Anti-Inflammatory Effects of the Novel Barbiturate Derivative MHY2699 in an MPTP-Induced Mouse Model of Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Primary Astrocyte Culture
2.3. Immunocytochemistry
2.4. ROS Measurements
2.5. Western Blot Analysis
2.6. RNA Isolation and Real-Time Polymerase Chain Reaction (Real-Time PCR)
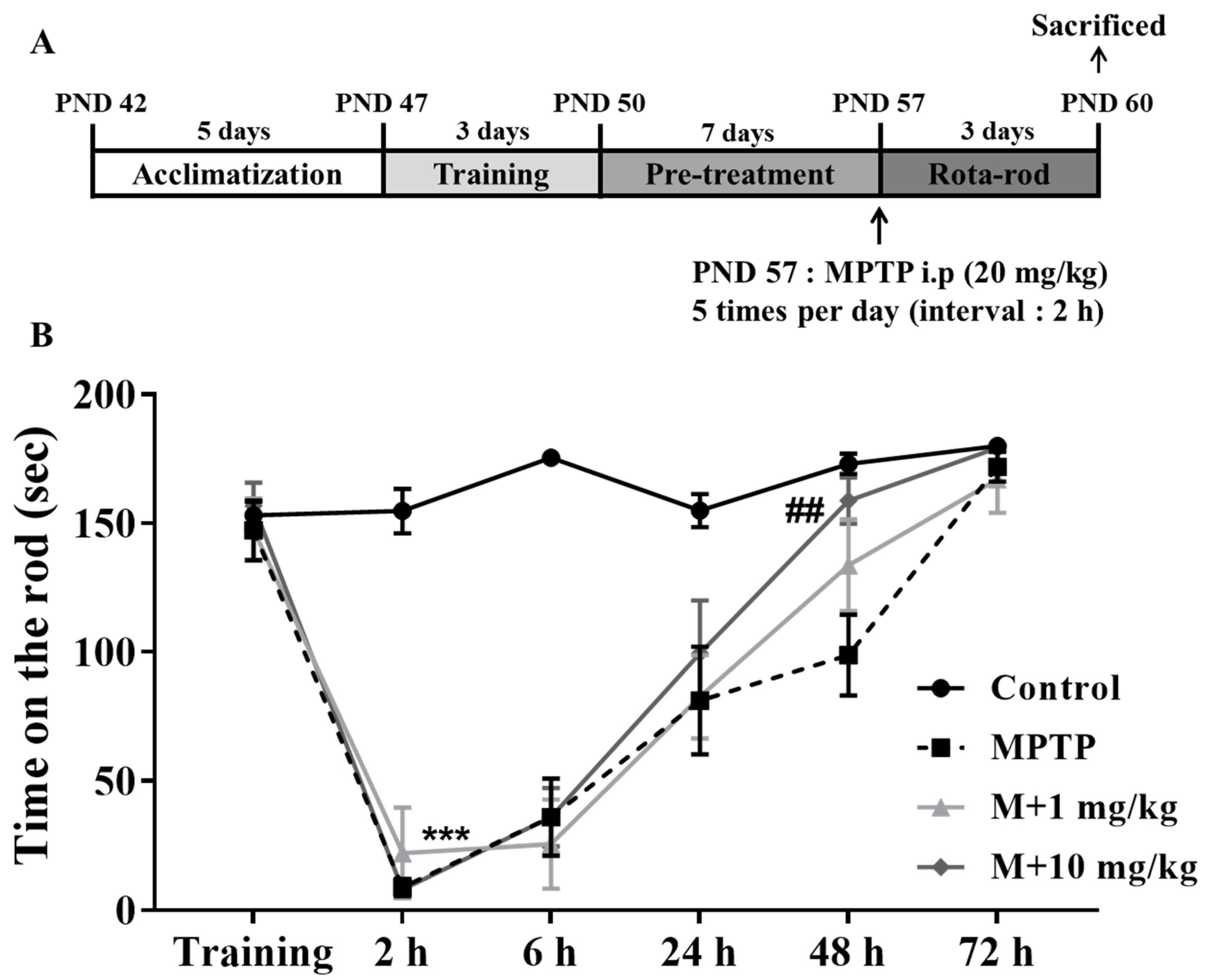
2.7. Animals and Drug Administration
2.8. Motor Performance Testing
2.9. Tissue Preparation
2.10. Diaminobenzidine Immunohistochemistry
2.11. Double Fluorescence Immunohistochemistry
2.12. Statistical Analysis
3. Results
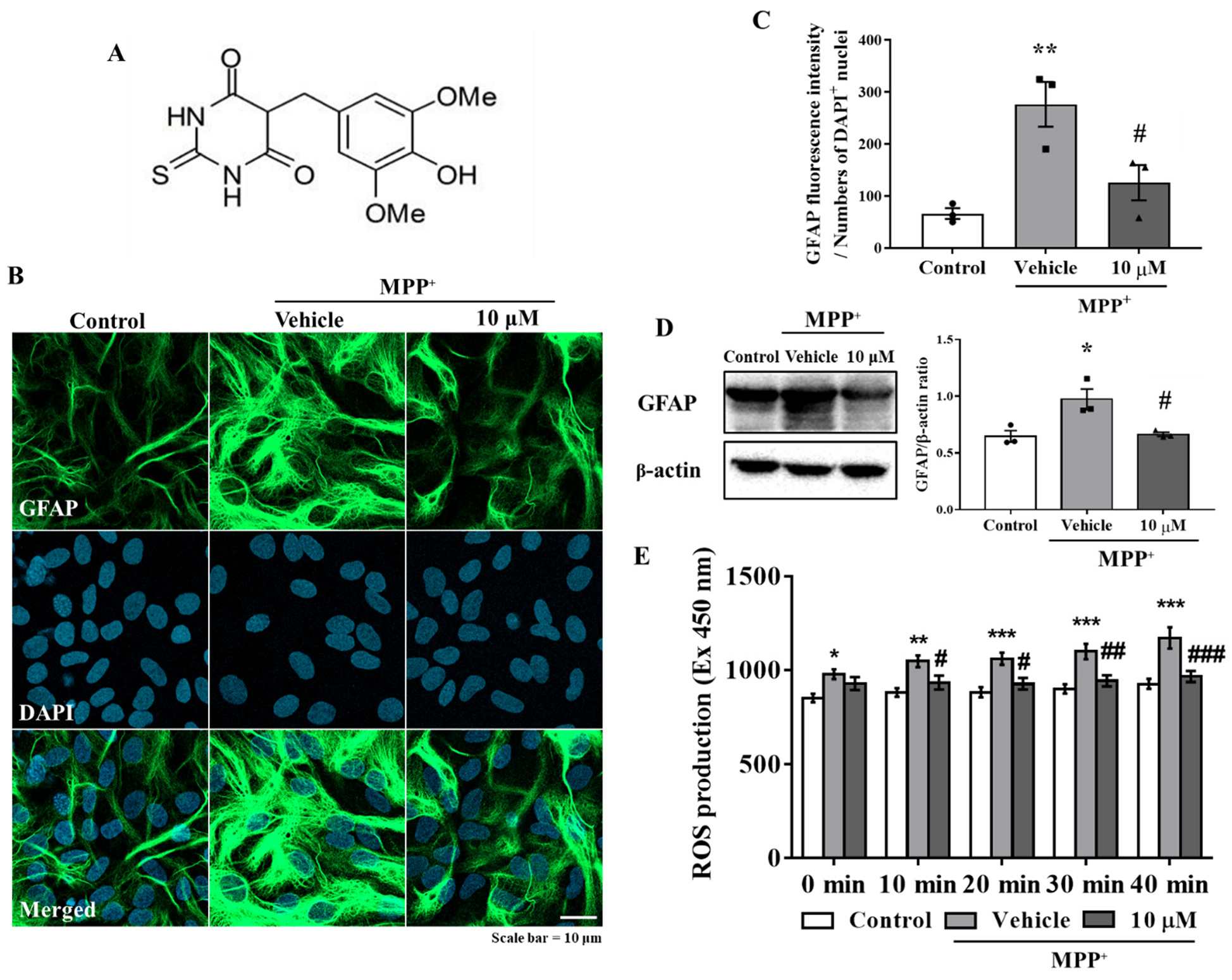
3.1. MHY2699 Attenuates MPP⁺-Induced Astroglial Activation and Oxidative Stress in Primary Astrocytes
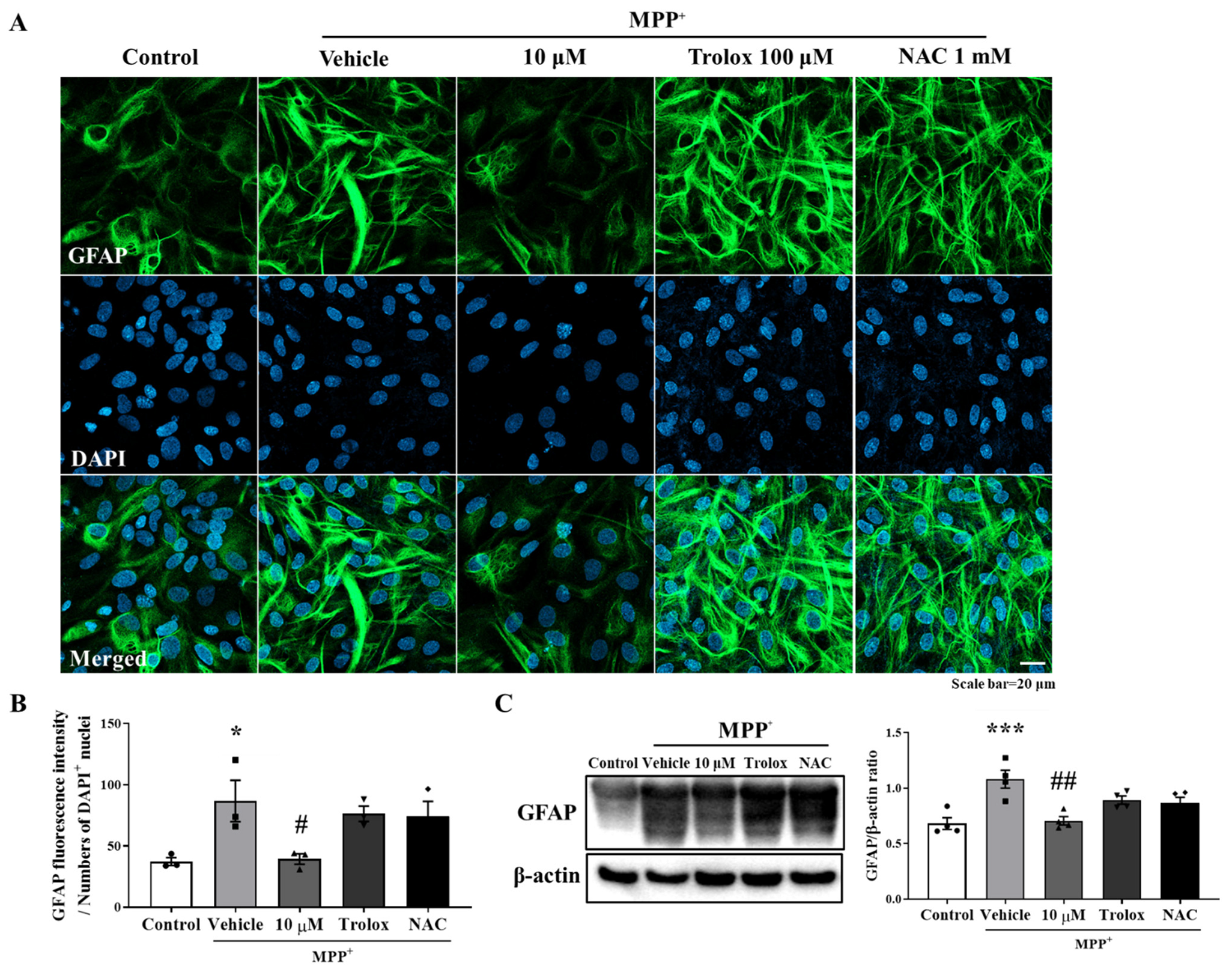
3.2. Anti-Inflammatory Effect of MHY2699 Was Independent of Its Antioxidant Activity
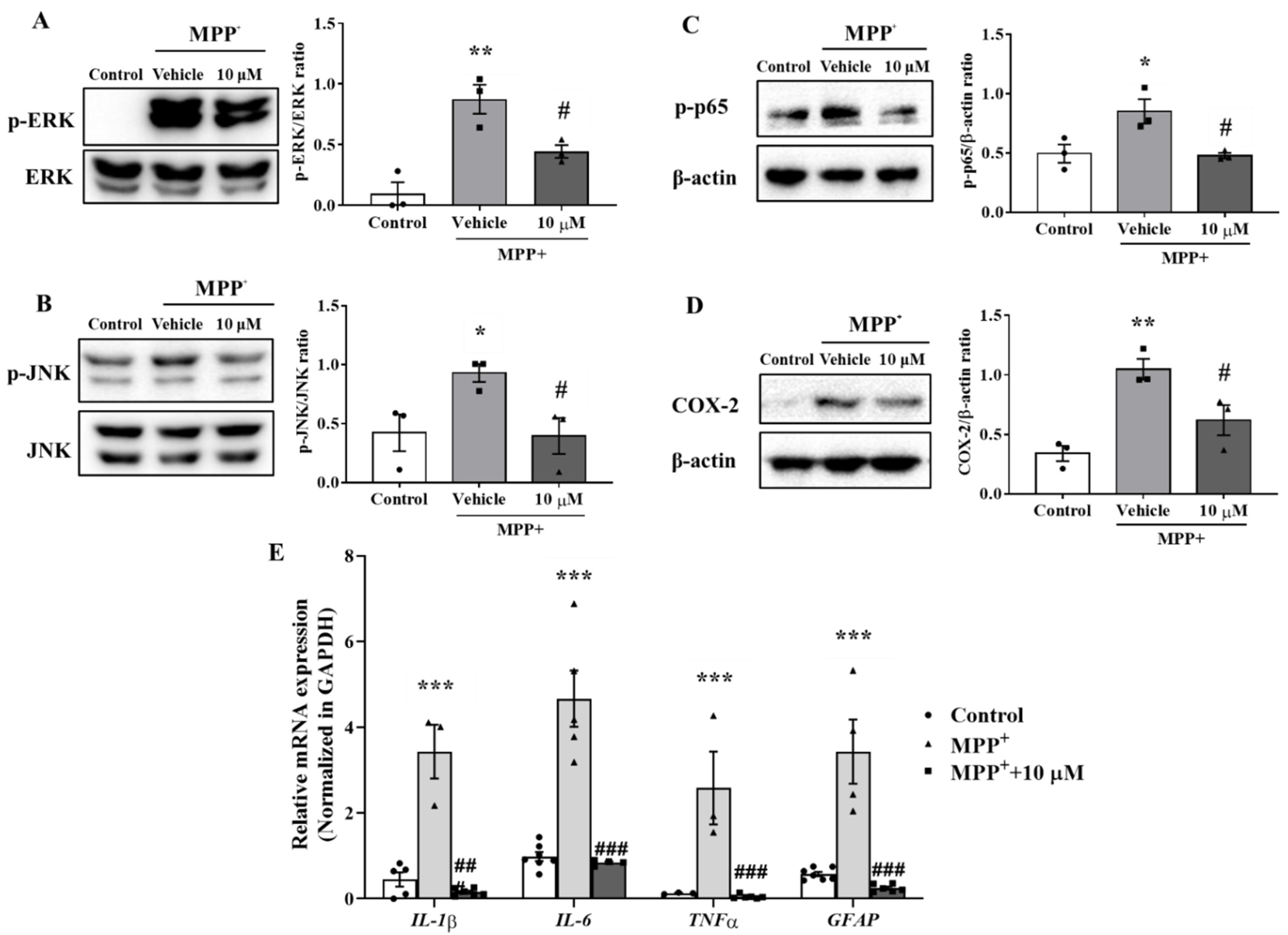
3.3. MHY2699 Suppressed the MAPK-NF-κB Pathway during Astroglial Activation
3.4. MHY2699 Improved MPTP-Induced Motor Dysfunction in the PD Mouse Model
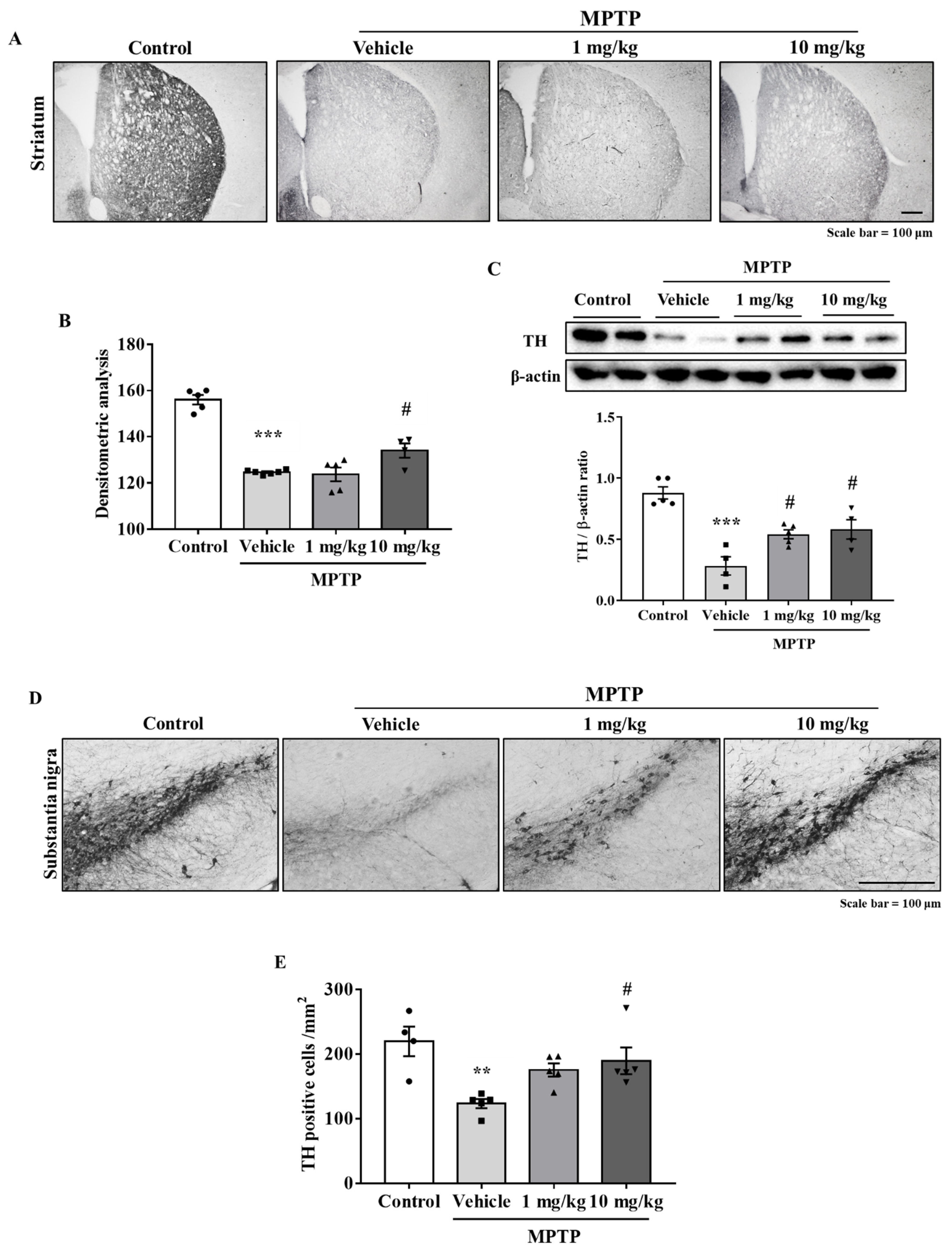
3.5. MHY2699 Prevented MPTP-Induced Dopaminergic Neuron Loss in the Nigrostriatal Pathway
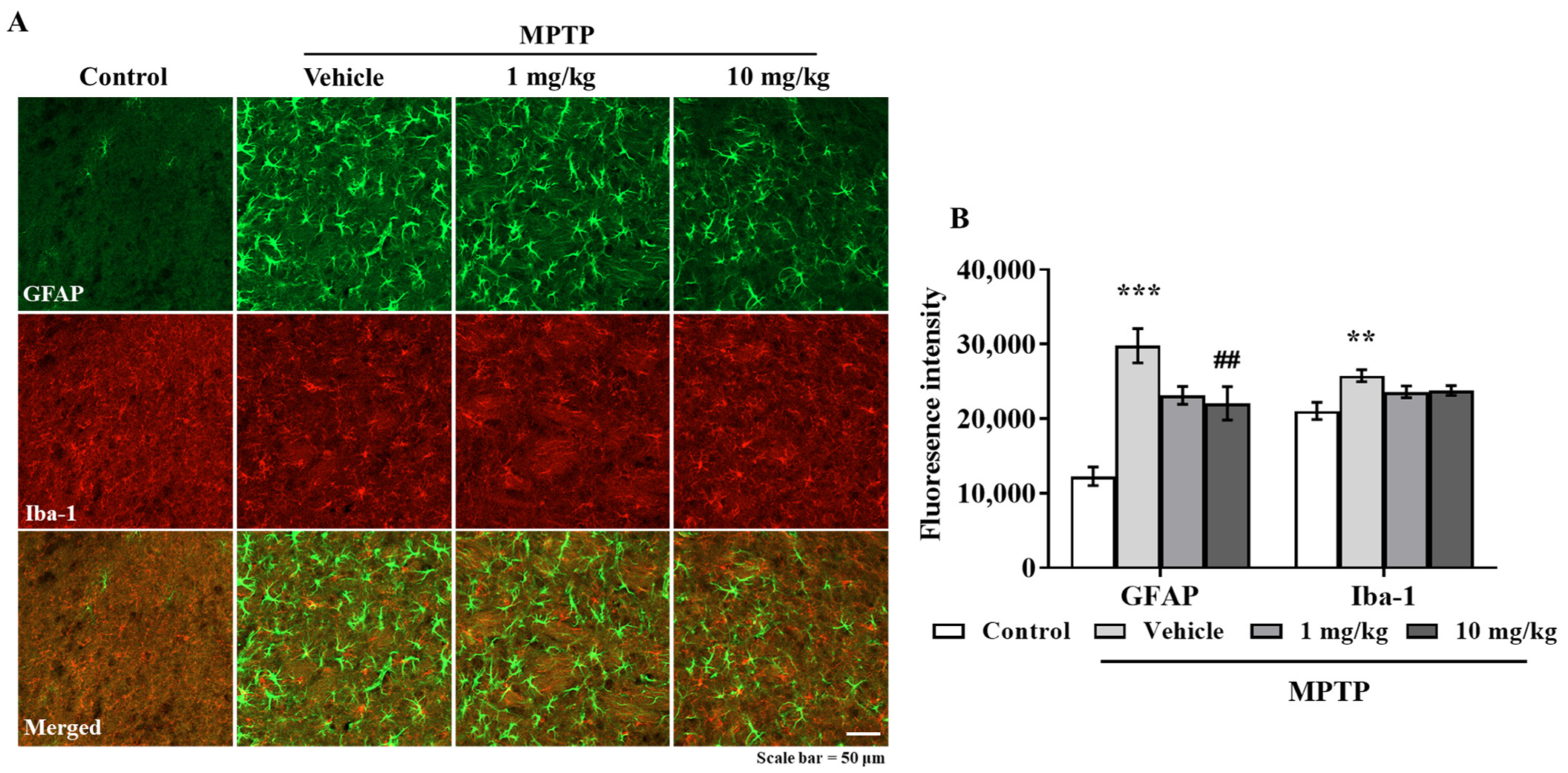
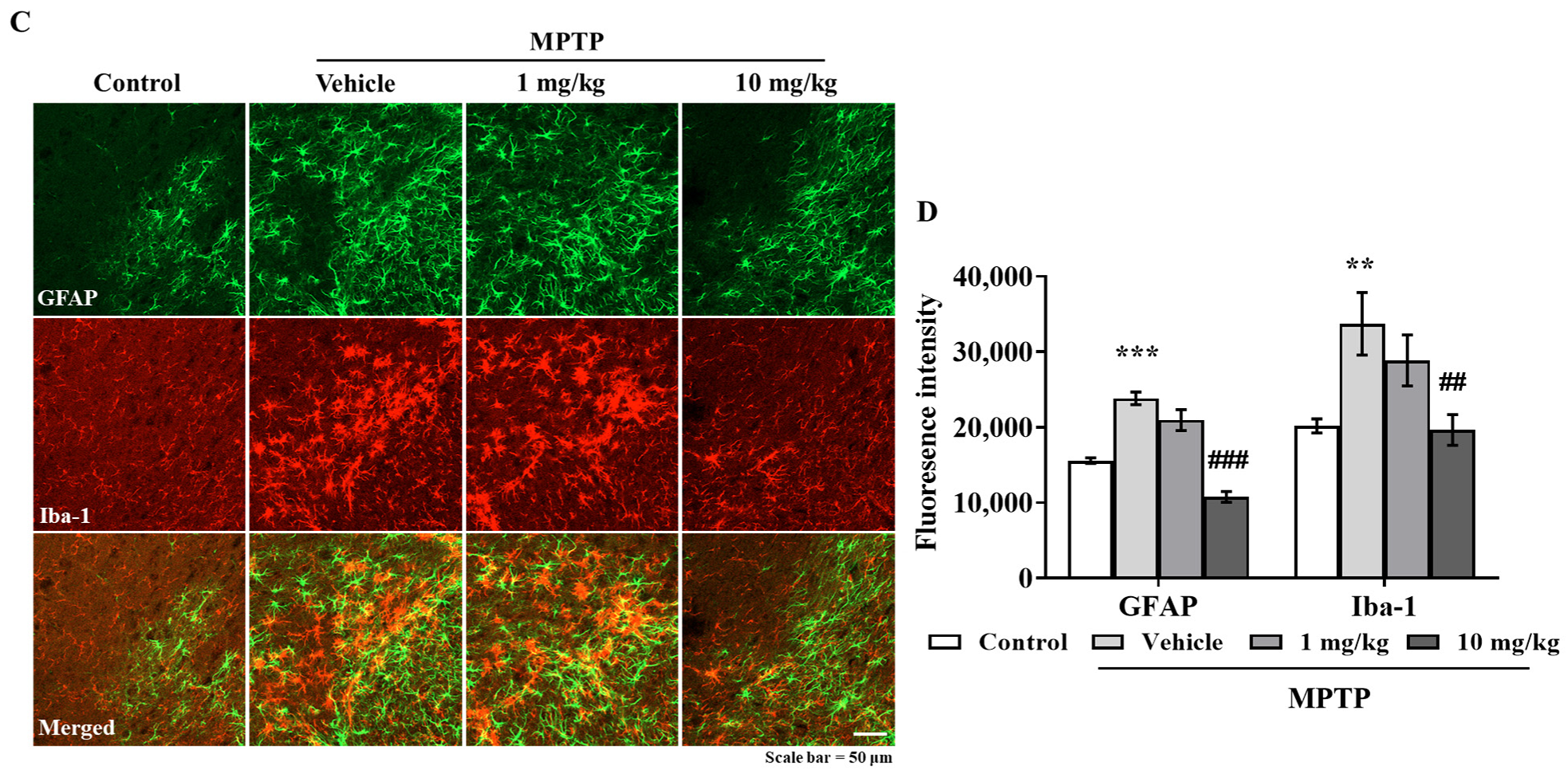
3.6. MHY2699 Suppresses MPTP-Induced Glial Activation in a PD Mouse Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.M.; Rothwell, N.J.; Gibson, R.M. The role of inflammation in CNS injury and disease. Br. J. Pharmacol. 2006, 147 (Suppl. 1), S232–S240. