Herbal Preparation (Bromelain, Papain, Curcuma, Black Pepper) Enhances Mineralization and Reduces Glucocorticoid-Induced Osteoporosis in Zebrafish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals
2.3. Herbal Preparation
- Bromelain (1200 GDU/g) 215 mg
- Curcuma longa (extract, 95% total curcuminoids) 131.5 mg (125 mg)
- Papain (1:100) 125 mg
- Piper Nigrum (dry extract, 95% piperine) 1.05 mg (1 mg)
2.4. Chemicals
2.5. Embryo Treatments
2.6. Embryo Histochemical Analysis
2.7. Adult Treatments and Scales Collection
2.8. Bone Matrix Vital Staining
2.9. Biochemical TRAP and ALP Assays in Scales
2.10. Histological TRAP and ALP Assays in Scales
2.11. Statistics
3. Results
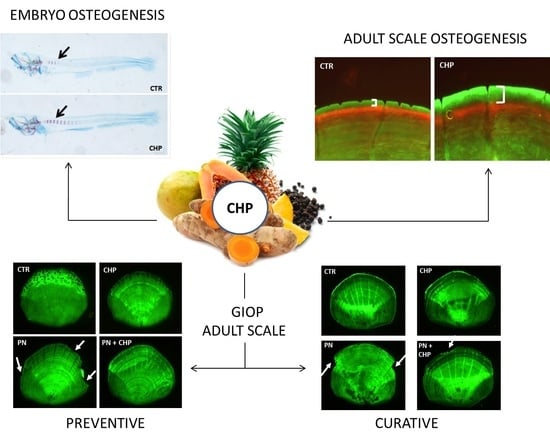
3.1. CHP Treatment Stimulates Osteogenesis in Zebrafish Embryos
3.2. CHP Stimulates the Deposition of New Mineralized Matrix in Juvenile Zebrafish Scale
3.3. Protective Effect of CHP on PN-Dependent Osteoporotic Phenotype in Adult Fish Scales
3.4. CHP Showed a Curative Effect on PN-Induced Bone Loss in Adult Fish Scales
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tarantino, U.; Iolascon, G.; Cianferotti, L.; Masi, L.; Marcucci, G.; Giusti, F.; Marini, F.; Parri, S.; Feola, M.; Rao, C.; et al. Clinical guidelines for the prevention and treatment of osteoporosis: Summary statements and recommendations from the Italian Society for Orthopaedics and Traumatology. J. Orthop. Traumatol. 2017, 18 (Suppl. 19), 3–36. [Google Scholar] [CrossRef] [Green Version]
- New, S.A. Intake of fruit and vegetables: Implications for bone health. Proc. Nutr. Soc. 2003, 62, 889–899. [Google Scholar] [CrossRef]
- Scorei, I.D.; Scorei, R.I. Calcium fructoborate helps control inflammation associated with diminished bone health. Biol. Trace Elem. Res. 2013, 155, 315–321. [Google Scholar] [CrossRef]
- Leong, D.J.; Choudhury, M.; Hirsh, D.M.; Hardin, J.A.; Cobelli, N.J.; Sun, H.B. Nutraceuticals: Potential for chondroprotection and molecular targeting of osteoarthritis. Int. J. Mol. Sci. 2013, 14, 23063–23085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, S.K.; Sah, A.K.; Jha, R.K.; Sah, P.; Shah, D.K. Turmeric (curcumin) remedies gastroprotective action. Pharmacogn. Rev. 2013, 7, 42–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folwarczna, J.; Zych, M.; Trzeciak, H.I. Effects of curcumin on the skeletal system in rats. Pharmacol. Rep. 2010, 62, 900–909. [Google Scholar] [CrossRef]
- Hussan, F.; Ibraheem, N.G.; Kamarudin, T.A.; Shuid, A.N.; Soelaiman, I.N.; Othman, F. Curcumin Protects against Ovariectomy-Induced Bone Changes in Rat Model. Evid. Based Complement. Altern. Med. 2012, 2012, 174916. [Google Scholar] [CrossRef]
- Chen, Z.; Xue, J.; Shen, T.; Ba, G.; Yu, D.; Fu, Q. Curcumin alleviates glucocorticoid-induced osteoporosis by protecting osteoblasts from apoptosis in vivo and in vitro. Clin. Exp. Pharmacol. Physiol. 2016, 43, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Rathnavelu, V.; Alitheen, N.B.; Sohila, S.; Kanagesan, S.; Ramesh, R. Potential role of bromelain in clinical and therapeutic applications. Biomed. Rep. 2016, 5, 283–288. [Google Scholar] [CrossRef] [Green Version]
