and gut microbiota. J. Med. Food 2021, 24, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Schretter, C.E.; Vielmetter, J.; Bartos, I.; Marka, Z.; Marka, S.; Argade, S.; Mazmanian, S.K. A gut microbial factor modulates locomotor behavior in Drosophila. Nature 2018, 563, 402–406. [Google Scholar] [CrossRef]
- Luge, R.; Zheng, X.; Oba, B.T.; Shen, C.; Sun, L. Activating soil microbial community using bacillus and rhamnolipid to remediate TPH contaminated soil. Chemosphere 2021, 275, 130062. [Google Scholar] [CrossRef]
- Wang, L.J.; Li, Y.M.; Lei, L.; Liu, Y.W.; Wang, X.B.; Ma, K.Y.; Chen, Z.Y. Cranberry anthocyanin extract prolongs lifespan of fruit flies. Exp. Gerontol. 2015, 69, 189–195. [Google Scholar] [CrossRef]
- Apidianakis, Y.; Rahme, L.G. Drosophila melanogaster as a model for human intestinal infection and pathology. Dis. Models Mech. 2011, 4, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Pais, I.S.; Valente, R.S.; Marta, S.; Luis, T.; Nancy, M. Drosophila melanogaster establishes a species-specific mutualistic interaction with stable gut-colonizing bacteria. PLoS Biol. 2018, 16, e2005710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, X.; Huang, Q.; Wang, S. Isolation of a novel lutein–protein complex from Chlorella vulgaris and its functional properties. Food Funct. 2015, 6, 1893–1899. [Google Scholar] [CrossRef]
- Niu, A.-j.; Wu, J.-m.; Yu, D.-h.; Wang, R. Protective effect of Lycium barbarum polysaccharides on oxidative damage in skeletal muscle of exhaustive exercise rats. Int. J. Biol. Macromol. 2008, 42, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cui, X.; Duan, M.; Ai, C.; Song, S.; Chen, X. In vitro fermentation of κ-carrageenan oligosaccharides by human gut microbiota and its inflammatory effect on HT29 cells. J. Funct. Foods 2019, 59, 80–91. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Q.; Xu, Q.; Zhang, Y.; Lin, B.; Guan, X.; Qian, L.; Zheng, Y. In vitro fermentation of O-acetyl-arabinoxylan from bamboo shavings by human colonic microbiota. Int. J. Biol. Macromol. 2019, 125, 27–34. [Google Scholar] [CrossRef]
- Chang, C.J.; Lin, C.S.; Lu, C.C.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Tseng, S.F.; Wu, T.R.; Chen, Y.; Young, J.D. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2017, 8, 16130. [Google Scholar] [CrossRef] [Green Version]
- Nagai, H.; Yano, T. Selective autophagy tolerates symbiotic bacteria in the Drosophila intestine. Autophagy 2021, 17, 1057–1058. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Balachandra, S.; Ngo, S.; O’Brien, L.E. Feedback regulation of steady-state epithelial turnover and organ size. Nature 2017, 548, 588–591. [Google Scholar] [CrossRef] [Green Version]
- Amcheslavsky, A.; Jiang, J.; Ip, Y.T. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell 2009, 4, 49–61. [Google Scholar] [CrossRef] [Green Version]
- Apidianakis, Y.; Rahme, L.G. Drosophila melanogaster as a model host for studying Pseudomonas aeruginosa infection. Nat. Protoc. 2009, 4, 1285–1294. [Google Scholar] [CrossRef]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA 2009, 106, 3698–3703. [Google Scholar] [CrossRef] [Green Version]
- Poeggeler, B.; Sambamurti, K.; Siedlak, S.L.; Perry, G.; Smith, M.A.; Pappolla, M.A. A novel endogenous indole protects rodent mitochondria and extends rotifer lifespan. PLoS ONE 2010, 5, e10206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, Q.; Yan, H.; Meng, Q.; Wang, S.; Li, G.; Zhu, Q.; Li, X.; Li, Q. Hydrogen peroxide plus ascorbic acid enhanced organic matter deconstructions and composting performances via changing microbial communities. J. Environ. Manag. 2021, 295, 113126. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jiang, Z.; Li, M.; Li, Q. A compost-derived thermophilic microbial consortium enhances the humification process and alters the microbial diversity during composting. J. Environ. Manag. 2019, 243, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Simhadri, R.K.; Fast, E.M.; Rong, G.; Schultz, M.J.; Frydman, H.M. The gut commensal microbiome of Drosophila melanogaster is modified by the endosymbiont wolbachia. Msphere 2017, 2, e00287-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.K.; Lee, J.B.; Huh, Y.R.; Am Jang, H.; Kim, C.H.; Yoo, J.W.; Lee, B.L. Burkholderia gut symbionts enhance the innate immunity of host Riptortus pedestris. Dev. Comp. Immunol. 2015, 53, 265–269. [Google Scholar] [CrossRef]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef]