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, S.; Chang, S.C.; Lee, J. Significant roles of neuroinflammation in Parkinson’s disease: Therapeutic targets for PD prevention. Arch. Pharm. Res. 2019, 42, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Damier, P.; Hirsch, E.C.; Zhang, P.; Agid, Y.; Javoy-Agid, F. Glutathione peroxidase, glial cells and Parkinson’s disease. Neuroscience 1993, 52, 1–6. [Google Scholar] [CrossRef]
- Brochard, V.; Combadiere, B.; Prigent, A.; Laouar, Y.; Perrin, A.; Beray-Berthat, V.; Bonduelle, O.; Alvarez-Fischer, D.; Callebert, J.; Launay, J.M.; et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J. Clin. Investig. 2009, 119, 182–192. [Google Scholar] [CrossRef]
- Kuter, K.; Olech, L.; Glowacka, U. Prolonged Dysfunction of Astrocytes and Activation of Microglia Accelerate Degeneration of Dopaminergic Neurons in the Rat Substantia Nigra and Block Compensation of Early Motor Dysfunction Induced by 6-OHDA. Mol. Neurobiol. 2018, 55, 3049–3066. [Google Scholar] [CrossRef]
- Chen, W.W.; Zhang, X.; Huang, W.J. Role of neuroinflammation in neurodegenerative diseases (Review). Mol. Med. Rep. 2016, 13, 3391–3396. [Google Scholar] [CrossRef]
- de Pablos, R.M.; Herrera, A.J.; Espinosa-Oliva, A.M.; Sarmiento, M.; Munoz, M.F.; Machado, A.; Venero, J.L. Chronic stress enhances microglia activation and exacerbates death of nigral dopaminergic neurons under conditions of inflammation. J. Neuroinflamm. 2014, 11, 34. [Google Scholar] [CrossRef]
- Choi, S.Y.; Son, T.G.; Park, H.R.; Jang, Y.J.; Oh, S.B.; Jin, B.; Lee, J. Naphthazarin has a protective effect on the 1-methyl-4-phenyl-1,2,3,4-tetrahydropyridine-induced Parkinson’s disease model. J. Neurosci. Res. 2012, 90, 1842–1849. [Google Scholar] [CrossRef]