- Singletary, K. Black Pepper: Overview of Health Benefits. Nutrition Today 2010, 45, 46–47. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, R.K.; Glaeser, H.; Becquemont, L.; Klotz, U.; Gupta, S.K.; Fromm, M.F. Piperine, a major constituent of black pepper, inhibits human P-glycoprotein and CYP3A4. In. J. Pharmacol. Exp. Ther. 2002, 302, 645–650. [Google Scholar] [CrossRef] [Green Version]
- Bartolozzi, E. The natural approach to osteoporosis. Clin. Cases Miner. Bone Metab. 2015, 12, 111–115. [Google Scholar] [CrossRef]
- Kimmel, C.B.; DeLaurier, A.; Ullmann, B.; Dowd, J.; McFadden, M. Modes of developmental outgrowth and shaping of a craniofacial bone in zebrafish. PLoS ONE 2010, 5, e9475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariotti, M.; Carnovali, M.; Banfi, G. Danio rerio: The Janus of the bone from embryo to scale. Clin. Cases Miner. Bone Metab. 2015, 12, 188–194. [Google Scholar] [CrossRef]
- Sire, J.Y.; Akimenko, M.A. Scale development in fish: A review, with description of sonic hedgehog (shh) expression in the zebrafish (Danio rerio). Int. J. Dev. Biol. 2004, 48, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Perrson, P.; Takagi, Y.; Björnsson, B.T. Tartrate resistant acid phosphatases as a marker for scale resorption in rainbow trout, Oncorhynchus mykiss: Effects of estradiol-17β treatment and refeeding. Fish Physiol. Biochem. 1995, 14, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Pasqualetti, S.; Banfi, G.; Mariotti, M. Osteoblast and osteoclast behavior in zebrafish cultured scales. Cell. Tissue Res. 2012, 350, 69–75. [Google Scholar] [CrossRef]
- Pasqualetti, S.; Congiu, T.; Banfi, G.; Mariotti, M. Alendronate rescued osteoporotic phenotype in a model of glucocorticoid-induced osteoporosis in adult zebrafish scale. Int. J. Exp. Path. 2015, 96, 11–20. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book, A Guide for the Laboratory Use of Zebrafish (Danio Rerio), 5th ed.; University of Oregon Press: Eugene, OR, USA, 2007. [Google Scholar]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Matthews, M.; Varga, Z.M. Anesthesia and Euthanasia in Zebrafish. ILAR J. 2012, 53, 192. [Google Scholar] [CrossRef] [Green Version]
- Walker, M.B.; Kimmel, C.B. A two-color acid-free cartilage and bone stain for zebrafish larvae. Biotech. Histochem. 2007, 82, 23–28. [Google Scholar] [CrossRef]
- Shimizu, K.; Funamoto, M.; Sunagawa, Y.; Shimizu, S.; Katanasaka, Y.; Miyazaki, Y.; Wada, H.; Hasegawa, K.; Morimoto, T. Anti-inflammatory action of Curcumin and Its Use in the Treatment of Lifestyle-related Diseases. Eur. Cardiol. 2019, 14, 117–122. [Google Scholar] [CrossRef] [Green Version]