- Auguet, M.; Viossat, I.; Marin, J.-G.; Chabrier, P.-E. Selective inhibition of inducible nitric oxide synthase by agmatine. Jpn. J. Pharmacol. 1995, 69, 285–287. [Google Scholar] [CrossRef] [Green Version]
- Han, B.; Sivaramakrishnan, P.; Lin, C.; Neve, I.; He, J.; Li, W.; Sowa, J.N.; Sizovs, A.; Du, G.; Wang, J. Microbial genetic composition tunes host longevity. Cell 2017, 169, 1249–1262. [Google Scholar] [CrossRef] [Green Version]
- Rand, M.; Vorojeikina, D.; Peppriell, A.; Gunderson, J.; Prince, L.M. Drosophotoxicology: Elucidating kinetic and dynamic pathways of methylmercury toxicity in a Drosophila model. Front. Genet. 2019, 10, 666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albrecht, S.C.; Barata, A.G.; Großhans, J.; Teleman, A.A.; Dick, T.P. In vivo mapping of hydrogen peroxide and oxidized glutathione reveals chemical and regional specificity of redox homeostasis. Cell Metab. 2011, 14, 819–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickering, A.M.; Linder, R.A.; Zhang, H.; Forman, H.J.; Davies, K. Nrf2-dependent induction of proteasome and Pa28αβ regulator are required for adaptation to oxidative stress. J. Biol. Chem. 2012, 287, 10021–10031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickering, A.M.; Staab, T.A.; Tower, J.; Sieburth, D.; Davies, K. A conserved role for the 20S proteasome and Nrf2 transcription factor in oxidative stress adaptation in mammals, Caenorhabditis elegans and Drosophila melanogaster. J. Exp. Biol. 2013, 216, 543–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhukel, A.; Madeo, F.; Sigrist, S.J. Spermidine boosts autophagy to protect from synapse aging. Autophagy 2017, 13, 444–445. [Google Scholar] [CrossRef] [Green Version]
- De Cabo, R.; Carmona-Gutierrez, D.; Bernier, M.; Hall, M.; Madeo, F. The search for antiaging interventions: From elixirs to fasting regimens. Cell 2014, 157, 1515–1526. [Google Scholar] [CrossRef] [Green Version]
- Qian, J.; Su, S.; Liu, P. Experimental approaches in delineating mTOR signaling. Genes 2020, 11, 738. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Guo, Y.; Cui, S.W.; Li, H.; Shan, Y.; Wang, H. Purple sweet potato extract extends lifespan by activating autophagy pathway in male Drosophila melanogaster. Exp. Gerontol. 2021, 144, 111190. [Google Scholar] [CrossRef] [PubMed]



| Genes | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Gene Accession Numbers |
|---|---|---|---|
| RP49 | AGGGTATCGACAACAGAGTG | CACCAGGAACTTCTTGAATC | NM_079843.4 |
| AMPKα | AGAGGTCTGCACCAAGTTCG | GTTTATTTGGTTGGCCGCGT | NM_057965.4 |
| Atg1 | AAGGGCAGACAAGAGTCCAT | GTTCTCCCGCTTCCTCCTTT | NM_140344.3 |
| Atg5 | ATATGCTTCCAGGCGGATCG | AACCACACAGCTCCATCCTG | NM_132162.4 |
| Atg8a | TCTAGCCACAGCAGTTAGCG | TTGTGTAGAGTGACCGTGCG | NM_167245.3 |
| GCL | GACACCGATACGCATTGCAC | CTCACCACGGAATCCTGCTT | NM_001298073.1 |
| GSTs | CAGACCGTCAAGGACAACGA | TCGCGCTTGACCATGTAGTT | NM_166216.2 |
| Nrf2 | AGCTTCTGTCGCATGGTTGA | AGCCGTTGCTAACATGTCCA | NM_170055.2 |
| SOD | ACCGACTCCAAGATTACGCTCT | GTTGCCCGTTGACTTGCTC | NM_057387.5 |
| mTOR | AAAGAGCCAGACAGACGTGG | CGACGCGAAGAGTTAAAGCG | NM_057719.4 |
| S6K | CGCAGGACGAGATGATGGA | TGGGATGGGTTGGTTGGT | NM_079217.3 |
| 4E-BP | ACCCTCTACTCCACCACTCC | GGAGTTTGGCTCAATGGGGA | NM_057947.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Li, Q.; Zhang, G.; Ma, C.; Dai, X. The Protective Effects of Carrageenan Oligosaccharides on Intestinal Oxidative Stress Damage of Female Drosophila melanogaster. Antioxidants 2021, 10, 1996. https://doi.org/10.3390/antiox10121996
Yang K, Li Q, Zhang G, Ma C, Dai X. The Protective Effects of Carrageenan Oligosaccharides on Intestinal Oxidative Stress Damage of Female Drosophila melanogaster. Antioxidants. 2021; 10(12):1996. https://doi.org/10.3390/antiox10121996
Chicago/Turabian StyleYang, Kun, Qiaowei Li, Guocai Zhang, Chao Ma, and Xianjun Dai. 2021. "The Protective Effects of Carrageenan Oligosaccharides on Intestinal Oxidative Stress Damage of Female Drosophila melanogaster" Antioxidants 10, no. 12: 1996. https://doi.org/10.3390/antiox10121996