- Lee, Y.; Cho, J.H.; Lee, S.; Lee, W.; Chang, S.C.; Chung, H.Y.; Moon, H.R.; Lee, J. Neuroprotective effects of MHY908, a PPAR α/γ dual agonist, in a MPTP-induced Parkinson’s disease model. Brain Res. 2019, 1704, 47–58. [Google Scholar] [CrossRef]
- Lee, S.; Lee, Y.; Ha, S.; Chung, H.Y.; Kim, H.; Hur, J.S.; Lee, J. Anti-inflammatory effects of usnic acid in an MPTP-induced mouse model of Parkinson’s disease. Brain Res. 2020, 1730, 146642. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Suh, Y.J.; Yang, S.; Hong, D.G.; Ishigami, A.; Kim, H.; Hur, J.S.; Chang, S.C.; Lee, J. Neuroprotective and Anti-Inflammatory Effects of Evernic Acid in an MPTP-Induced Parkinson’s Disease Model. Int. J. Mol. Sci. 2021, 22, 2098. [Google Scholar] [CrossRef]
- Shahzad, S.; Shahzadi, L.; Mahmood, N.; Siddiqi, S.A.; Rauf, A.; Manzoor, F.; Chaudhry, A.A.; Rehman, I.U.; Yar, M. A new synthetic methodology for the preparation of biocompatible and organo-soluble barbituric- and thiobarbituric acid based chitosan derivatives for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 66, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Schulz, D.W.; Macdonald, R.L. Barbiturate enhancement of GABA-mediated inhibition and activation of chloride ion conductance: Correlation with anticonvulsant and anesthetic actions. Brain Res. 1981, 209, 177–188. [Google Scholar] [CrossRef]
- Sokmen, B.B.; Ugras, S.; Sarikaya, H.Y.; Ugras, H.I.; Yanardag, R. Antibacterial, antiurease, and antioxidant activities of some arylidene barbiturates. Appl. Biochem. Biotechnol. 2013, 171, 2030–2039. [Google Scholar] [CrossRef]