- Akpinar, A.; Calisir, M.; Cansın Karakan, N.; Lektemur Alpan, A.; Goze, F.; Poyraz, O. Effects of Curcumin on Alveolar Bone Loss in Experimental Periodontitis in Rats: A Morphometric and Histopathologic Study. Int. J. Vitam. Nutr. Res. 2017, 87, 262–270. [Google Scholar] [CrossRef]
- Yang, X.; He, B.; Liu, P.; Yan, L.; Yang, M.; Li, D. Treatment with curcumin alleviates sublesional bone loss following spinal cord injury in rats. Eur. J. Pharmacol. 2015, 765, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016, 24, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jheng-Yu, W.; Chin-Yi, L.; Tien-Wei, L.; Chuian-Fu, K.; Yu-Der, W. Curcumin affects development of zebrafish embryo. Biol. Pharm. Bull. 2007, 30, 1336–1339. [Google Scholar] [CrossRef] [Green Version]
- Harishkumar, R.; Reddy, L.P.K.; Karadkar, S.H.; Murad, M.A.; Karthik, S.S.; Manigandan, S.; Selvaraj, C.I.; Christopher, J.G. Toxicity and Selective Biochemical Assessment of Quercetin, Gallic Acid, and Curcumin in Zebrafish. Biol. Pharm. Bull. 2019, 42, 1969–1976. [Google Scholar] [CrossRef] [Green Version]
- Son, H.E.; Kim, E.J.; Jang, W.G. Curcumin induces osteoblast differentiation through mild-endoplasmic reticulum stress-mediated such as BMP2 on osteoblast cells. Life Sci. 2018, 193, 34–39. [Google Scholar] [CrossRef]
- Gu, Q.; Cai, Y.; Huang, C.; Shi, Q.; Yang, H. Curcumin increases rat mesenchymal stem cell osteoblast differentiation but inhibits adipocyte differentiation. Pharmacognosy 2012, 8, 202–208. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Kim, D.H.; Heck, B.E.; Shaffer, M.; Hur, J.; Yoo, K.H. A natural supplement formula reduces anti-oxidative stress and enhances osteo-chondrogenic differentiation potential in mesenchymal stem cells. J. Clin. Biochem. Nutr. 2020, 66, 206–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.; Wang, F.; Gao, Y.; Yin, P.; Pan, C.; Liu, W.; Zhou, Z.; Wang, J. Curcumin protects human adipose-derived mesenchymal stem cells against oxidative stress-induced inhibition of osteogenesis. J. Pharmacol. Sci. 2016, 132, 192–200. [Google Scholar] [CrossRef] [Green Version]
- De Laurier, A.; Eames, B.F.; Blanco-Sánchez, B.; Peng, G.; He, X.; Swartz, M.E.; Ullmann, B.; Westerfield, M.; Kimmel, C.B.; Blanco-Sánchez, B. Zebrafish sp.7:EGFP: A transgenic for studying otic vesicle formation, skeletogenesis and bone regeneration. Genesis 2010, 48, 505–511. [Google Scholar] [CrossRef]
- Cox, B.D.; De Simone, A.; Tornini, V.A.; Singh, S.P.; Di Talia, S.; Poss, K.D. In Toto Imaging of Dynamic Osteoblast Behaviors in Regenerating Skeletal Bone. Curr. Biol. 2018, 28, 3937–3947. [Google Scholar] [CrossRef] [Green Version]
- Guowei, L.; Juyuan, B.; Yingxian, Z.; Xiaoyu, X.; Liang, Z.; Zhang, R. Curcumin improves bone microarchitecture in glucocorticoid-induced secondary osteoporosis mice through the activation of microRNA-365 via regulating MMP-9. Int. J. Clin. Exp. Pathol. 2015, 8, 15684–15695. Available online: http://www.ncbi.nlm.nih.gov/pmc/articles/pmc4730051/ (accessed on 13 December 2021).