- Xu, C.; Wyman, A.R.; Alaamery, M.A.; Argueta, S.A.; Ivey, F.D.; Meyers, J.A.; Lerner, A.; Burdo, T.H.; Connolly, T.; Hoffman, C.S.; et al. Anti-inflammatory effects of novel barbituric acid derivatives in T lymphocytes. Int. Immunopharmacol. 2016, 38, 223–232. [Google Scholar] [CrossRef]
- Wang, Y.H.; Suk, F.M.; Liu, C.L.; Chen, T.L.; Twu, Y.C.; Hsu, M.H.; Liao, Y.J. Antifibrotic Effects of a Barbituric Acid Derivative on Liver Fibrosis by Blocking the NF-kappaB Signaling Pathway in Hepatic Stellate Cells. Front. Pharmacol. 2020, 11, 388. [Google Scholar] [CrossRef]
- Moon, K.M.; Lee, B.; Jeong, J.W.; Kim, D.H.; Park, Y.J.; Kim, H.R.; Park, J.Y.; Kim, M.J.; An, H.J.; Lee, E.K.; et al. Thio-barbiturate-derived compounds are novel antioxidants to prevent LPS-induced inflammation in the liver. Oncotarget 2017, 8, 91662–91673. [Google Scholar] [CrossRef][Green Version]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef]
- Geronikaki, A.A.; Gavalas, A.M. Antioxidants and inflammatory disease: Synthetic and natural antioxidants with anti-inflammatory activity. Comb. Chem. High Throughput Screen. 2006, 9, 425–442. [Google Scholar] [CrossRef]
- Borlongan, C.V.; Koutouzis, T.K.; Freeman, T.B.; Cahill, D.W.; Sanberg, P.R. Behavioral pathology induced by repeated systemic injections of 3-nitropropionic acid mimics the motoric symptoms of Huntington’s disease. Brain Res. 1995, 697, 254–257. [Google Scholar] [CrossRef]
- Mouse Brain Atlas. Available online: http://labs.gaidi.ca/mouse-brain-atlas/ (accessed on 19 September 2021).