- Dong, Y.; Huihui, Z.; Li, C. Piperine inhibit inflammation, alveolar bone loss and collagen fibers breakdown in a rat periodontitis model. J. Periodontal Res. 2015, 50, 758–765. [Google Scholar] [CrossRef]
- Sampaio Lacativa, P.G.; Fleiuss de Farias, M.L. Osteoporosis and inflammation. Arq. Bras. Endocrinol. Metabol. 2010, 54, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Fang, Z.; Song, C.; Kang, H.; Guo, Q.; Dong, Y.; Zhang, Y.; Peng, R.; Guan, H.; Li, F. Schisandrin B Inhibits Osteoclastogenesis and Protects Against Ovariectomy-Induced Bone Loss. Front. Pharmacol. 2020, 11, 1175. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cheng, R.; Liu, J.; Fang, J.; Wang, X.; Cui, Y.; Zhang, P.; Du, B. Linarin Protects against Cadmium-Induced Osteoporosis Via Reducing Oxidative Stress and Inflammation and Altering RANK/RANKL/OPG Pathway. Biol. Trace Elem. Res. 2021. ahead of print. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Mao, Y.; Dai, P.; Sun, X.; Zhang, X.; Cheng, H.; Wang, Y.; Banda, I.; Wu, G.; et al. Curcumin Protects Osteoblasts From Oxidative Stress-Induced Dysfunction via GSK3beta-Nrf2 Signaling Pathway. Front. Bioeng. Biotechnol. 2020, 8, 625. [Google Scholar] [CrossRef]
- Kim, W.K.; Ke, K.; Sul, O.J.; Kim, H.J.; Kim, S.H.; Lee, M.H.; Kim, H.J.; Kim, S.Y.; Chung, H.T.; Choi, H.S. Curcumin protects against ovariectomy-induced bone loss and decreases osteoclastogenesis. J. Cell. Biochem. 2011, 112, 3159–3166. [Google Scholar] [CrossRef]
- Xin, M.; Yang, Y.; Zhang, D.; Wang, J.; Chen, S.; Zhou, D. Attenuation of hind-limb suspension-induced bone loss by curcumin is associated with reduced oxidative stress and increased vitamin D receptor expression. Osteoporos. Int. 2015, 26, 2665–2676. [Google Scholar] [CrossRef]
- Deepak, V.; Kruger, M.C.; Joubert, A.; Coetzee, M. Piperine alleviates osteoclast formation through the p38/c-Fos/NFATc1 signaling axis. Biofactors 2015, 41, 403–413. [Google Scholar] [CrossRef]
- Ferah Okkay, I.; Okkay, U.; Cicek, B.; Yilmaz, A.; Yesilyurt, F.; Mendil, A.S.; Hacimuftuoglu, A. Neuroprotective effect of bromelain in 6-hydroxydopamine induced in vitro model of Parkinson’s disease. Mol. Biol. Rep. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Kim, K.M.; Lee, C.H.; Kim, Y.K.; Oh, J.; Kwak, H.B.; Kim, J.J. Effect of Water Extract of Papaya on RANKL-induced Osteoclast Differentiation. Korean J. Anat. 2009, 42, 179–185. Available online: https://www.koreamed.org/SearchBasic.php?RID=1472490 (accessed on 13 December 2021).




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carnovali, M.; Ramoni, G.; Banfi, G.; Mariotti, M. Herbal Preparation (Bromelain, Papain, Curcuma, Black Pepper) Enhances Mineralization and Reduces Glucocorticoid-Induced Osteoporosis in Zebrafish. Antioxidants 2021, 10, 1987. https://doi.org/10.3390/antiox10121987
Carnovali M, Ramoni G, Banfi G, Mariotti M. Herbal Preparation (Bromelain, Papain, Curcuma, Black Pepper) Enhances Mineralization and Reduces Glucocorticoid-Induced Osteoporosis in Zebrafish. Antioxidants. 2021; 10(12):1987. https://doi.org/10.3390/antiox10121987
Chicago/Turabian StyleCarnovali, Marta, Gina Ramoni, Giuseppe Banfi, and Massimo Mariotti. 2021. "Herbal Preparation (Bromelain, Papain, Curcuma, Black Pepper) Enhances Mineralization and Reduces Glucocorticoid-Induced Osteoporosis in Zebrafish" Antioxidants 10, no. 12: 1987. https://doi.org/10.3390/antiox10121987