- Lee, Y.; Heo, G.; Lee, K.M.; Kim, A.H.; Chung, K.W.; Im, E.; Chung, H.Y.; Lee, J. Neuroprotective effects of 2,4-dinitrophenol in an acute model of Parkinson’s disease. Brain Res. 2017, 1663, 184–193. [Google Scholar] [CrossRef]
- Filograna, R.; Beltramini, M.; Bubacco, L.; Bisaglia, M. Anti-Oxidants in Parkinson’s Disease Therapy: A Critical Point of View. Curr. Neuropharmacol. 2016, 14, 260–271. [Google Scholar] [CrossRef]
- Pawate, S.; Shen, Q.; Fan, F.; Bhat, N.R. Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J. Neurosci. Res. 2004, 77, 540–551. [Google Scholar] [CrossRef]
- Lee, E.; Park, H.R.; Ji, S.T.; Lee, Y.; Lee, J. Baicalein attenuates astroglial activation in the 1-methyl-4-phenyl-1,2,3,4-tetrahydropyridine-induced Parkinson’s disease model by downregulating the activations of nuclear factor-kappaB, ERK, and JNK. J. Neurosci. Res. 2014, 92, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Chun, H.J.; Lee, K.M.; Jung, Y.S.; Lee, J. Silibinin suppresses astroglial activation in a mouse model of acute Parkinson’s disease by modulating the ERK and JNK signaling pathways. Brain Res. 2015, 1627, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Kritsilis, M.; Rizou, S.V.; Koutsoudaki, P.N.; Evangelou, K.; Gorgoulis, V.G.; Papadopoulos, D. Ageing, Cellular Senescence and Neurodegenerative Disease. Int. J. Mol. Sci. 2018, 19, 2937. [Google Scholar] [CrossRef] [PubMed]
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in Parkinson’s disease. J. Parkinsons Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef]
- Gelders, G.; Baekelandt, V.; Van der Perren, A. Linking Neuroinflammation and Neurodegeneration in Parkinson’s Disease. J. Immunol. Res. 2018, 2018, 4784268. [Google Scholar] [CrossRef]
- Singh, S.; Nagalakshmi, D.; Sharma, K.K.; Ravichandiran, V. Natural antioxidants for neuroinflammatory disorders and possible involvement of Nrf2 pathway: A review. Heliyon 2021, 7, e06216. [Google Scholar] [CrossRef] [PubMed]
- Hunot, S.; Brugg, B.; Ricard, D.; Michel, P.P.; Muriel, M.P.; Ruberg, M.; Faucheux, B.A.; Agid, Y.; Hirsch, E.C. Nuclear translocation of NF-kappaB is increased in dopaminergic neurons of patients with parkinson disease. Proc. Natl. Acad. Sci. USA 1997, 94, 7531–7536. [Google Scholar] [CrossRef] [PubMed]
- Bassani, T.B.; Vital, M.A.; Rauh, L.K. Neuroinflammation in the pathophysiology of Parkinson’s disease and therapeutic evidence of anti-inflammatory drugs. Arq. Neuropsiquiatr. 2015, 73, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Son, T.G.; Park, H.R.; Kim, S.J.; Kim, K.; Kim, M.S.; Ishigami, A.; Handa, S.; Maruyama, N.; Chung, H.Y.; Lee, J. Senescence marker protein 30 is up-regulated in kainate-induced hippocampal damage through ERK-mediated astrocytosis. J. Neurosci. Res. 2009, 87, 2890–2897. [Google Scholar] [CrossRef]
- Bauerfeld, C.P.; Rastogi, R.; Pirockinaite, G.; Lee, I.; Huttemann, M.; Monks, B.; Birnbaum, M.J.; Franchi, L.; Nunez, G.; Samavati, L. TLR4-mediated AKT activation is MyD88/TRIF dependent and critical for induction of oxidative phosphorylation and mitochondrial transcription factor A in murine macrophages. J. Immunol. 2012, 188, 2847–2857. [Google Scholar] [CrossRef]
- Haque, M.A.; Jantan, I.; Harikrishnan, H. Zerumbone suppresses the activation of inflammatory mediators in LPS-stimulated U937 macrophages through MyD88-dependent NF-kappaB/MAPK/PI3K-Akt signaling pathways. Int. Immunopharmacol. 2018, 55, 312–322. [Google Scholar] [CrossRef]
- Kumar, A. Editorial: Neuroinflammation and Cognition. Front. Aging Neurosci. 2018, 10, 413. [Google Scholar] [CrossRef]
- Muthuraju, S.; Zakaria, R.; Karuppan, M.K.M.; Al-Rahbi, B. The Role of Neuroinflammation in Cellular Damage in Neurodegenerative Diseases. Biomed Res. Int. 2020, 2020, 9231452. [Google Scholar] [CrossRef]
- Lee, K.M.; Lee, Y.; Chun, H.J.; Kim, A.H.; Kim, J.Y.; Lee, J.Y.; Ishigami, A.; Lee, J. Neuroprotective and anti-inflammatory effects of morin in a murine model of Parkinson’s disease. J. Neurosci. Res. 2016, 94, 865–878. [Google Scholar] [CrossRef]
- Chung, Y.C.; Baek, J.Y.; Kim, S.R.; Ko, H.W.; Bok, E.; Shin, W.H.; Won, S.Y.; Jin, B.K. Capsaicin prevents degeneration of dopamine neurons by inhibiting glial activation and oxidative stress in the MPTP model of Parkinson’s disease. Exp. Mol. Med. 2017, 49, e298. [Google Scholar] [CrossRef]
- McKee, C.A.; Lukens, J.R. Emerging Roles for the Immune System in Traumatic Brain Injury. Front. Immunol. 2016, 7, 556. [Google Scholar] [CrossRef] [PubMed]
- Tschoe, C.; Bushnell, C.D.; Duncan, P.W.; Alexander-Miller, M.A.; Wolfe, S.Q. Neuroinflammation after Intracerebral Hemorrhage and Potential Therapeutic Targets. J. Stroke 2020, 22, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Commercialization Promotion Agency for R&D Outcomes. Neurodegenerative Disease Drug Market Trends. Volume 33. Available online: https://www.compa.re.kr/cop/bbs/selectBoardList.do?bbsId=BBSMSTR_000000000042 (accessed on 15 September 2021).
- McFarthing, K.; Buff, S.; Rafaloff, G.; Dominey, T.; Wyse, R.K.; Stott, S.R.W. Parkinson’s Disease Drug Therapies in the Clinical Trial Pipeline: 2020. J. Parkinsons Dis. 2020, 10, 757–774. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Suh, Y.J.; Lee, Y.; Yang, S.; Hong, D.G.; Thirumalai, D.; Chang, S.-C.; Chung, K.W.; Jung, Y.-S.; Moon, H.R.; et al. Anti-Inflammatory Effects of the Novel Barbiturate Derivative MHY2699 in an MPTP-Induced Mouse Model of Parkinson’s Disease. Antioxidants 2021, 10, 1855. https://doi.org/10.3390/antiox10111855
Lee S, Suh YJ, Lee Y, Yang S, Hong DG, Thirumalai D, Chang S-C, Chung KW, Jung Y-S, Moon HR, et al. Anti-Inflammatory Effects of the Novel Barbiturate Derivative MHY2699 in an MPTP-Induced Mouse Model of Parkinson’s Disease. Antioxidants. 2021; 10(11):1855. https://doi.org/10.3390/antiox10111855
Chicago/Turabian StyleLee, Seulah, Yeon Ji Suh, Yujeong Lee, Seonguk Yang, Dong Geun Hong, Dinakaran Thirumalai, Seung-Cheol Chang, Ki Wung Chung, Young-Suk Jung, Hyung Ryong Moon, and et al. 2021. "Anti-Inflammatory Effects of the Novel Barbiturate Derivative MHY2699 in an MPTP-Induced Mouse Model of Parkinson’s Disease" Antioxidants 10, no. 11: 1855. https://doi.org/10.3390/antiox10111855
APA StyleLee, S., Suh, Y. J., Lee, Y., Yang, S., Hong, D. G., Thirumalai, D., Chang, S.-C., Chung, K. W., Jung, Y.-S., Moon, H. R., Chung, H. Y., & Lee, J. (2021). Anti-Inflammatory Effects of the Novel Barbiturate Derivative MHY2699 in an MPTP-Induced Mouse Model of Parkinson’s Disease. Antioxidants, 10(11), 1855. https://doi.org/10.3390/antiox10111855






